Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases
Abstract
:1. Introduction
2. Proliferative Retinopathies
3. Animal Models
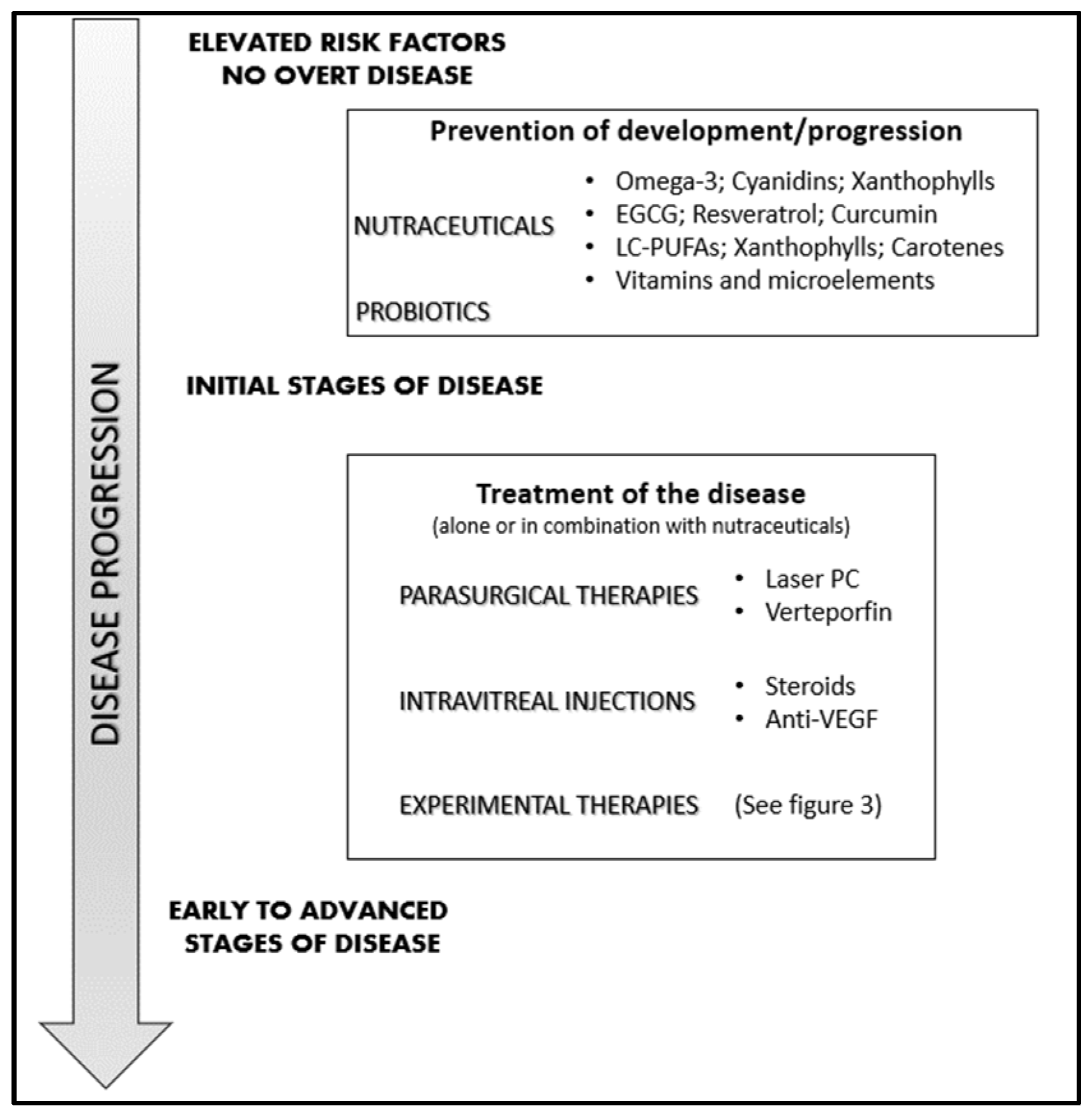
4. Management of Neovascular Retinal Diseases
4.1. Laser Photocoagulation and Vitrectomy
4.2. Photodynamic Therapy
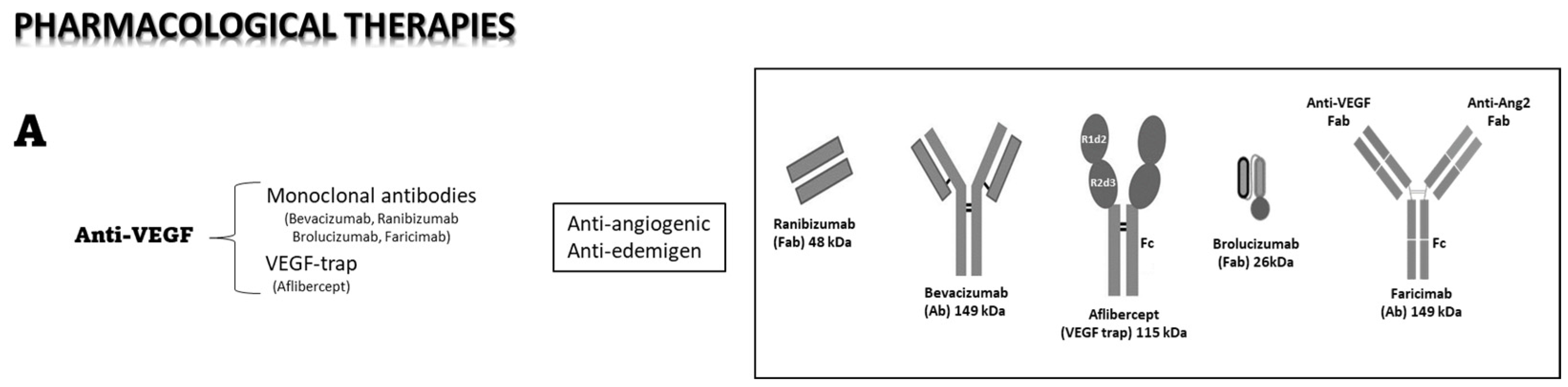
4.3. Pharmacological Therapies
4.4. Intraocular Anti-VEGF Therapy
4.5. Steroid Intravitreal Implants
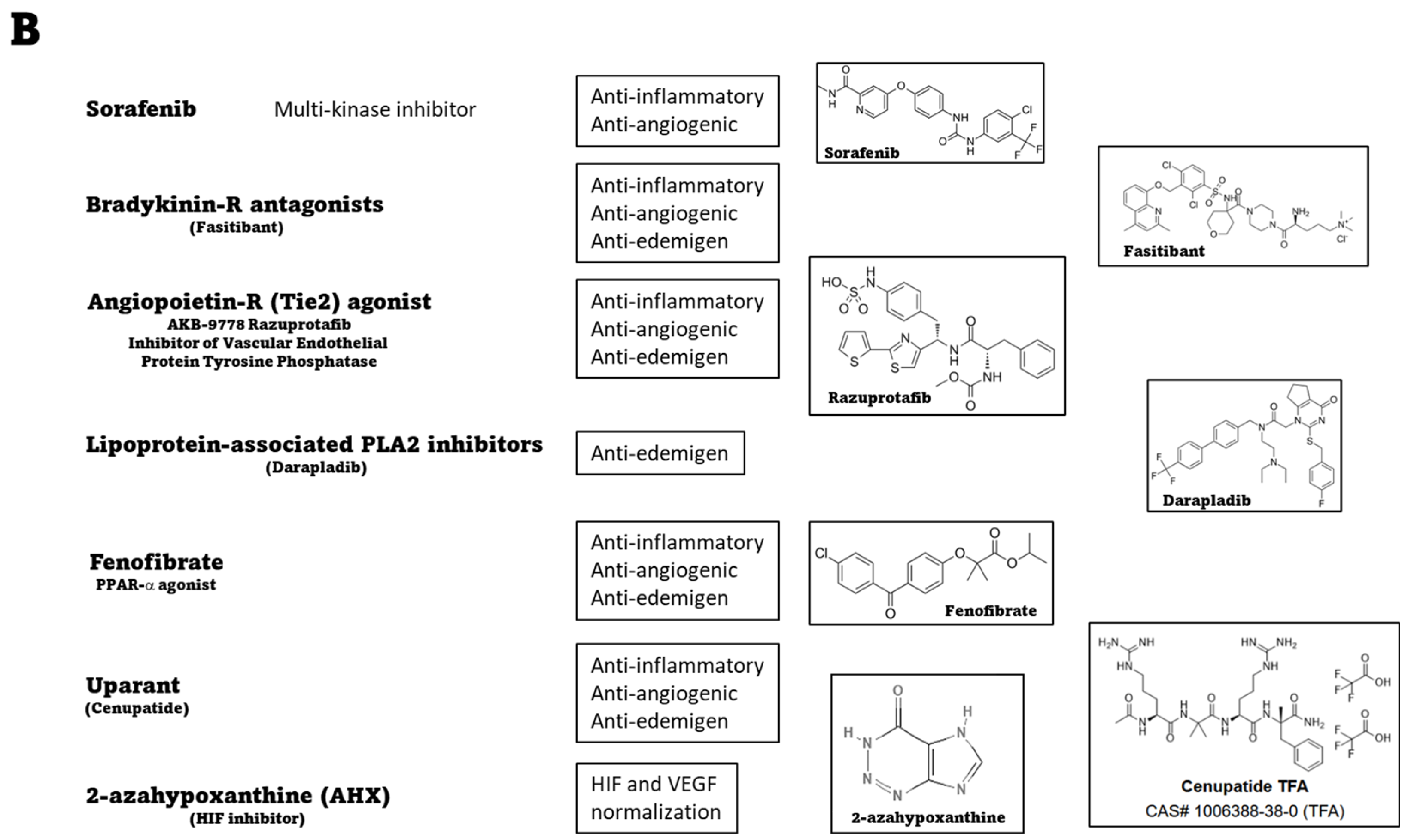
4.6. Systemic Therapies: Preclinical Evidence of Novel Treatments
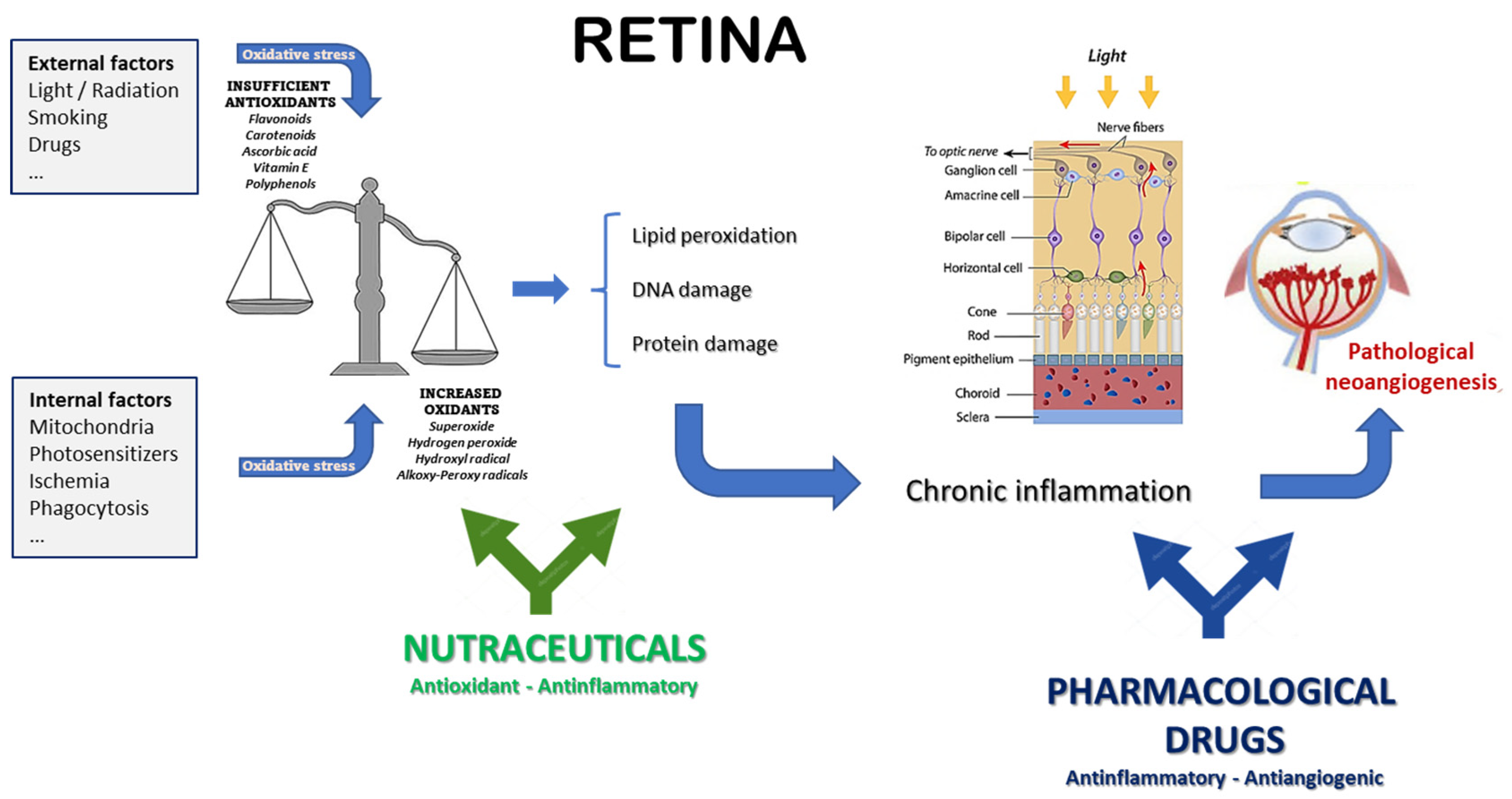
5. Nutraceuticals: Which Place in the Management of Neovascular Eye Diseases?
5.1. Association #1
5.2. Association #2
5.3. Association #3
5.4. Further Elements in the Associations
5.5. Role of the Gut’s Microbiota
5.6. Final Considerations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loscalzo, J.; Kohane, I.; Barabasi, A.L. Human disease classification in the postgenomic era: A complex systems approach to human pathobiology. Mol. Syst. Biol. 2007, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Novellino, E. To Nutraceuticals and Back: Rethinking a Concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, A.; Brennan, M.; Reinhardt, J.P. Prevalence and risk factors for self-reported visual impairment among middle-aged and older adults. Res. Aging 2005, 27, 307–326. [Google Scholar] [CrossRef]
- Johnson, G.J.; Minassian, D.C.; Weale, R.A.; West, S.K. (Eds.) Epidemiology of Eye Disease, 3rd ed.; World Scientific: Singapore, 2012. [Google Scholar]
- Khoo, H.E.; Ng, H.S.; Yap, W.S.; Goh, H.J.H.; Yim, H.S. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants 2019, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Choo, P.P.; Woi, P.J.; Bastion, M.C.; Omar, R.; Mustapha, M.; Din, N. Review of Evidence for the Usage of Antioxidants for Eye Aging. Biomed. Res. Int. 2022, 2022, 5810373. [Google Scholar] [CrossRef]
- Kim, D.; Choi, S.W.; Cho, J.; Been, J.H.; Choi, K.; Jiang, W.; Han, J.; Oh, J.; Park, C.; Choi, S.; et al. Discovery of Novel Small-Molecule Antiangiogenesis Agents to Treat Diabetic Retinopathy. J. Med. Chem. 2021, 64, 5535–5550. [Google Scholar] [CrossRef]
- Kumar Dubey, S.; Pradhan, R.; Hejmady, S.; Singhvi, G.; Choudhury, H.; Gorain, B.; Kesharwani, P. Emerging innovations in nano-enabled therapy against age-related macular degeneration: A paradigm shift. Int. J. Pharm. 2021, 600, 120499. [Google Scholar] [CrossRef]
- EUROSTAT. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Preventive_health_care_expenditure_statistics#Among_EU_Member_States.2C_spending_on_preventive_healthcare_ranged_between_1.0_.25_and_5.6_.25_of_current_healthcare_expenditure_in_2020. (accessed on 10 March 2023).
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Eichler, W.; Yafai, Y.; Wiedemann, P.; Fengler, D. Antineovascular agents in the treatment of eye diseases. Curr. Pharm. Des. 2006, 12, 2645–2660. [Google Scholar] [CrossRef]
- Morbidelli, L.; Terzuoli, E.; Donnini, S. Use of Nutraceuticals in Angiogenesis-Dependent Disorders. Molecules 2018, 23, 2676. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, R.B.; Bartoli, M.; Behzadian, M.A.; El-Remessy, A.E.; Al-Shabrawey, M.; Platt, D.H.; Caldwell, R.W. Vascular endothelial growth factor and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Diabetes Metab. Res. Rev. 2003, 19, 442–455. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Hietala, K.; Harjutsalo, V.; Forsblom, C.; Summanen, P.; Groop, P.H.; Finndlane Study Group. Age at onset and the risk of proliferative retinopathy in type 1 diabetes. Diabetes Care 2010, 33, 1315–1319. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhang, L.; Jia, P.; Xin, Z.; Yang, J.K. Early Onset Age Increased the Risk of Diabetic Retinopathy in Type 2 Diabetes Patients with Duration of 10-20 Years and HbA1C ≥7%: A Hospital-Based Case-Control Study. Int. J. Endocrinol. 2021, 2021, 5539654. [Google Scholar] [CrossRef]
- Gange, W.S.; Lopez, J.; Xu, B.Y.; Lung, K.; Seabury, S.A.; Toy, B.C. Incidence of Proliferative Diabetic Retinopathy and Other Neovascular Sequelae at 5 Years Following Diagnosis of Type 2 Diabetes. Diabetes Care 2021, 44, 2518–2526. [Google Scholar] [CrossRef]
- Im, J.H.B.; Jin, Y.P.; Chow, R.; Yan, P. Prevalence of diabetic macular edema based on optical coherence tomography in people with diabetes: A systematic review and meta-analysis. Surv. Ophthalmol. 2022, 67, 1244–1251. [Google Scholar] [CrossRef]
- Kokotas, H.; Grigoriadou, M.; Petersen, M.B. Age-related macular degeneration: Genetic and clinical findings. Clin. Chem. Lab. Med. 2011, 49, 601–616. [Google Scholar] [CrossRef]
- Silvestri, G.; Williams, M.A.; McAuley, C.; Oakes, K.; Sillery, E.; Henderson, D.C.; Ferguson, S.; Silvestri, V.; Muldrew, K.A. Drusen prevalence and pigmentary changes in Caucasians aged 18-54 years. Eye 2012, 26, 1357–1362. [Google Scholar] [CrossRef]
- de Jong, S.; Tang, J.; Clark, S.J. Age-related macular degeneration: A disease of extracellular complement amplification. Immunol. Rev. 2022, 313, 279–297. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular Macular Degeneration: A Review of Etiology, Risk Factors, and Recent Advances in Research and Therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- Gorin, M.B.; daSilva, M.J. Predictive genetics for AMD: Hype and hopes for genetics-based strategies for treatment and prevention. Exp. Eye Res. 2020, 191, 107894. [Google Scholar] [CrossRef] [PubMed]
- Csader, S.; Korhonen, S.; Kaarniranta, K.; Schwab, U. The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4273. [Google Scholar] [CrossRef] [PubMed]
- Vinekar, A.; Gangwe, A.; Agarwal, S.; Kulkarni, S.; Azad, R. Improving Retinopathy of Prematurity Care: A Medico-Legal Perspective. Asia Pac. J. Ophthalmol. 2021, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflamm. 2017, 14, 165. [Google Scholar] [CrossRef] [Green Version]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age-related macular degeneration. Mol. Aspects Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.; Barathi, V.A.; Chaurasia, S.S.; Wong, T.Y.; Kern, T.S. Update on animal models of diabetic retinopathy: From molecular approaches to mice and higher mammals. Dis. Model. Mech. 2012, 5, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Fruttiger, M. Oxygen-induced retinopathy: A model for vascular pathology in the retina. Eye 2010, 24, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.L.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Smith, A.G.; Kaiser, P.K. Emerging treatments for wet age-related macular degeneration. Expert. Opin. Emerg. Drugs. 2014, 19, 157–164. [Google Scholar] [CrossRef]
- Palmer, E.A.; Hardy, R.J.; Dobson, V.; Phelps, D.L.; Quinn, G.E.; Summers, C.G.; Krom, C.P.; Tung, B.; Cryotherapy for Retinopathy of Prematurity Cooperative Group. 15-year outcomes following threshold retinopathy of prematurity: Final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch. Ophthalmol. 2005, 123, 311–318. [Google Scholar] [CrossRef]
- Lenis, T.L.; Gunzenhauser, R.C.; Fung, S.S.M.; Dhindsa, Y.K.; Sarraf, D.; Pineles, S.L.; Tsui, I. Myopia and anterior segment optical coherence tomography findings in laser-treated retinopathy of prematurity eyes. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 86.e1–86.e7. [Google Scholar] [CrossRef]
- Tsang, J.K.W.; Liu, J.; Lo, A.C.Y. Vascular and Neuronal Protection in the Developing Retina: Potential Therapeutic Targets for Retinopathy of Prematurity. Int. J. Mol. Sci. 2019, 20, 4321. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.B.; Zheng, G.Y.; He, B.M.; Zhang, Y.; Zhou, Q. Clinical Efficacy and Safety of Propranolol in the Prevention and Treatment of Retinopathy of Prematurity: A Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 2021, 9, 631673. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Hasan, T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 195–214. [Google Scholar] [CrossRef]
- Mammo, Z.; Forooghian, F. Incidence of acute exudative maculopathy after reduced-fluence photodynamic therapy. Retin. Cases Brief Rep. 2017, 11, 217–220. [Google Scholar] [CrossRef]
- Wallsh, J.O.; Gallemore, R.P. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 2021, 10, 1049. [Google Scholar] [CrossRef]
- Jermak, C.M.; Dellacroce, J.T.; Heffez, J.; Peyman, G.A. Triamcinolone acetonide in ocular therapeutics. Surv. Ophthalmol. 2007, 52, 503–522. [Google Scholar] [CrossRef]
- Cáceres-del-Carpio, J.; Costa, R.D.; Haider, A.; Narayanan, R.; Kuppermann, B.D. Corticosteroids: Triamcinolone, Dexamethasone and Fluocinolone. Dev. Ophthalmol. 2016, 55, 221–231. [Google Scholar] [CrossRef]
- Irigoyen, C.; Alonso, A.A.; Sanchez-Molina, J.; Rodríguez-Hidalgo, M.; Lara-López, A.; Ruiz-Ederra, J. Subretinal Injection Techniques for Retinal Disease: A Review. J. Clin. Med. 2022, 11, 4717. [Google Scholar] [CrossRef]
- Ghoraba, H.H.; Akhavanrezayat, A.; Karaca, I.; Yavari, N.; Lajevardi, S.; Hwang, J.; Regenold, J.; Matsumiya, W.; Pham, B.; Zaidi, M.; et al. Ocular Gene Therapy: A Literature Review with Special Focus on Immune and Inflammatory Responses. Clin. Ophthalmol. 2022, 16, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Peral, A.; Mateo, J.; Domínguez-Godínez, C.O.; Carracedo, G.; Gómez, J.A.; Crooke, A.; Pintor, J. Therapeutic potential of topical administration of siRNAs against HIF-1α for corneal neovascularization. Exp. Eye Res. 2022, 219, 109036. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tsirukis, D.I.; Sun, Y. Targeting Neuroinflammation in Neovascular Retinal Diseases. Front. Pharmacol. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.; Henriques, J.; Carneiro, Â.; Marques-Neves, C.; Flores, R.; Castro-Sousa, J.P.; Meireles, A.; Gomes, N.; Nascimento, J.; Amaro, M.; et al. Guidelines for the Management of Center-Involving Diabetic Macular Edema: Treatment Options and Patient Monitorization. Clin. Ophthalmol. 2021, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef]
- Cai, S.; Yang, Q.; Li, X.; Zhang, Y. The efficacy and safety of aflibercept and conbercept in diabetic macular edema. Drug Des. Dev. Ther. 2018, 12, 3471–3483. [Google Scholar] [CrossRef] [Green Version]
- Halim, S.; Nugawela, M.; Chakravarthy, U.; Peto, T.; Madhusudhan, S.; Lenfestey, P.; Hamill, B.; Zheng, Y.; Parry, D.; Nicholson, L.; et al. Topographical Response of Retinal Neovascularization to Aflibercept or Panretinal Photocoagulation in Proliferative Diabetic Retinopathy: Post Hoc Analysis of the CLARITY Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 501–507, PMCID:PMC7953330. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W.; Rosenfeld, P.J.; Penha, F.M.; Wang, F.; Yehoshua, Z.; Bueno-Lopez, E.; Lopez, P.F. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina 2012, 32, 434–457. [Google Scholar] [CrossRef]
- Wang, J.K.; Huang, T.L.; Chang, P.Y.; Chen, Y.T.; Chang, C.W.; Chen, F.T.; Hsu, Y.R.; Chen, Y.J. Intravitreal aflibercept versus bevacizumab for treatment of myopic choroidal neovascularization. Sci. Rep. 2018, 8, 14389. [Google Scholar] [CrossRef] [Green Version]
- Ross, E.L.; Hutton, D.W.; Stein, J.D.; Bressler, N.M.; Jampol, L.M.; Glassman, A.R.; Diabetic Retinopathy Clinical Research Network. Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema Treatment: Analysis from the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol. 2016, 134, 888–896. [Google Scholar] [CrossRef]
- Binder, S. Loss of reactivity in intravitreal anti-VEGF therapy: Tachyphylaxis or tolerance? Br. J. Ophthalmol. 2012, 96, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Cheema, M.R.; DaCosta, J.; Talks, J. Ten-Year Real-World Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 279–287. [Google Scholar] [CrossRef]
- Bae, K.W.; Kim, D.I.; Hwang, D.D. The effect of intravitreal brolucizumab on choroidal thickness in patients with neovascular age-related macular degeneration. Sci. Rep. 2022, 12, 19855. [Google Scholar] [CrossRef]
- Nair, A.A.; Finn, A.P.; Sternberg, P., Jr. Spotlight on Faricimab in the Treatment of Wet Age-Related Macular Degeneration: Design, Development and Place in Therapy. Drug Des. Dev. Ther. 2022, 16, 3395–3400. [Google Scholar] [CrossRef]
- Lin, F.L.; Wang, P.Y.; Chuang, Y.F.; Wang, J.H.; Wong, V.H.Y.; Bui, B.V.; Liu, G.S. Gene Therapy Intervention in Neovascular Eye Disease: A Recent Update. Mol. Ther. 2020, 28, 2120–2138. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, A.; Zhang, H.; Wang, M.; Tang, Q.; Huang, Y.; Wang, L. Inhibition of retinal neovascularization by VEGF siRNA delivered via bioreducible lipid-like nanoparticles. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2407–2418. [Google Scholar] [CrossRef]
- Garba, A.O.; Mousa, S.A. Bevasiranib for the treatment of wet, age-related macular degeneration. Ophthalmol. Eye Dis. 2010, 2, 75–83. [Google Scholar] [CrossRef]
- Froger, N.; Matonti, F.; Roubeix, C.; Forster, V.; Ivkovic, I.; Brunel, N.; Baudouin, C.; Sahel, J.A.; Picaud, S. VEGF is an autocrine/paracrine neuroprotective factor for injured retinal ganglion neurons. Sci. Rep. 2020, 10, 12409. [Google Scholar] [CrossRef]
- Wingard, J.B.; Delzell, D.A.; Houlihan, N.V.; Lin, J.; Gieser, J.P. Incidence of Glaucoma or Ocular Hypertension After Repeated Anti-Vascular Endothelial Growth Factor Injections for Macular Degeneration. Clin. Ophthalmol. 2019, 13, 2563–2572. [Google Scholar] [CrossRef] [Green Version]
- Lind, J.T.; Gill, Z.; Seibold, L.K. Anti-VEGF Injection IOP Elevations. Eye-Wiki, the American Academy of Ophthalmology. 2022. Available online: https://eyewiki.aao.org/Anti-VEGF_Injection_IOP_Elevations (accessed on 10 March 2023).
- Fico, E.; Rosso, P.; Triaca, V.; Segatto, M.; Lambiase, A.; Tirassa, P. NGF Prevents Loss of TrkA/VEGFR2 Cells, and VEGF Isoform Dysregulation in the Retina of Adult Diabetic Rats. Cells 2022, 11, 3246. [Google Scholar] [CrossRef]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.C.; Yeh, P.T.; Chen, S.N.; Yang, C.M.; Lai, C.C.; Kuo, H.K. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in Taiwan. Ophthalmology 2011, 118, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.A.; Schwartz, S.; García-Aguirre, G.; Quiroz-Mercado, H. Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br. J. Ophthalmol. 2013, 97, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castellanos, M.A.; Schwartz, S.; Hernández-Rojas, M.L.; Kon-Jara, V.A.; García-Aguirre, G.; Guerrero-Naranjo, J.L.; Chan, R.V.; Quiroz-Mercado, H. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina 2013, 33, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Urbančič, M.; Gardašević Topčić, I. Dexamethasone implant in the management of diabetic macular edema from clinician’s perspective. Clin. Ophthalmol. 2019, 13, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.Y.; Jee, D.; Kwon, J.W. Characteristics of diabetic macular edema patients refractory to anti-VEGF treatments and a dexamethasone implant. PLoS ONE 2019, 14, e0222364. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.M.; Chaya, C.J.; Kahook, M.Y.; Wirostko, B.M. Intraocular Pressure Elevation Following Intravitreal Anti-VEGF Injections: Short- and Long-term Considerations. J. Glaucoma. 2021, 30, 1019–1026. [Google Scholar] [CrossRef]
- Catanzaro, O.; Labal, E.; Andornino, A.; Capponi, J.A.; Di Martino, I.; Sirois, P. Blockade of early and late retinal biochemical alterations associated with diabetes development by the selective bradykinin B1 receptor antagonist R-954. Peptides 2012, 34, 349–352. [Google Scholar] [CrossRef]
- Othman, R.; Cagnone, G.; Joyal, J.S.; Vaucher, E.; Couture, R. Kinins and Their Receptors as Potential Therapeutic Targets in Retinal Pathologies. Cells 2021, 10, 1913. [Google Scholar] [CrossRef]
- Terzuoli, E.; Morbidelli, L.; Nannelli, G.; Giachetti, A.; Donnini, S.; Ziche, M. Involvement of Bradykinin B2 Receptor in Pathological Vascularization in Oxygen-Induced Retinopathy in Mice and Rabbit Cornea. Int. J. Mol. Sci. 2018, 19, 330. [Google Scholar] [CrossRef] [Green Version]
- Campochiaro, P.A.; Peters, K.G. Targeting Tie2 for Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diab. Rep. 2016, 16, 126. [Google Scholar] [CrossRef]
- Khan, M.; Aziz, A.A.; Shafi, N.A.; Abbas, T.; Khanani, A.M. Targeting Angiopoietin in Retinal Vascular Diseases: A Literature Review and Summary of Clinical Trials Involving Faricimab. Cells 2020, 9, 1869. [Google Scholar] [CrossRef]
- Khanani, A.M.; Russell, M.W.; Aziz, A.A.; Danzig, C.J.; Weng, C.Y.; Eichenbaum, D.A.; Singh, R.P. Angiopoietins as Potential Targets in Management of Retinal Disease. Clin. Ophthalmol. 2021, 15, 3747–3755. [Google Scholar] [CrossRef]
- Canning, P.; Kenny, B.A.; Prise, V.; Glenn, J.; Sarker, M.H.; Hudson, N.; Brandt, M.; Lopez, F.J.; Gale, D.; Luthert, P.J.; et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc. Natl. Acad. Sci. USA 2016, 113, 7213–7218. [Google Scholar] [CrossRef]
- Stewart, S.; Lois, N. Fenofibrate for Diabetic Retinopathy. Asia Pac. J. Ophthalmol. 2018, 7, 422–426. [Google Scholar] [CrossRef]
- Duran, C.L.; Howell, D.W.; Dave, J.M.; Smith, R.L.; Torrie, M.E.; Essner, J.J.; Bayless, K.J. Molecular Regulation of Sprouting Angiogenesis. Compr. Physiol. 2017, 8, 153–235. [Google Scholar] [CrossRef]
- Cammalleri, M.; Dal Monte, M.; Pavone, V.; De Rosa, M.; Rusciano, D.; Bagnoli, P. The uPAR System as a Potential Therapeutic Target in the Diseased Eye. Cells 2019, 8, 925. [Google Scholar] [CrossRef] [Green Version]
- Santonocito, M.; Zappulla, C.; Viola, S.; La Rosa, L.R.; Solfato, E.; Abbate, I.; Tarallo, V.; Apicella, I.; Platania, C.B.M.; Maugeri, G.; et al. Assessment of a New Nanostructured Microemulsion System for Ocular Delivery of Sorafenib to Posterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 4404. [Google Scholar] [CrossRef]
- Vinores, S.A.; Xiao, W.H.; Aslam, S.; Shen, J.; Oshima, Y.; Nambu, H.; Liu, H.; Carmeliet, P.; Campochiaro, P.A. Implication of the hypoxia response element of the Vegf promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J. Cell Physiol. 2006, 206, 749–758. [Google Scholar] [CrossRef]
- Martinez-Alejo, J.M.; Baiza-Duran, L.M.; Quintana-Hau, J.D. Novel therapies for proliferative retinopathies. Ther. Adv. Chronic Dis. 2022, 13, 20406223221140395. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, S.; Li, S.; Yu, C.; Huang, D.; Chen, H.; Yin, X. CircPDE4B inhibits retinal pathological angiogenesis via promoting degradation of HIF-1α though targeting miR-181c. IUBMB Life 2020, 72, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Medori, M.C.; Naureen, Z.; Dhuli, K.; Placidi, G.; Falsini, B.; Bertelli, M. Dietary supplements in retinal diseases, glaucoma, and other ocular conditions. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E189–E199. [Google Scholar] [CrossRef]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Milluzzo, A.; Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; Mazzone, M.G.; Sciacca, L.; Agodi, A. Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review. Nutrients 2022, 14, 4430. [Google Scholar] [CrossRef]
- Castro-Castaneda, C.R.; Altamirano-Lamarque, F.; Ortega-Macías, A.G.; Santa Cruz-Pavlovich, F.J.; Gonzalez-De la Rosa, A.; Armendariz-Borunda, J.; Santos, A.; Navarro-Partida, J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014, PMCID:PMC9740859. [Google Scholar] [CrossRef] [PubMed]
- Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. [Google Scholar] [CrossRef]
- Rusciano, D.; Pezzino, S.; Olivieri, M.; Cristaldi, M.; Spampinato, G. Food Supplements in the Treatment of Ophthalmic Diseases: Preclinical and Clinical Studies. J. Pharmacol. Pharm. Res. 2020, 3, 1–34. [Google Scholar] [CrossRef]
- Reinders, M.E.; Sho, M.; Izawa, A.; Wang, P.; Mukhopadhyay, D.; Koss, K.E.; Geehan, C.S.; Luster, A.D.; Sayegh, M.H.; Briscoe, D.M. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Investig. 2003, 112, 1655–1665. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef] [Green Version]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Díaz-López, A.; Babio, N.; Martínez-González, M.A.; Corella, D.; Amor, A.J.; Fitó, M.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2015, 38, 2134–2141, Erratum in: Diabetes Care 2018, 41, 2260–2261. [Google Scholar] [CrossRef] [Green Version]
- Pall, M.L.; Levine, S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Sapieha, P.; Chen, J.; Stahl, A.; Seaward, M.R.; Favazza, T.L.; Juan, A.M.; Hatton, C.J.; Joyal, J.-S.; Krah, N.M.; Dennison, R.J.; et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabetes. 2012, 2, e36. [Google Scholar] [CrossRef] [Green Version]
- Eynard, A.R.; Repossi, G. Role of ω3 polyunsaturated fatty acids in diabetic retinopathy: A morphological and metabolically cross talk among blood retina barriers damage, autoimmunity and chronic inflammation. Lipids Health Dis. 2019, 18, 114. [Google Scholar] [CrossRef] [Green Version]
- Chew, E.Y. Dietary Intake of Omega-3 Fatty Acids from Fish and Risk of Diabetic Retinopathy. JAMA 2017, 317, 2226–2227. [Google Scholar] [CrossRef]
- Rosenberg, K. Omega-3 Fatty Acid Intake Lowers Risk of Diabetic Retinopathy. Am. J. Nurs. 2017, 117, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Ortín, L.; Argente, M.; Guindo, J.L.; López-Bernal, M.D.; López-Román, F.J.; García, M.J.; Domingo, J.C.; Lajara, J. Combined intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema: Two-Year Randomized Single-Blind Controlled Trial Results. Retina 2017, 37, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Ortín, L.; Argente, M.; Guindo, J.L.; López-Bernal, M.D.; López-Román, F.J.; Domingo, J.C.; Lajara, J. Three-year outcomes in a randomized single-blind controlled trial of intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema. Retina 2019, 39, 1083–1090. [Google Scholar] [CrossRef]
- Duda, M.; Kawula, K.; Pawlak, A.; Sarna, T.; Wisniewska-Becker, A. EPR Studies on the Properties of Model Photoreceptor Membranes Made of Natural and Synthetic Lipids. Cell Biochem. Biophys. 2017, 75, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Lardner, E.; Kvanta, A.; Rusciano, D.; André, H.; Bagnoli, P. Efficacy of a Fatty Acids Dietary Supplement in a Polyethylene Glycol-Induced Mouse Model of Retinal Degeneration. Nutrients 2017, 9, 1079. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Fu, Z.; Liegl, R.; Chen, J.; Hellström, A.; Smith, L.E. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am. J. Clin. Nutr. 2017, 106, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Aiello, L.P.; Pierce, E.A.; Foley, E.D.; Takagi, H.; Chen, H.; Riddle, L.; Ferrara, N.; King, G.L.; Smith, L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10457–10461. [Google Scholar] [CrossRef]
- NoA, L. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436, Erratum in: Arch. Ophthalmol. 2008, 126, 1251. [Google Scholar] [CrossRef] [Green Version]
- NoA, L. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015, Erratum in: JAMA 2013, 310, 208. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bell, B.A.; Song, Y.; Zhang, K.; Anderson, B.; Axelsen, P.H.; Bohannan, W.; Agbaga, M.; Park, H.G.; James, G.; et al. Deuterated docosahexaenoic acid protects against oxidative stress and geographic atrophy-like retinal degeneration in a mouse model with iron overload. Aging Cell 2022, 21, e13579. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, X.; Fan, Y.; Wang, D.; Li, B.; Zhou, J.; Pei, C.; Ma, L. Dietary omega-3 polyunsaturated fatty acids and fish intake and risk of age-related macular degeneration. Clin. Nutr. 2021, 40, 5662–5673. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.; AREDS and AREDS2 Research Groups. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Meng, X.T.; Shi, Y.Y.; Hong-Yan, Z. Dietary omega-3 LCPUFA intake in the prevention of neovascular age-related macular degeneration: A systematic review and meta-analysis. Nutr. Hosp. 2022, 39, 910–915. (In English) [Google Scholar]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Paik, S.S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sim, S.; Jeon, J.H.; Chun, M.H.; Kim, I.B. Anthocyanins from the seed coat of black soybean reduce retinal degeneration induced by N-methyl-N-nitrosourea. Exp. Eye Res. 2012, 97, 55–62. [Google Scholar] [CrossRef]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296, Erratum in: J. Nat. Prod. 1999, 62, 802. [Google Scholar] [CrossRef]
- Canovai, A.; Amato, R.; Melecchi, A.; Dal Monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Preventive Efficacy of an Antioxidant Compound on Blood Retinal Barrier Breakdown and Visual Dysfunction in Streptozotocin-Induced Diabetic Rats. Front. Pharmacol. 2022, 12, 811818. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [Green Version]
- Silván, J.M.; Reguero, M.; de Pascual-Teresa, S. A protective effect of anthocyanins and xanthophylls on UVB-induced damage in retinal pigment epithelial cells. Food Funct. 2016, 7, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Nakamura, Y.; Tachibanaki, S.; Kawamura, S.; Hirayama, M. Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J. Agric. Food Chem. 2003, 51, 3560–3563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Wu, H.; Li, D.J.; Song, J.F.; Xiao, Y.D.; Liu, C.Q.; Zhou, J.Z.; Sui, Z.Q. Protective Effects of Blueberry Anthocyanins against H2O2-Induced Oxidative Injuries in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2018, 66, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, C.; Wu, W.; Liu, T.; Ji, B.; Zhou, F. Cyanidin-3-glucoside Alleviates 4-Hydroxyhexenal-Induced NLRP3 Inflammasome Activation via JNK-c-Jun/AP-1 Pathway in Human Retinal Pigment Epithelial Cells. J. Immunol. Res. 2018, 2018, 5604610. [Google Scholar] [CrossRef] [Green Version]
- Amato, R.; Canovai, A.; Melecchi, A.; Pezzino, S.; Corsaro, R.; Monte, M.D.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Dietary Supplementation of Antioxidant Compounds Prevents Light-Induced Retinal Damage in a Rat Model. Biomedicines 2021, 9, 1177. [Google Scholar] [CrossRef]
- Huang, S.; Yang, N.; Liu, Y.; Hu, L.; Zhao, J.; Gao, J.; Li, Y.; Li, C.; Zhang, X.; Huang, T. Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr. Res. 2012, 32, 530–536. [Google Scholar] [CrossRef]
- Mohn, E.S.; Erdman, J.W., Jr.; Kuchan, M.J.; Neuringer, M.; Johnson, E.J. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: Relationship to DHA oxidation products. PLoS ONE 2017, 12, e0186767. [Google Scholar] [CrossRef] [Green Version]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary identification of the human macular pigment. Vis. Vision. Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010, 53, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; O’Dea, K. Plasma carotenoids and diabetic retinopathy. Br. J. Nutr. 2009, 101, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.C.; Wu, C.R.; Wang, Z.L.; Wang, L.Y.; Han, Y.; Sun, S.L.; Li, Q.S.; Ma, L. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar] [CrossRef]
- Hu, B.J.; Hu, Y.N.; Lin, S.; Ma, W.J.; Li, X.R. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int. J. Ophthalmol. 2011, 4, 303–306. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Garcia-Medina, M.; Zanon-Moreno, V.; Pons-Vazquez, S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 637–643. [Google Scholar] [CrossRef]
- Lawlor, S.M.; O’Brien, N.M. Astaxanthin: Antioxidant effects in chicken embryo fibroblasts. Nutr. Res. 1995, 15, 1695–1704. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef] [Green Version]
- Yeh, P.T.; Huang, H.W.; Yang, C.M.; Yang, W.S.; Yang, C.H. Astaxanthin Inhibits Expression of Retinal Oxidative Stress and Inflammatory Mediators in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, F.; Hu, X.; Chen, J.; Wen, X.; Sun, Y.; Liu, Y.; Tang, R.; Zheng, K.; Song, Y. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol. Behav. 2015, 151, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.P.; Sun, L.; Yu, H.S.; Liang, L.P.; Li, W.; Ding, H.; Song, X.B.; Zhang, L.J. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, A.; Subczynski, W.K. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic. Biol. Med. 2006, 41, 1257–1265. [Google Scholar] [CrossRef]
- Wisniewska-Becker, A.; Nawrocki, G.; Duda, M.; Subczynski, W.K. Structural aspects of the antioxidant activity of lutein in a model of photoreceptor membranes. Acta Biochim. Pol. 2012, 59, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Subczynski, W.; Wisniewska-Becker, A.; Widomska, J. Xanthophyll–membrane interactions. In Carotenoids and Retinal Disease; Landrum, J.T., Nolan, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 203–222. [Google Scholar]
- Biswal, M.R.; Justis, B.D.; Han, P.; Li, H.; Gierhart, D.; Dorey, C.K.; Lewin, A.S. Daily zeaxanthin supplementation prevents atrophy of the retinal pigment epithelium (RPE) in a mouse model of mitochondrial oxidative stress. PLoS ONE 2018, 13, e0203816. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef] [Green Version]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [Green Version]
- Thurnham, D.I. Macular zeaxanthins and lutein—A review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr. Res. Rev. 2007, 20, 163–179. [Google Scholar] [CrossRef] [Green Version]
- Fernando, C.D.; Soysa, P. Simple isocratic method for simultaneous determination of caffeine and catechins in tea products by HPLC. Springerplus 2016, 5, 970. [Google Scholar] [CrossRef] [Green Version]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin Gallate Is the Most Effective Catechin Against Antioxidant Stress via Hydrogen Peroxide and Radical Scavenging Activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, K.P.; Yang, Y.P.; Qin, Y.J.; Li, W.Y.; Chan, S.O.; Wang, C.C.; Pang, C.P. Effects of EGCG content in green tea extract on pharmacokinetics, oxidative status and expression of inflammatory and apoptotic genes in the rat ocular tissues. J. Nutr. Biochem. 2015, 26, 1357–1367. [Google Scholar] [CrossRef]
- Zhang, B.; Osborne, N.N. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006, 1124, 176–187. [Google Scholar] [CrossRef]
- Peng, P.H.; Ko, M.L.; Chen, C.F. Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp. Eye Res. 2008, 86, 637–646. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, L.; Shen, C.; Wan, H.; Xu, L.; Wang, N.; Jonas, J.B. Neuroprotective effect of epigallocatechin-3-gallate against N-methyl-D-aspartate-induced excitotoxicity in the adult rat retina. Acta Ophthalmol. 2012, 90, e609–e615. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.; Faria, J.B.; Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.M.; Huang, J.H.; Lin, H.H.; Chiang, H.S.; Chen, B.H.; Hong, J.Y.; Hung, C.F. Protective effects of (-)-epigallocatechin gallate on UVA-induced damage in ARPE19 cells. Mol. Vis. 2008, 14, 2528–2534. [Google Scholar]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, R. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar] [CrossRef]
- Garbisa, S.; Sartor, L.; Biggin, S.; Salvato, B.; Benelli, R.; Albini, A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 2001, 91, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aica, I.; Donà, M.; Sartor, L.; Pezzato, E.; Garbisa, S. (-)Epigallocatechin-3-gallate directly inhibits MT1-MMP activity, leading to accumulation of nonactivated MMP-2 at the cell surface. Lab. Investig. 2002, 82, 1685–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.D.; Ellis, L.M. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int. J. Exp. Pathol. 2001, 82, 309–316. [Google Scholar] [CrossRef]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Chen, Q.; Srivastava, R.K. Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to enhance antiangiogenic effects of EGCG through activation of FOXO transcription factor. J. Mol. Signal. 2008, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, Z.K.; Liang, S. Epigallocatechin-3-gallate protects retinal vascular endothelial cells from high glucose stress in vitro via the MAPK/ERK-VEGF pathway. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Xu, J.; Tu, Y.; Wang, Y.; Xu, X.; Sun, X.; Xie, L.; Zhao, Q.; Guo, Y.; Gu, Y.; Du, J.; et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed. Pharmacother. 2020, 121, 109606. [Google Scholar] [CrossRef] [PubMed]
- Bola, C.; Bartlett, H.; Eperjesi, F. Resveratrol and the eye: Activity and molecular mechanisms. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 699–713. [Google Scholar] [CrossRef]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The Role of Resveratrol in Eye Diseases-A Review of the Literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Ying, J.; Shi, T.; Wang, P. Resveratrol Prevents ROS-Induced Apoptosis in High Glucose-Treated Retinal Capillary Endothelial Cells via the Activation of AMPK/Sirt1/PGC-1α Pathway. Oxid. Med. Cell Longev. 2017, 2017, 7584691. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Lin, C.W.; Hsieh, M.C.; Wu, H.J.; Wu, W.S.; Wu, W.C.; Kao, Y.H. High mobility group B1 up-regulates angiogenic and fibrogenic factors in human retinal pigment epithelial ARPE-19 cells. Cell Signal. 2017, 40, 248–257. [Google Scholar] [CrossRef]
- Losso, J.N.; Truax, R.E.; Richard, G. Trans-resveratrol inhibits hyperglycemia-induced inflammation and connexin downregulation in retinal pigment epithelial cells. J. Agric. Food Chem. 2010, 58, 8246–8252. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, C.H.; Li, H.J.; Hsiao, C.Y.; Su, C.C.; Lee, P.L.; Hung, C.F. Protective effects of resveratrol against UVA-induced damage in ARPE19 cells. Int. J. Mol. Sci. 2015, 16, 5789–5802. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Kubota, S.; Tsubota, K.; Ozawa, Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J. Nutr. Biochem. 2014, 25, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Subramani, M.; Ponnalagu, M.; Krishna, L.; Jeyabalan, N.; Chevour, P.; Sharma, A.; Jayadev, C.; Shetty, R.; Begum, N.; Archunan, G.; et al. Resveratrol reverses the adverse effects of bevacizumab on cultured ARPE-19 cells. Sci. Rep. 2017, 7, 12242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bråkenhielm, E.; Cao, R.; Cao, Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001, 15, 1798–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Fu, Z.D.; Wang, F.; Liu, H.Y.; Han, R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J. Asian Nat. Prod. Res. 2005, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Guerin, K.I.; Chen, J.; Michán, S.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Juan, A.M.; Hatton, C.J.; et al. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(−/−) mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2809–2816. [Google Scholar] [CrossRef] [Green Version]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases-A review. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1457–1473. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Borgia, A.; Sorrentino, T.; Montesano, G.; Tsoutsanis, P.; Cancian, G.; Verma, Y.; De Rosa, F.P.; Romano, M.R. Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside. Int. J. Mol. Sci. 2022, 23, 3557. [Google Scholar] [CrossRef] [PubMed]
- Franzone, F.; Nebbioso, M.; Pergolizzi, T.; Attanasio, G.; Musacchio, A.; Greco, A.; Limoli, P.G.; Artico, M.; Spandidos, D.A.; Taurone, S.; et al. Anti-inflammatory role of curcumin in retinal disorders (Review). Exp. Ther. Med. 2021, 22, 790. [Google Scholar] [CrossRef]
- Nebbioso, M.; Franzone, F.; Greco, A.; Gharbiya, M.; Bonfiglio, V.; Polimeni, A. Recent Advances and Disputes About Curcumin in Retinal Diseases. Clin. Ophthalmol. 2021, 15, 2553–2571. [Google Scholar] [CrossRef]
- NoA, L. Joint Expert Committee on Food Additives Evaluation of Certain Food Additives and Contaminants: Eightieth Report of the Joint FAO/WHO Expert Committee on Food Additives: Rome, 16–25 June 2015; WHO Technical Report Series; WHO: Geneva, Switzerland, 2016; ISBN 978-92-4-120995-3.
- Cheng, A.L.; Hsu, C.-H.; Lin, J.K.; Hsu, M.M.; Ho, Y.-F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.-R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal Protection and Distribution of Curcumin in Vitro and in Vivo. Front. Pharmacol. 2018, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2179–2184. [Google Scholar] [CrossRef]
- Woo, J.M.; Shin, D.Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901–908. [Google Scholar]
- Li, Y.; Zou, X.; Cao, K.; Xu, J.; Yue, T.; Dai, F.; Zhou, B.; Lu, W.; Feng, Z.; Liu, J. Curcumin analog 1, 5-bis (2-trifluoromethylphenyl)-1, 4-pentadien-3-one exhibits enhanced ability on Nrf2 activation and protection against acrolein-induced ARPE-19 cell toxicity. Toxicol. Appl. Pharmacol. 2013, 272, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.F.; Zhang, Q.; Liu, X.Z. Protective effects of curcumin on retinal Müller cell in early diabetic rats. Int. J. Ophthalmol. 2013, 6, 422–424. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Agrawal, S.S.; Agrawal, R.; Agrawal, P.; Saxena, R.; Srivastava, S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 2011, 27, 123–130. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab. 2007, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.R.; Aggarwal, B.B. Curcumin: A component of the golden spice, targets multiple angiogenic pathways. Cancer Biol. Ther. 2011, 11, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Arbiser, J.L.; Klauber, N.; Rohan, R.; van Leeuwen, R.; Huang, M.T.; Fisher, C.; Flynn, E.; Byers, H.R. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998, 4, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gururaj, A.E.; Belakavadi, M.; Venkatesh, D.A.; Marmé, D.; Salimath, B.P. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem. Biophys. Res. Commun. 2002, 297, 934–942. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, J.H.; Cho, H.Y.; Yum, Y.N.; Kim, S.H.; Park, H.J.; Shim, B.S.; Choi, S.H.; Kwon, H.J. Irreversible inhibition of CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem. Biol. 2003, 10, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallée, A. Curcumin and Wnt/β catenin signaling in exudative age related macular degeneration (Review). Int. J. Mol. Med. 2022, 49, 79. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Angi, M.; Ricciardelli, G.; Montericcio, A.; Borgia, A.; Romano, M.R. Curcuma-Based Nutritional Supplement in Patients with Neovascular Age-Related Macular Degeneration. J. Med. Food 2021, 24, 1191–1196. [Google Scholar] [CrossRef]
- Cota, F.; Costa, S.; Giannantonio, C.; Purcaro, V.; Catenazzi, P.; Vento, G. Lutein supplementation and retinopathy of prematurity: A meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Meng, S.S.; Burnim, S.B.; Smith, L.E.; Lo, A.C. Lutein facilitates physiological revascularization in a mouse model of retinopathy of prematurity. Clin. Exp. Ophthalmol. 2017, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, B.; Ramaprasad, T.R.; Baskaran, V. Dietary fatty acid determines the intestinal absorption of lutein in lutein deficient mice. Food Res. Int. 2014, 64, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, B.; Mamatha, B.S.; Baskaran, V. Olive oil improves the intestinal absorption and bioavailability of lutein in lutein-deficient mice. Eur. J. Nutr. 2014, 53, 117–126. [Google Scholar] [CrossRef]
- Baack, M.L.; Puumala, S.E.; Messier, S.E.; Pritchett, D.K.; Harris, W.S. What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot. Essent. Fat. Acids 2015, 100, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Khalesi, N.; Bordbar, A.; Khosravi, N.; Kabirian, M.; Karimi, A. The Efficacy of Omega-3 Supplement on Prevention of Retinopathy of Prematurity in Premature Infants: A Randomized Double-blinded Controlled trial. Curr. Pharm. Des. 2018, 24, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Pivodic, A.; Gränse, L.; Lundgren, P.; Sjöbom, U.; Nilsson, A.K.; Söderling, H.; Hård, A.L.; Smith, L.E.H.; Löfqvist, C.A. Association of Docosahexaenoic Acid and Arachidonic Acid Serum Levels with Retinopathy of Prematurity in Preterm Infants. JAMA Netw. Open 2021, 4, e2128771. [Google Scholar] [CrossRef]
- Hellström, A.; Nilsson, A.K.; Wackernagel, D.; Pivodic, A.; Vanpee, M.; Sjöbom, U.; Hellgren, G.; Hallberg, B.; Domellöf, M.; Klevebro, S.; et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 359–367. [Google Scholar] [CrossRef]
- Tu, C.F.; Lee, C.H.; Chen, H.N.; Tsao, L.Y.; Chen, J.Y.; Hsiao, C.C. Effects of fish oil-containing lipid emulsions on retinopathy of prematurity in very low birth weight infants. Pediatr. Neonatol. 2020, 61, 224–230. [Google Scholar] [CrossRef]
- Sun, H.; Cheng, R.; Wang, Z. Early vitamin A supplementation improves the outcome of retinopathy of prematurity in extremely preterm infants. Retina 2020, 40, 1176–1184. [Google Scholar] [CrossRef]
- Garofoli, F.; Barillà, D.; Angelini, M.; Mazzucchelli, I.; De Silvestri, A.; Guagliano, R.; Decembrino, L.; Tzialla, C. Oral vitamin A supplementation for ROP prevention in VLBW preterm infants. Ital. J. Pediatr. 2020, 46, 77. [Google Scholar] [CrossRef] [PubMed]
- Okai, Y.; Higashi-Okai, K.; FSato, E.; Konaka, R.; Inoue, M. Potent radical-scavenging activities of thiamin and thiamin diphosphate. J. Clin. Biochem. Nutr. 2007, 40, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Berrone, E.; Beltramo, E.; Solimine, C.; Ape, A.U.; Porta, M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J. Biol. Chem. 2006, 281, 9307–9313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Wang, P.; Airen, S.; Brown, C.; Liu, Z.; Townsend, J.H.; Wang, J.; Jiang, H. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis. 2020, 7, 33. [Google Scholar] [CrossRef]
- NoA, L. Vitamin C-Health Professional Fact Sheet. National Institutes of Health. 2020. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 10 March 2023).
- Shang, F.; Lu, M.; Dudek, E.; Reddan, J.; Taylor, A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic Biol. Med. 2003, 34, 521–530. [Google Scholar] [CrossRef]
- Guan, Y.; Dai, P.; Wang, H. Effects of vitamin C supplementation on essential hypertension: A systematic review and meta-analysis. Medicine 2020, 99, e19274. [Google Scholar] [CrossRef]
- Thosar, S.S.; Bielko, S.L.; Wiggins, C.C.; Klaunig, J.E.; Mather, K.J.; Wallace, J.P. Antioxidant vitamin C prevents decline in endothelial function during sitting. Med. Sci. Monit. 2015, 21, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Ghim, W.; Oh, S.; Kim, Y.; Park, U.C.; Kang, J.; Yu, H.G. Association of vitreous vitamin C depletion with diabetic macular ischemia in proliferative diabetic retinopathy. PLoS ONE 2019, 14, e0218433. [Google Scholar] [CrossRef]
- Gurreri, A.; Pazzaglia, A.; Schiavi, C. Role of Statins and Ascorbic Acid in the Natural History of Diabetic Retinopathy: A New, Affordable Therapy? Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, S23–S27. [Google Scholar] [CrossRef]
- Ulker, E.; Parker, W.H.; Raj, A.; Qu, Z.C.; May, J.M. Ascorbic acid prevents VEGF-induced increases in endothelial barrier permeability. Mol. Cell Biochem. 2016, 412, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.; Wang, C.; Liu, D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr. Diabetes. 2017, 7, e281. [Google Scholar] [CrossRef] [PubMed]
- Bursell, S.E.; Clermont, A.C.; Aiello, L.P.; Aiello, L.M.; Schlossman, D.K.; Feener, E.P.; Laffel, L.; King, G.L. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999, 22, 1245–1251. [Google Scholar] [CrossRef]
- Chatziralli, I.P.; Theodossiadis, G.; Dimitriadis, P.; Charalambidis, M.; Agorastos, A.; Migkos, Z.; Platogiannis, N.; Moschos, M.M.; Theodossiadis, P.; Keryttopoulos, P. The Effect of Vitamin E on Oxidative Stress Indicated by Serum Malondialdehyde in Insulin-dependent Type 2 Diabetes Mellitus Patients with Retinopathy. Open Ophthalmol. J. 2017, 11, 51–58. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Goldman, R.; Darrow, R.M.; Organisciak, D.T.; Kagan, V.E. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr. Eye Res. 1995, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Schaffer, D.; Quinn, G.; Goldstein, D.; Mathis, M.J.; Otis, C.; Boggs, T.R., Jr. Vitamin E supplementation and the retinopathy of prematurity. Ann. N. Y. Acad. Sci. 1982, 393, 473–495. [Google Scholar] [CrossRef]
- Hittner, H.M.; Rudolph, A.J.; Kretzer, F.L. Suppression of severe retinopathy of prematurity with vitamin E supplementation. Ultrastructural mechanism of clinical efficacy. Ophthalmology 1984, 91, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, M.; Polat, O. Clinical Efficacy of Topical CoQ10 and Vitamin-E Eye-drop in Retinopathy of Prematurity. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 291–297. [Google Scholar]
- Robison, W.G.; Kuwabara, T.; Bieri, J.G. The roles of vitamin E and unsaturated fatty acids in the visual process. Retina 1982, 2, 263–281. [Google Scholar] [CrossRef]
- Tanito, M.; Yoshida, Y.; Kaidzu, S.; Chen, Z.H.; Cynshi, O.; Jishage, K.; Niki, E.; Ohira, A. Acceleration of age-related changes in the retina in alpha-tocopherol transfer protein null mice fed a Vitamin E-deficient diet. Investig. Ophthalmol. Vis. Sci. 2007, 48, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Katz, M.L.; Eldred, G.E. Failure of vitamin E to protect the retina against damage resulting from bright cyclic light exposure. Investig. Ophthalmol. Vis. Sci. 1989, 30, 29–36. [Google Scholar]
- Belda, J.I.; Romá, J.; Vilela, C.; Puertas, F.J.; Díaz-Llopis, M.; Bosch-Morell, F.; Romero, F.J. Serum vitamin E levels negatively correlate with severity of age-related macular degeneration. Mech. Ageing Dev. 1999, 107, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, W.; El-Sherbiny, S. Evidence-based nutritional advice for patients affected by age-related macular degeneration. Ophthalmologica 2014, 231, 185–190. [Google Scholar] [CrossRef] [PubMed]
- de Koning-Backus, A.P.M.; Buitendijk, G.H.S.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C.W. Intake of Vegetables, Fruit, and Fish is Beneficial for Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 198, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group; Sangiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Rd, F.F.; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Błasiak, J. Can vitamin D protect against age-related macular degeneration or slow its progression? Acta Biochim. Pol. 2019, 66, 147–158. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857, Erratum in: JAMA 2008, 299, 765–766. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef]
- Miao, X.; Sun, W.; Miao, L.; Fu, Y.; Wang, Y.; Su, G.; Liu, Q. Zinc and diabetic retinopathy. J. Diabetes Res. 2013, 2013, 425854. [Google Scholar] [CrossRef] [Green Version]
- Dascalu, A.M.; Anghelache, A.; Stana, D.; Costea, A.C.; Nicolae, V.A.; Tanasescu, D.; Costea, D.O.; Tribus, L.C.; Zgura, A.; Serban, D.; et al. Serum levels of copper and zinc in diabetic retinopathy: Potential new therapeutic targets (Review). Exp. Ther. Med. 2022, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- NoA, L.; EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Draft Scientific Opinion on Dietary Reference Values for Zinc. EFSA Journal 2014. Available online: https://www.efsa.europa.eu/sites/default/files/consultation/140514%2C0.pdf (accessed on 10 March 2023).
- Terrin, G.; Berni Canani, R.; Passariello, A.; Messina, F.; Conti, M.G.; Caoci, S.; Smaldore, A.; Bertino, E.; De Curtis, M. Zinc supplementation reduces morbidity and mortality in very-low-birth-weight preterm neonates: A hospital-based randomized, placebo-controlled trial in an industrialized country. Am. J. Clin. Nutr. 2013, 98, 1468–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staub, E.; Evers, K.; Askie, L.M. Enteral zinc supplementation for prevention of morbidity and mortality in preterm neonates. Cochrane Database Syst. Rev. 2021, 3, CD012797. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.A. Chromium, glucose intolerance and diabetes. J. Am. Coll. Nutr. 1998, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. Interaction of chromium with insulin: A progress report. Nutr. Rev. 1998, 56, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Cheng, N.; Bryden, N.A.; Polansky, M.M.; Cheng, N.; Chi, J.; Feng, J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 1997, 46, 1786–1791. [Google Scholar] [CrossRef]
- Erie, J.C.; Good, J.A.; Butz, J.A.; Pulido, J.S. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am. J. Ophthalmol. 2009, 147, 276–282.e1. [Google Scholar] [CrossRef]
- Arteel, G.E.; Sies, H. The biochemistry of selenium and the glutathione system. Environ. Environ. Toxicol. Pharmacol. 2001, 10, 153–158. [Google Scholar] [CrossRef]
- Farnsworth, C.C.; Stone, W.L.; Dratz, E.A. Effects of vitamin E and selenium deficiency on the fatty acid composition of rat retinal tissues. Biochim. Biophys. Acta 1979, 552, 281–293. [Google Scholar] [CrossRef]
- Rinninella, E.; Mele, M.C.; Merendino, N.; Cintoni, M.; Anselmi, G.; Caporossi, A.; Gasbarrini, A.; Minnella, A.M. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut–Retina Axis. Nutrients 2018, 10, 1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floyd, J.L.; Grant, M.B. The Gut-Eye Axis: Lessons Learned from Murine Models. Ophthalmol. Ther. 2020, 9, 499–513. [Google Scholar] [CrossRef]
- Bu, Y.; Chan, Y.K.; Wong, H.L.; Poon, S.H.; Lo, A.C.; Shih, K.C.; Tong, L. A Review of the Impact of Alterations in Gut Microbiome on the Immunopathogenesis of Ocular Diseases. J. Clin. Med. 2021, 10, 4694. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Miwa, Y.; Jounai, K.; Fujiwara, D.; Kurihara, T.; Kanauchi, O. Lactobacillus paracasei KW3110 Prevents Blue Light-Induced Inflammation and Degeneration in the Retina. Nutrients 2018, 10, 1991. [Google Scholar] [CrossRef] [Green Version]
- Morita, Y.; Jounai, K.; Sakamoto, A.; Tomita, Y.; Sugihara, Y.; Suzuki, H.; Ohshio, K.; Otake, M.; Fujiwara, D.; Kanauchi, O.; et al. Long-term intake of Lactobacillus paracasei KW3110 prevents age-related chronic inflammation and retinal cell loss in physiologically aged mice. Aging 2018, 10, 2723–2740. [Google Scholar] [CrossRef] [PubMed]
- Lima-Fontes, M.; Meira, L.; Barata, P.; Falcão, M.; Carneiro, Â. Gut microbiota and age-related macular degeneration: A growing partnership. Surv. Ophthalmol. 2022, 67, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Jabbehdari, S.; Sallam, A.B. Gut microbiome and diabetic retinopathy. Eur. J. Ophthalmol. 2022, 32, 2494–2497. [Google Scholar] [CrossRef]
- Bai, J.; Wan, Z.; Zhang, Y.; Wang, T.; Xue, Y.; Peng, Q. Composition and diversity of gut microbiota in diabetic retinopathy. Front. Microbiol. 2022, 13, 926926. [Google Scholar] [CrossRef]
- Liu, K.; Zou, J.; Fan, H.; Hu, H.; You, Z. Causal effects of gut microbiota on diabetic retinopathy: A Mendelian randomization study. Front. Immunol. 2022, 13, 930318. [Google Scholar] [CrossRef]
- Feuerbach, C.M. “Man is what he eats”: A rectification. J. Hist. Ideas 1963, 24, 397–406. [Google Scholar]
- Cizza, G.; Rother, K.I. Was Feuerbach right: Are we what we eat? J. Clin. Investig. 2011, 121, 2969–2971. [Google Scholar] [CrossRef]
- Pache, M.; Flammer, J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Surv. Ophthalmol. 2006, 51, 179–212. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxid. Med. Cell Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of Diet and The Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef] [Green Version]
- Rezende, F.A.; Lapalme, E.; Qian, C.X.; Smith, L.E.; Sangiovanni, J.P.; Sapieha, P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Semeraro, F.; Gambicordi, E.; Cancarini, A.; Morescalchi, F.; Costagliola, C.; Russo, A. Treatment of exudative age-related macular degeneration with aflibercept combined with pranoprofen eye drops or nutraceutical support with omega-3: A randomized trial. Br. J. Clin. Pharmacol. 2019, 85, 908–913. [Google Scholar] [CrossRef]

|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusciano, D.; Bagnoli, P. Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases. Medicina 2023, 59, 1334. https://doi.org/10.3390/medicina59071334
Rusciano D, Bagnoli P. Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases. Medicina. 2023; 59(7):1334. https://doi.org/10.3390/medicina59071334
Chicago/Turabian StyleRusciano, Dario, and Paola Bagnoli. 2023. "Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases" Medicina 59, no. 7: 1334. https://doi.org/10.3390/medicina59071334






