Factors Predisposing to The Formation of Degenerative Spondylolisthesis—A Narrative Review
Abstract
1. Introduction
Etiology
- Arthritis and orientation of the facet joint favoring increased mobility;
- Disturbances in the functions of the ligament system stabilizing the column;
2. Literature Search for Evidence
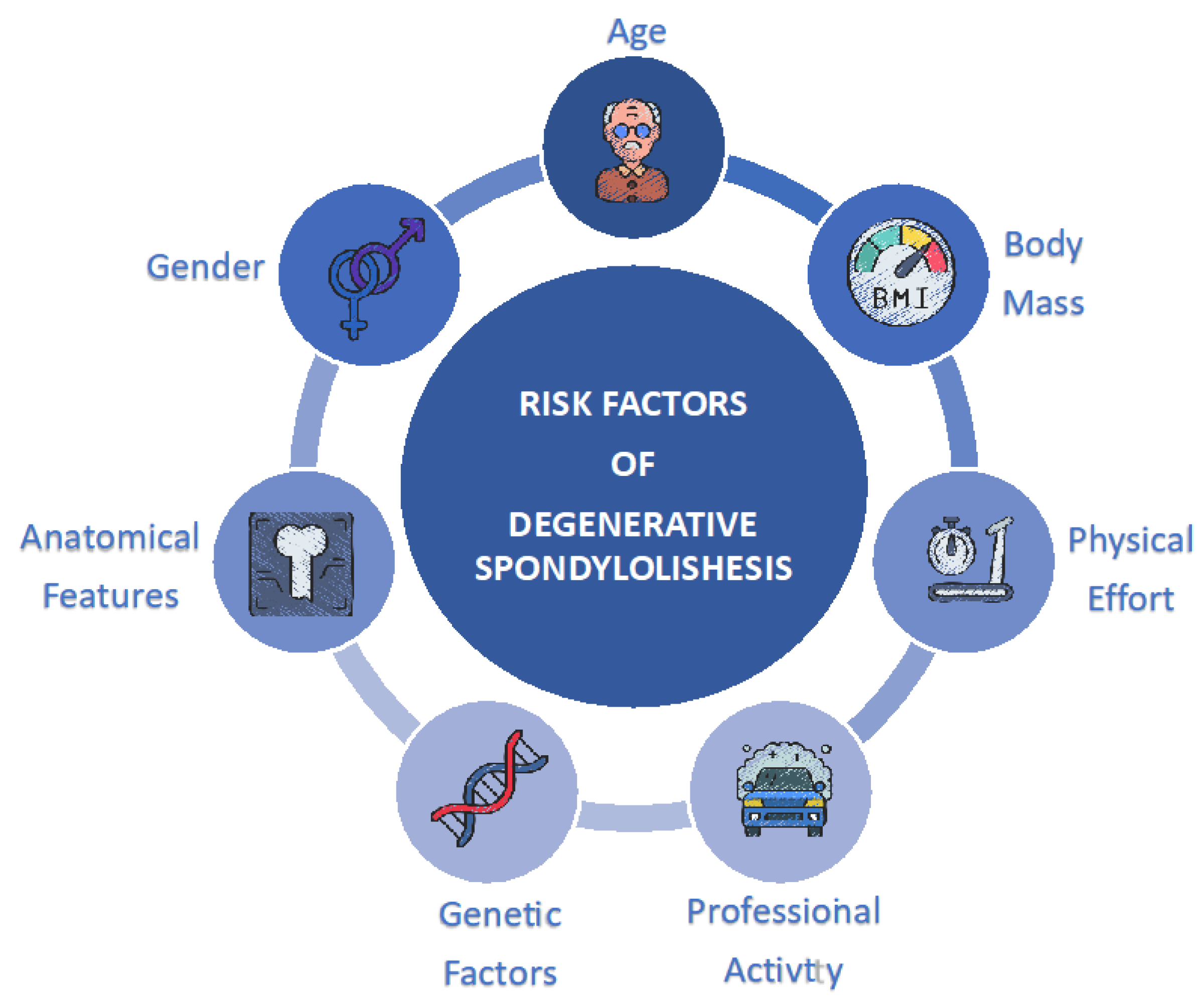
3. Risk Factors
3.1. Physical Effort and Professional Activity
3.2. Body Mass
3.3. Anatomical Features
3.4. Age
3.5. Ethnic Origin and Genetic Factors
3.6. Gender and the Influence of Sex Hormones
3.7. Other Factors
3.8. The Importance of Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Errico, T.J.; Lonner, B.S.; Moulton, A.W. Surgical Management of Spinal Deformities; Introduction to Chapter 17; Sounders Elsevier: Philadelphia, PA, USA, 2009; ISBN 9781416033721.CS1. [Google Scholar]
- Shamrock, A.G.; Donnally, C.J.; Varacallo, M. Lumbar Spondylolysis and Spondylolisthesis; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- DeVine, J.G.; Schenk-Kisser, J.M.; Skelly, A.C. Risk factors for degenerative spondylolisthesis: A systematic review. Evid. Based Spine-Care J. 2012, 3, 25–34. [Google Scholar] [CrossRef]
- Vogt, M.T.; Rubin, D.; Valentin, R.S.; Palermo, L.; Donaldson, W.F.; Nevitt, M.; Cauley, J.A. Lumbar Olisthesis and Lower Back Symptoms in Elderly White Women. Spine 1998, 23, 2640–2647. [Google Scholar] [CrossRef]
- Vogt, M.T.; Rubin, D.A.; Palermo, L.; Christianson, L.; Kang, J.D.; Nevitt, M.C.; Cauley, J.A. Lumbar spine listhesis in older African American women. Spine J. 2003, 3, 255–261. [Google Scholar] [CrossRef]
- Chen, J.C.; Chan, W.P.; Katz, J.N.; Chang, W.; Christiani, D. Occupational and personal factors associated with acquired lumbar spondylolisthesis of urban taxi drivers. Occup. Environ Med. 2004, 61, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.; Sonne-Holm, S.; Rovsing, H.; Monrad, H.; Gebuhr, P. Degenerative lumbar spondylolisthesis: An epidemiological perspective: The Copenhagen Osteoarthritis Study. Spine 2007, 32, 120–125. [Google Scholar] [CrossRef]
- Kalichman, L.; Kim, D.H.; Li, L.; Guermazi, A.; Berkin, V.; Hunter, D.J. Spondylolysis and spondylolisthesis: Prevalence and association with low back pain in the adult community-based population. Spine 2009, 34, 199–205. [Google Scholar] [CrossRef]
- Denard, P.J.; Holton, K.F.; Miller, J.; Fink, H.A.; Kado, D.M.; Yoo, J.U.; Marshall, L.M. Lumbar spondylolisthesis among elderly men: Prevalence, correlates and progression. Spine 2010, 35, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Wakami, T.; Kurihara, A.; Kasahara, K.; Yoshiya, S.; Nishida, K. Lumbar Multilevel Degenerative Spondylolisthesis: Radiological Evaluation and Factors Related to Anterolisthesis and Retrolisthesis. J. Spinal Disord. Tech. 2002, 15, 93–99. [Google Scholar] [CrossRef]
- Farfan, H.F. The pathological anatomy of degenerative spondylolisthesis. A cadaver study. Spine 1980, 5, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Wiltse, L.L. Classification, Terminology and Measurements in Spondylolisthesis. Iowa Orthop. J. 1981, 1, 52–57. [Google Scholar]
- Syrmou, E.; Tsitsopoulos, P.P.; Marinopoulos, D.; Tsonidis, C.; Anagnostopoulos, I.; Tsitsopoulos, P.D. Spondylolysis: A review and reappraisal. Hippokratia 2010, 14, 17–21. [Google Scholar]
- Dent, C.E.; Engelbrecht, H.E.; Godfrey, R.C. Osteoporosis of Lumbar Vertebrae and Calcification of Abdominal Aorta in Women Living in Durban. BMJ 1968, 4, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.J. Degenerative spondylolisthesis. Predisposing factors. J. Bone Jt. Surg. Am. 1975, 57, 467–474. [Google Scholar] [CrossRef]
- Imada, K.; Matsui, H.; Tsuji, H. Oophorectomy predisposes to degenerative spondylolisthesis. J. Bone Jt. Surg. Br. 1995, 77-B, 126–130. [Google Scholar] [CrossRef]
- Sanderson, P.L.; Fraser, R.D. The influence of pregnancy on the development of degenerative spondylolisthesis. J. Bone Jt. Surg. Br. 1996, 78, 951–954. [Google Scholar] [CrossRef]
- Kauppila, L.I.; Eustace, S.; Kiel, D.P.; Felson, D.T.; Wright, A.M. Degenerative Displacement of Lumbar Vertebrae. A 25-year follow-up study in Framingham. Spine 1998, 23, 1868–1873. [Google Scholar] [CrossRef]
- Yamamuro, T.; Hama, H.; Takeda, T.; Shikata, J.; Sanada, H. Biomechanical and hormonal factors in the etiology of congenital dislocation of the hip joint. Int. Orthop. 1977, 1, 231–236. [Google Scholar] [CrossRef]
- Horikawa, K.; Kasai, Y.; Yamakawa, T.; Sudo, A.; Uchida, A. Prevalence of Osteoarthritis, Osteoporotic Vertebral Fractures, and Spondylolisthesis among the Elderly in a Japanese Village. J. Orthop. Surg. 2006, 14, 9–12. [Google Scholar] [CrossRef]
- Mariconda, M.; Galasso, O.; Imbimbo, L.; Lotti, G.; Milano, C. Relationship between alterations of the lumbar spine, visualized with magnetic resonance imaging, and occupational variables. Eur. Spine J. 2007, 16, 255–266. [Google Scholar] [CrossRef]
- Hosoe, H.; Ohmori, K. Degenerative lumbosacral spondylolisthesis: Possible factors which predispose the fifth lumbar vertebra to slip. J. Bone Jt. Surg. Br. 2008, 90, 356–359. [Google Scholar] [CrossRef]
- Chen, I.-R.; Wei, T.-S. Disc Height and Lumbar Index as Independent Predictors of Degenerative Spondylolisthesis in Middle-Aged Women with Low Back Pain. Spine 2009, 34, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Schuller, S.; Charles, Y.P.; Steib, J.-P. Sagittal spinopelvic alignment and body mass index in patients with degenerative spondylolisthesis. Eur. Spine J. 2010, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Aono, K.; Kobayashi, T.; Jimbo, S.; Atsuta, Y.; Matsuno, T. Radiographic Analysis of Newly Developed Degenerative Spondylolisthesis in a Mean Twelve-Year Prospective Study. Spine 2010, 35, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Marty-Poumarat, C.; Ostertag, A.; Baudoin, C.; Marpeau, M.; de Vernejoul, M.-C.; Cohen-Solal, M. Does hormone replacement therapy prevent lateral rotatory spondylolisthesis in postmenopausal women? Eur. Spine J. 2012, 21, 1127–1134. [Google Scholar] [CrossRef]
- He, L.-C.; Wang, Y.-X.J.; Gong, J.-S.; Griffith, J.F.; Zeng, X.-J.; Kwok, A.W.; Leung, J.C.; Kwok, T.; Ahuja, A.T.; Leung, P.C. Prevalence and risk factors of lumbar spondylolisthesis in elderly Chinese men and women. Eur. Radiol. 2014, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, E.; Ould-Slimane, M.; Gille, O.; Guigui, P.; French Spine Society (SFCR). Sagittal spinopelvic alignment in 654 degenerative spondylolisthesis. Eur. Spine J. 2015, 24, 1219–1227. [Google Scholar] [CrossRef]
- Enyo, Y.; Yoshimura, N.; Yamada, H.; Hashizume, H.; Yoshida, M. Radiographic natural course of lumbar degenerative spondylolisthesis and its risk factors related to the progression and onset in a 15-year community-based cohort study: The Miyama study. J. Orthop. Sci. 2015, 20, 978–984. [Google Scholar] [CrossRef]
- Wang, Y.-X.J.; Griffith, J.F.; Zeng, X.-J.; Deng, M.; Kwok, A.W.L.; Leung, J.C.S.; Ahuja, A.T.; Kwok, T.; Leung, P.C. Prevalence and Sex Difference of Lumbar Disc Space Narrowing in Elderly Chinese Men and Women: Osteoporotic Fractures in Men (Hong Kong) and Osteoporotic Fractures in Women (Hong Kong) Studies. Arthritis Rheum. 2013, 65, 1004–1010. [Google Scholar] [CrossRef]
- Cholewicki, J.; Lee, A.S.; Popovich, J.M.; Mysliwiec, L.W.; Winkelpleck, M.D.; Flood, J.N.; Pathak, P.K.; Kaaikala, K.H.; Reeves, N.P.; Kothe, R. Degenerative Spondylolisthesis Is Related to Multiparity and Hysterectomies in Older Women. Spine 2017, 42, 1643–1647. [Google Scholar] [CrossRef]
- Fraser, R.D.; Brooks, F.; Dalzell, K. Degenerative spondylolisthesis: A prospective cross-sectional cohort study on the role of weakened anterior abdominal musculature on causation. Eur. Spine J. 2018, 28, 1406–1412. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Cooper, C.; Ntani, G.; Yamada, H.; Hashizume, H.; Nagata, K.; Muraki, S.; Tanaka, S.; Yoshida, M.; Yoshimura, N.; et al. Is radiographic lumbar spondylolisthesis associated with occupational exposures? Findings from a nested case control study within the Wakayama spine study. BMC Musculoskelet. Disord. 2019, 20, 618. [Google Scholar] [CrossRef]
- Junghanns, H. Spondylolisthesen ohne spalt im Zwishengelenkstuck. Arch. Orthop. Unfallchir. 1931, 29, 118–127. [Google Scholar] [CrossRef]
- Macnab, I. Spondylolisthesis with an intact neural arch; the so-called pseudo-spondylolisthesis. J. Bone Jt. Surg. Br. 1950, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.H.; Stone, K.H. The etiology of spondylolisthesis. J. Bone Jt. Surg. Br. 1963, 45-B, 39–59. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Newman, P.H. Degenerative spondylolisthesis. J. Bone Jt. Surg. Br. 1976, 58, 184–192. [Google Scholar] [CrossRef]
- Kalichman, L.; Hunter, D.J. Degenerative lumbar spondylolisthesis: Anatomy, biomechanics and risk factors. J. Back Musculoskelet. Rehabil. 2008, 21, 1–12. [Google Scholar] [CrossRef]
- Matsunaga, S.; Sakou, T.; Morizono, Y.; Masuda, A.; Demirtas, A.M. Natural History of Degenerative Spondylolisthesis: Pathogenesis and natural course of the slippage. Spine 1990, 15, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Grobler, L.J.; Robertson, P.A.; Novotny, J.E.; Pope, M.H. Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine 1993, 18, 80–91. [Google Scholar] [CrossRef]
- Kirkaldy-Willis, W.H.; Farfan, H.F. Instability of the lumbar spine. Clin. Orthop. 1982, 165, 110–123. [Google Scholar] [CrossRef]
- Taillard, W.F. Etiology of spondylolisthesis. Clin. Orthop. 1976, 117, 30–39. [Google Scholar] [CrossRef]
- Farfan, H.F.; Sullivan, J.B. The relation of facet orientation to intervertebral disc failure. Can. J. Surg. 1969, 12, 336–344. [Google Scholar]
- Ha, K.-Y.; Chang, C.-H.; Kim, K.-W.; Kim, Y.-S.; Na, K.-H.; Lee, J.-S. Expression of Estrogen Receptor of the Facet Joints in Degenerative Spondylolisthesis. Spine 2005, 30, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.K.; Herkowitz, H.N. Degenerative spondylolisthesis: Review of current trends and controversies. Spine 2005, 30, 71–81. [Google Scholar] [CrossRef]
- Fujiwara, A.; Tamai, K.; An, H.S.; Kurihashi, A.; Lim, T.-H.; Yoshida, H.; Saotome, K. The Relationship Between Disc Degeneration, Facet Joint Osteoarthritis, and Stability of the Degenerative Lumbar Spine. J. Spinal Disord. 2000, 13, 444–450. [Google Scholar] [CrossRef]
- Ito, S.; Yamada, Y.; Tuboi, S.; Muro, T. Specific pattern of ruptured annulus fibrosus in lumbar degenerative spondylolisthesis. Neuroradiology 1990, 32, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Frymoyer, J.W. Degenerative Spondylolisthesis: Diagnosis and Treatment. J. Am. Acad. Orthop. Surg. 1994, 2, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Hou, D.; Zhao, B.; Sun, X.; Sun, H.; Li, N.; Guo, L.; Liu, C. The pedicle-facet angle and tropism in the sagittal plane in degenerative spondylolisthesis: A computed tomography study using multiplanar reformations techniques. J. Spinal Disord Tech. 2012, 25, E18–E22. [Google Scholar] [CrossRef]
- Inoue, S.; Watanabe, T.; Goto, S.; Takahashi, K.; Takata, K.; Sho, E. Degenerative spondylolisthesis. Pathophysiology and results of anterior interbody fusion. Clin. Orthop. 1988, 227, 90–98. [Google Scholar] [CrossRef]
- Sato, K.; Wakamatsu, E.; Yoshizumi, A.; Watanabe, N.; Irei, O. The configuration of the laminas and facet joints in degenerative spondylolisthesis: A clinicoradiologic study. Spine 1989, 14, 1265–1271. [Google Scholar] [CrossRef]
- Cinotti, G.; Postacchini, F.; Fassari, F.; Urso, S. Predisposing factors in degenerative spondylolisthesis: A radiographic and CT study. Int Orthop. 1997, 21, 337–342. [Google Scholar] [CrossRef]
- Berlemann, U.; Jeszenszky, D.J.; Buhler, D.W.; Harms, J. Facet joint remodeling in degenerative spondylolisthesis: An investi-gation of joint orientation and tropism. Eur. Spine J. 1998, 7, 376–380. [Google Scholar] [CrossRef]
- Love, T.W.; Fagan, A.B.; Fraser, R.D. Degenerative spondylolisthesis. Developmental or acquired? J. Bone Jt. Surg. Br. 1999, 81, 670–674. [Google Scholar] [CrossRef]
- Aihara, T.; Takahashi, K.; Yamagata, M.; Moriya, H.; Tamaki, T. Biomechanical functions of the iliolumbar ligament in L5 spondylolysis. J. Orthop. Sci. 2000, 5, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Bird, H.A.; Eastmond, C.J.; Hudson, A.; Wright, V. Is generalized joint laxity a factor in spondylolisthesis? Scand. J. Rheumatol. 1980, 9, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Hodges, P.; Li, L.; Guermazi, A.; Hunter, D.J. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur. Spine J. 2009, 19, 1136–1144. [Google Scholar] [CrossRef]
- Izzo, R.; Guarnieri, G.; Guglielmi, G.; Muto, M. Biomechanics of the spine. Part I: Spinal stability. Eur. J. Radiol. 2013, 82, 118–126. [Google Scholar] [CrossRef]
- Lai, Q.; Gao, T.; Lv, X.; Liu, X.; Wan, Z.; Dai, M.; Zhang, B.; Nie, T. Correlation between the sagittal spinopelvic alignment and degenerative lumbar spondylolisthesis: A retrospective study. BMC Musculoskelet. Disord. 2018, 19, 151. [Google Scholar] [CrossRef]
- Lindgren, K.A.; Sihvonen, T.; Leino, E.; Pitkänen, M.; Manninen, H. Exercise therapy effects on functional radiographic findings and segmental electromyographic activity in lumbar spine instability. Arch. Phys. Med. Rehabil. 1993, 74, 933–939. [Google Scholar]
- Sihvonen, T.; Partanen, J.; Hanninen, O.; Soimakallio, S. Electric behaviour of low back muscles during lumbar pelvic rhythm in low back pain patients and healthy controls. Arch. Phys. Med. Rehabil. 1991, 72, 1080–1087. [Google Scholar]
- Guo, M.; Kong, C.; Sun, S.; Sun, X.; Li, X.; Lu, S. Predictors of L4−L5 Degenerative Lumbar Spondylolisthesis: L4 Inclination Angle and Facet Joint Angle. World Neurosurg. 2019, 130, e680–e686. [Google Scholar] [CrossRef]
- Stokes, I.A.; Gardner-Morse, M. Stability increase of the lumbar spine with different muscle groups: A biomechanical in vitro study. Spine 1995, 20, 2168–2169. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.J.; Shih, T.; Chen, B.; Hwang, Y.H.; Ma, L.P.; Huang, W.C.; Liou, S.H.; Ho, I.K.; Guo, Y.L. The dose response rela-tionship between cumulative lifting load and lumbar disk degeneration based on magnetic resonance imaging findings. Phys Ther. 2014, 94, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Cannata, F.; Vadalà, G.; Ambrosio, L.; Fallucca, S.; Napoli, N.; Papalia, R.; Pozzilli, P.; Denaro, V. Intervertebral disc degeneration: A focus on obesity and type 2 diabetes. Diabetes Metab. Res. Rev. 2019, 36, e3224. [Google Scholar] [CrossRef] [PubMed]
- Videman, T.; Battié, M.C.; Parent, E.; Gibbons, L.E.; Vainio, P.; Kaprio, J. Progression and determinants of quantitative magnetic resonance imaging measures of lumbar disc degeneration: A five-year follow-up of adult male monozygotic twins. Spine 2008, 33, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F. Spondylolysis, spondylolisthesis and sports. J. Sports Med. Phys. Fit. 1978, 18, 317–340. [Google Scholar]
- Jakab, G. 6 Occupational spondylolysis and spondylolisthesis. Baillieres Clin. Rheumatol. 1989, 3, 89–98. [Google Scholar] [CrossRef]
- Muschik, M.; Hahnel, H.; Robinson, P.N.; Perka, C.; Muschik, C. Competitive sports and the progression of spondylolisthesis. J. Pediatr. Orthop. 1996, 16, 364–369. [Google Scholar] [CrossRef]
- Beutler, W.J.; Fredrickson, B.E.; Murtland, A.; Sweeney, C.A.; Grant, W.D.; Baker, D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine 2003, 28, 1027–1035. [Google Scholar] [CrossRef]
- Ishihara, H.; Tsuji, H.; Hirano, N.; Ohshima, H.; Terahata, N. Effects of Continuous Quantitative Vibration on Rheologic and Biological Behaviors of the Intervertebral Disc. Spine 1992, 17, 7–12. [Google Scholar] [CrossRef]
- Jensen, A.; Jepsen, J.R. Vibration on board and health effects. Int. Marit. Health 2014, 65, 58–60. [Google Scholar] [CrossRef][Green Version]
- Vallone, M.; Bono, F.; Quendler, E.; Febo, P.; Catania, P. Risk exposure to vibration and noise in the use of agricultural track-laying tractors. Ann. Agric. Environ. Med. 2016, 23, 591–597. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.M.; Deng, M.M.; Griffith, J.F.M.; Kwok, A.W.; Leung, J.C.M.; Ahuja, A.T.M.; Kwok, T.M.; Leung, P.C.M. Lumbar Spondylolisthesis Progression and De Novo Spondylolisthesis in Elderly Chinese Men and Women: A Year-4 Follow-up Study. Spine 2016, 41, 1096–1103. [Google Scholar] [CrossRef]
- Farfan, H.F.; Osteria, V.; Lamy, C. The mechanical etiology of spondylolysis and spondylolisthesis. Clin. Orthop. Relat. Res. 1976, 117, 40–55. [Google Scholar] [CrossRef]
- Tassanawipas, W.; Chansriwong, P.; Mokkhavesa, S. The orientation of facet joints and transverse articular dimension in degenerative spondylolisthesis. J. Med. Assoc. Thail. 2005, 88, 31–34. [Google Scholar]
- Toyone, T.; Ozawa, T.; Kamikawa, K.; Watanabe, A.; Matsuki, K.; Yamashita, T.; Wada, Y. Facet joint orientation difference between cephalad and caudal portions: A possible cause of degenerative spondylolisthesis. Spine 2009, 34, 2259–2262. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Belfer, I.; Schwartz, C.E.; Banco, R.; Martha, J.F.; Tighioughart, H.; Tromanhauser, S.G.; Jenis, L.G.; Kim, D.H. Association of catechol-O-methyltransferase genetic variants with outcome in patients undergoing surgical treatment for lumbar degenerative disc disease. Spine J. 2010, 10, 949–957. [Google Scholar] [CrossRef]
- Smorgick, Y.; Mirovsky, Y.; Fischgrund, J.S.; Baker, K.C.; Gelfer, Y.; Anekstein, Y. Radiographic Predisposing Factors for Degenerative Spondylolisthesis. Orthopedics 2014, 37, e260–e264. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Nakamura, T. Associated Factors for Lumbar Degenerative Spondylolisthesis in Japanese Patients with Osteoarthritis of the Hip: A Radiographic Study. Asian Spine J. 2016, 10, 935–939. [Google Scholar] [CrossRef]
- Epstein, N.E.; Epstein, J.A.; Carras, R.; Lavine, L.S. Degenerative Spondylolisthesis with an Intact Neural Arch: A Review of 60 Cases with an Analysis of Clinical Findings and the Development of Surgical Management. Neurosurgery 1983, 13, 555–561. [Google Scholar] [CrossRef]
- Rihn, J.A.; Radcliff, K.; Hilibrand, A.S.; Anderson, D.T.; Zhao, W.; Lurie, J.; Vaccaro, A.R.; Freedman, M.K.; Albert, T.J.; Weinstein, J.N. Does Obesity Affect Outcomes of Treatment for Lumbar Stenosis and Degenerative Spondylolisthesis? Analysis of the Spine Patient Outcomes Research Trial (SPORT). Spine 2012, 37, 1933–1946. [Google Scholar] [CrossRef]
- Simmonds, A.M.; Rampersaud, Y.R.; Dvorak, M.F.; Dea, N.; Melnyk, A.D.; Fisher, C.G. Defining the inherent stability of degenerative spondylolisthesis: A systematic review. J. Neurosurg. Spine 2015, 23, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.; McCarthy, M. Management of symptomatic degenerative low-grade lumbar spondylolisthesis. EFORT Open Rev. 2018, 3, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Heuer, F.; Wilke, H.-J. Interaction Between Finite Helical Axes and Facet Joint Forces Under Combined Loading. Spine 2008, 33, 2741–2748. [Google Scholar] [CrossRef]
- Lorenz, M.; Patwardhan, A.; Vanderby, R. Load-Bearing Characteristics of Lumbar Facets in Normal and Surgically Altered Spinal Segments. Spine 1983, 8, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Maciejczak, A.; Jabłońska-Sudoł, K. Correlation between correction of pelvic balance and clinical outcomes in mid- and low-grade adult isthmic spondylolisthesis. Eur. Spine J. 2016, 26, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.D.; Riew, K.D.; Yamaguchi, K.; Branch, T.P.; Schellinger, D.; Wiesel, S.W. Orientation of the lumbar facet joints: Association with degenerative disc disease. J. Bone Jt. Surg. Am. 1996, 78, 403–411. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, L.; Bian, C.; Wang, H.-R.; Lin, H.; Li, X.-L.; Jiang, Y.-Q.; Dong, J. Degenerative Spondylolisthesis in the Fifth Lumbar Vertebra and Radiographic Parameters: A Correlation Analysis. Clin. Spine Surg. 2017, 30, E1233–E1238. [Google Scholar] [CrossRef] [PubMed]
- Barrey, C.; Jund, J.; Perrin, G.; Roussouly, P. Spinopelvic alignment of patients with degenerative spondylolisthesis. Neurosurgery 2007, 61, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.T.; Rubin, D.A.; San Valentin, R.; Palermo, L.; Kang, J.D.; Donaldson, W.F., 3rd; Nevitt, M.; Cauley, J.A. Degenerative lumbar listhesis and bone mineral density in elderly women. The study of osteoporotic fractures. Spine 1999, 24, 2536–2541. [Google Scholar] [CrossRef]
- Wang, Y.-X.J.; Griffith, J.F. Effect of Menopause on Lumbar Disk Degeneration: Potential Etiology. Radiology 2010, 257, 318–320. [Google Scholar] [CrossRef]
- Wang, Y.-X.J.; Griffith, J.F.; Ma, H.T.; Kwok, A.W.L.; Leung, J.C.S.; Yeung, D.K.W.; Ahuja, A.T.; Leung, P.C. Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: A study in elderly subjects using an eight-level MRI-based disc degeneration grading system. Osteoporos. Int. 2010, 22, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tower, S.S.; Pratt, W.B. Spondylolysis and associated spondylolisthesis in Eskimo and Athabascan populations. Clin. Orthop. Relat. Res. 1990, 250, 171–175. [Google Scholar] [CrossRef]
- Hakim, A.J.; Cherkas, L.F.; Grahame, R.; Spector, T.D.; MacGregor, A.J. The genetic epidemiology ofjoint hypermobility: Apopulation studyoffemale twins. Arthritis Rheum. 2004, 50, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Eskola, P.J.; Lemmelä, S.; Kjaer, P.; Solovieva, S.; Männikkö, M.; Tommerup, N.; Lind-Thomsen, A.; Husgafvel-Pursiainen, K.; Cheung, K.M.C.; Chan, D.; et al. Genetic Association Studies in Lumbar Disc Degeneration: A Systematic Review. PLoS ONE 2012, 7, e49995. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Kanna, R.M.; Senthil, N.; Raveendran, M.; Ranjani, V.; Cheung, K.M.C.; Chan, D.; Kao, P.Y.P.; Yee, A.; Shetty, A.P. Genetic susceptibility of lumbar degenerative disc disease in young Indian adults. Eur. Spine J. 2014, 24, 1969–1975. [Google Scholar] [CrossRef]
- Chan, D.; Song, Y.; Sham, P.; Cheung, K.M. Genetics of disc degeneration. Eur. Spine J. 2006, 15 (Suppl. S3), 317–325. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Q.; Jiang, J.; Zhan, X.; Xiao, Z. Association between COL11A1 (rs1337185) and ADAMTS5 (rs162509) gene polymorphisms and lumbar spine pathologies in Chinese Han population: An observational study. BMJ Open 2017, 7, e015644. [Google Scholar] [CrossRef]
- Liu, S.H.; Al-Shaikh, R.; Yang, R.-S.; Nelson, S.D.; Soleiman, N.; Finerman, G.A.M.; Lane, J.M.; Panossian, V. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J. Orthop. Res. 1996, 14, 526–533. [Google Scholar] [CrossRef]
- Ushiyama, T.; Inoue, K.; Nishioka, J. Expression of estrogen receptor related protein (p29) and estradiol binding in human arthritic synovium. J. Rheumatol. 1995, 22, 421–426. [Google Scholar]
- Sievert, L.L.; Saliba, M.; Reher, D.; Sahel, A.; Hoyer, D.; Deeb, M.; Obermeyer, C.M. The medical management of menopause: A four-country comparison care in urban areas. Maturitas 2008, 59, 7–21. [Google Scholar] [CrossRef]
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X. Post-menopausal Chinese women have accelerated lumbar disc degeneration compared with Chinese men. J. Or-thop. Translat. 2015, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; Yamada, T.; Inoue, N.; Nishimura, A.; Sudo, A. Risk factors for lumbar intervertebral disc height narrowing: A population-based longitudinal study in the elderly. BMC Musculoskelet. Disord. 2015, 16, 344. [Google Scholar] [CrossRef]
- Dodge, H.J.; Mikkelsen, W.M.; Duff, I.F. Age-sex specific prevalence of radiographic abnormalities of the joints of the hands, wrists and cervical spine of adult residents of the Tecumseh, Michigan, Community Health Study area, 1962–1965. J. Chronic Dis. 1970, 23, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Xiang, W.Y.; Griffith, J.F.; Gang, L.; Ahuja, A.T.; Poon, W.S. Characteristics of rat lumbar vertebral body bone mineral density and differential segmental responses to sex hormone. Biomed. Environ. Sci. 2012, 25, 607–613. [Google Scholar] [CrossRef]
- Wang, Y.X.; Griffith, J.F.; Deng, M.; Yeung, D.K.; Yuan, J. Rapid increase in marrow fat content and decrease in marrow per-fusion in lumbar vertebra following bilateral oophorectomy: An MR imaging-based prospective longitudinal study. Korean J. Radiol. 2015, 16, 154–159. [Google Scholar] [CrossRef]
- Shikata, J.; Sanada, H.; Yamamuro, T.; Takeda, T. Experimental Studies of the Elastic Fiber of the Capsular Ligament. Connect. Tissue Res. 1979, 7, 21–27. [Google Scholar] [CrossRef]
- Baron, Y.M.; Brincat, M.P.; Galea, R.; Calleja, N. Intervertebral disc height in treated and untreated overweight post-menopausal women. Hum. Reprod. 2005, 20, 3566–3570. [Google Scholar] [CrossRef]
- Tostes, R.; Nigro, D.; Fortes, Z.; Carvalho, M. Effects of estrogen on the vascular system. Braz. J. Med. Biol. Res. 2003, 36, 1143–1158. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chiba, H.; Jimbo, S.; Senoo, I.; Shimizu, M.; Atsuta, Y.; Ito, H.; Sugisawa, H.; Sugawara, T.; Habaguchi, T. Clinical, physical, and radiographic analyses of lumbar degenerative kyphosis and spondylolisthesis among community-based cohort. Eur. Spine J. 2016, 25, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Skelton, D.A.; Phillips, S.K.; Bruce, S.A.; Naylor, C.H.; Woledge, R.C. Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin. Sci. 1999, 96, 357–364. [Google Scholar] [CrossRef]
- Phillips, S.K.; Rook, K.M.; Siddle, N.C.; Bruce, S.A.; Woledge, R.C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin. Sci. 1993, 84, 95–98. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Sipila, S.; Cheng, S.; Puolakka, J.; Toivanen, J.; Suominen, H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in post-menopausal women: A yearlong intervention. Clin. Physiol. Funct. Imaging 2005, 25, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Preisinger, E.; Alacamlioglu, Y.; Saradeth, T.; Resch, K.L.; Holzer, G.; Metka, M. Forearm bone-density and grip strength in women after menopause, with and without estrogen replacement therapy. Maturitas 1995, 21, 57–63. [Google Scholar] [CrossRef]
- Naessen, T.; Lindmark, B.; Larsen, H.C. Better postural balance in elderly women receiving estrogens. Am. J. Obstet. Gynecol. 1997, 177, 412–416. [Google Scholar] [CrossRef]
- Marshall, S.A.; Senadheera, S.N.; Parry, L.J.; Girling, J.E. The Role of Relaxin in Normal and Abnormal Uterine Function During the Menstrual Cycle and Early Pregnancy. Reprod. Sci. 2017, 24, 342–354. [Google Scholar] [CrossRef]
- Tsai, C.L.; Liu, T.K. Estradiol-induced knee osteoarthrosis in ovariectomized rabbits. Clin. Orthop. 1993, 291, 295–302. [Google Scholar] [CrossRef]
- Felson, D.T.; Nevitt, M.C. The effects of estrogen on osteoarthritis. Curr. Opin. Rheumatol. 1998, 10, 269–272. [Google Scholar] [CrossRef]
- Nordin, E.C.; Polley, K.J. Metabolic consequences of the menopause: A cross-sectional, longitudinal, and intervention study on 557 normal postmenopausal women. Calcif. Tissue Int. 1987, 41, 1–59. [Google Scholar]
- Spector, T.D.; Perry, L.A.; Jubb, R.W. Endogenous sex steroid levels in women with generalized osteoarthritis. Clin. Rheumatol. 1991, 10, 316–319. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Liu, T.-K. Up-regulation of estrogen receptors in rabbit osteoarthritic cartilage. Life Sci. 1992, 50, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Liu, T.; Chen, T. Estrogen and osteoarthritis: A study of synovial estradiol and estradiol receptor binding in human osteoarthritic knees. Biochem. Biophys. Res. Commun. 1992, 183, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.L.; Lee, J.S.; Suh, K.T.; Kim, J.I.; Lee, H.S.; Goh, T.S.; Park, S.H. Association Between Estrogen Receptor Gene Polymorphism and Back Pain Intensity in Female Patients with Degenerative Lumbar Spondylolisthesis. J. Spinal Disord. Tech. 2013, 26, E53–E57. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Suh, K.T.; Kim, J.I.; Lim, J.M.; Goh, T.S. Association of Estrogen Receptor Gene Polymorphism in Patients with Degenerative Lumbar Spondylolisthesise. J. Korean Neurosurg. Soc. 2011, 50, 415–419. [Google Scholar] [CrossRef]
- Anekstein, Y.; Smorgick, Y.; Lotan, R.; Agar, G.; Shalmon, E.; Floman, Y.; Mirovsky, Y. Diabetes mellitus as a risk factor for the development of lumbar spinal stenosis. Isr. Med. Assoc. J. 2010, 12, 16. [Google Scholar] [PubMed]
| Study | Study Groups | Inclusion Criteria | Exclusion Criteria: | Article Type | Main Conclusions |
|---|---|---|---|---|---|
| Dent [14] | N = 300 Age (range)—50–90 y Gender:
|
|
|
|
|
| Rosenberg [15] | N = 200 Age (range): 44–89 y Gender:
| Diagnosis of degenerative spondylolisthesis. |
|
|
|
| Imada [16] | Study group: N = 210 Age (mean):
N =138 Gender:
| Study Group:
|
|
|
|
| Sanderson [17] | N = 1069 Age (mean):
|
|
|
|
|
| Kauppila [18] | N = 617
|
|
|
|
|
| Vogt [4] | N = 788 Age (mean): 71.5 y Gender:
|
|
|
|
|
| Vogt [19] | N = 1366 Age (mean)—71.2 ± 5.1 y Gender:
|
|
|
|
|
| Iguchi [10] | N = 201 Age (mean): 64.6 y Gender:
|
|
|
|
|
| Vogt [5] | N = 470 Age (mean)—75.1 ± 4.9 y Gender:
|
|
|
|
|
| Chen [6] | N = 1242 Age (mean)—44.5 ± 8.7 y Gender:
|
|
|
|
|
| Horikawa [20] | N = 528 Age (mean): 70.6 y Gender:
|
|
|
|
|
| Jacobsen [7] | N = 4001 Age (range)—22–93 y Gender:
|
|
|
|
|
| Mariconda [21] | N = 120 Age (mean)—57.5 ± 11.8 y Gender:
|
|
|
|
|
| Hosoe [22] | N = 250 Age (mean):
|
DS group:
Control group:
|
|
|
|
| Chen [23] | N = 132 Age (mean):
| Study group:
|
|
|
|
| Schuller [24] | Study group: N = 49 Age (mean)—65.9 y Gender:
N = 77 Age (mean)—65.5 y Gender:
| Study group:
|
|
|
|
| Kalichman [8] | N = 188 |
|
|
|
|
| Aono [25] | N = 142 Age (mean): Gender:
|
|
|
|
|
| Denard [9] | N = 190 Age (mean): 74 ± 6 y Gender:
| Osteoporotic Fractures in Men
|
|
|
|
| Marty-Poumarat [26] | N = 146
|
No HRT group:
|
Indices;
|
|
|
| He [27] | N = 3990 Age. mean:
|
|
|
|
|
| Ferrero [28] | N = 654 Age (mean): 67.3 ± 10.6 y Gender:
|
|
|
|
|
| Enyo [29] | N = 200 Gender:
|
|
|
|
|
| Wang [30] | N = 3065 Age (mean):
|
|
|
|
|
| Cholewicki [31] | N = 322
Gender:
|
|
|
|
|
| Fraser [32] | N = 205 Age (mean): 68.3 y Gender:
|
|
|
|
|
| Ishimoto [33] | N = 722
Gender:
|
|
|
|
|
| No. | Study | n | Age | BMI | BMD | Gender | Height | Ehtnic Origin | Physical Activity | Diabetes | Smoking | Pregnancy Status | HRT | Opherectomy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dent [14] | 300 | + | |||||||||||
| 2 | Rosenberg [15] | 200 | + | + | + | + | ||||||||
| 3 | Imada 109] | 210 | + | |||||||||||
| 4 | Sanderson [17] | 1069 | + | + | ||||||||||
| 5 | Kauppila [18] | 617 | − | +/− | + | - | − | |||||||
| 6 | Vogt [4] | 788 | + | − | − | + | +/− | |||||||
| 7 | Vogt [19] | 1400 | + | + | ||||||||||
| 8 | Iguchi [10] | 3259 | + | |||||||||||
| 9 | Vogt [5] | 481 | + | − | + | + | ||||||||
| 10 | Chen [6] | 1242 | + | + | + | |||||||||
| 11 | Horikawa [20] | 528 | − | + | + | |||||||||
| 12 | Jacobsen [7] | 4001 | + | +/− | + | +/− | − | − | − | |||||
| 13 | Mariconda [21] | 120 | + | + | + | |||||||||
| 14 | Hosoe [22] | 250 | + | + | ||||||||||
| 15 | Chen [23] | 132 | − | |||||||||||
| 16 | Kalichman [8] | 188 | + | + | ||||||||||
| 17 | Aono [25] | 142 | − | − | ||||||||||
| 18 | Denard [9] | 190 | + | − | − | + | − | − | ||||||
| 19 | Schuller [24] | 126 | + | + | ||||||||||
| 20 | Marty-Poumarat [26] | 147 | + | + | ||||||||||
| 21 | He [27] | 3990 | + | + | + | − | + | − | − | − | − | − | ||
| 22 | Ferrero [28] | 654 | + | + | ||||||||||
| 23 | Enyo [29] | 200 | + | + | ||||||||||
| 24 | Wang [30] | 3065 | + | + | + | − | + | − | − | − | − | − | ||
| 25 | Cholewicki [31] | 322 | + | + | + | + | ||||||||
| 26 | Fraser [32] | 205 | − | − | + | |||||||||
| 27 | Ishimoto [33] | 722 | − | − | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, M.; Kulesza, B.; Gołębiowska, N.; Tyzo, B.; Kura, K.; Szczepanek, D. Factors Predisposing to The Formation of Degenerative Spondylolisthesis—A Narrative Review. Medicina 2023, 59, 1430. https://doi.org/10.3390/medicina59081430
Mazurek M, Kulesza B, Gołębiowska N, Tyzo B, Kura K, Szczepanek D. Factors Predisposing to The Formation of Degenerative Spondylolisthesis—A Narrative Review. Medicina. 2023; 59(8):1430. https://doi.org/10.3390/medicina59081430
Chicago/Turabian StyleMazurek, Marek, Bartłomiej Kulesza, Natalia Gołębiowska, Bartłomiej Tyzo, Krzysztof Kura, and Dariusz Szczepanek. 2023. "Factors Predisposing to The Formation of Degenerative Spondylolisthesis—A Narrative Review" Medicina 59, no. 8: 1430. https://doi.org/10.3390/medicina59081430
APA StyleMazurek, M., Kulesza, B., Gołębiowska, N., Tyzo, B., Kura, K., & Szczepanek, D. (2023). Factors Predisposing to The Formation of Degenerative Spondylolisthesis—A Narrative Review. Medicina, 59(8), 1430. https://doi.org/10.3390/medicina59081430







