Robot-Assisted Parathyroidectomy Using Indocyanine Green (ICG) Fluorescence in Primary Hyperparathyroidism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Indication
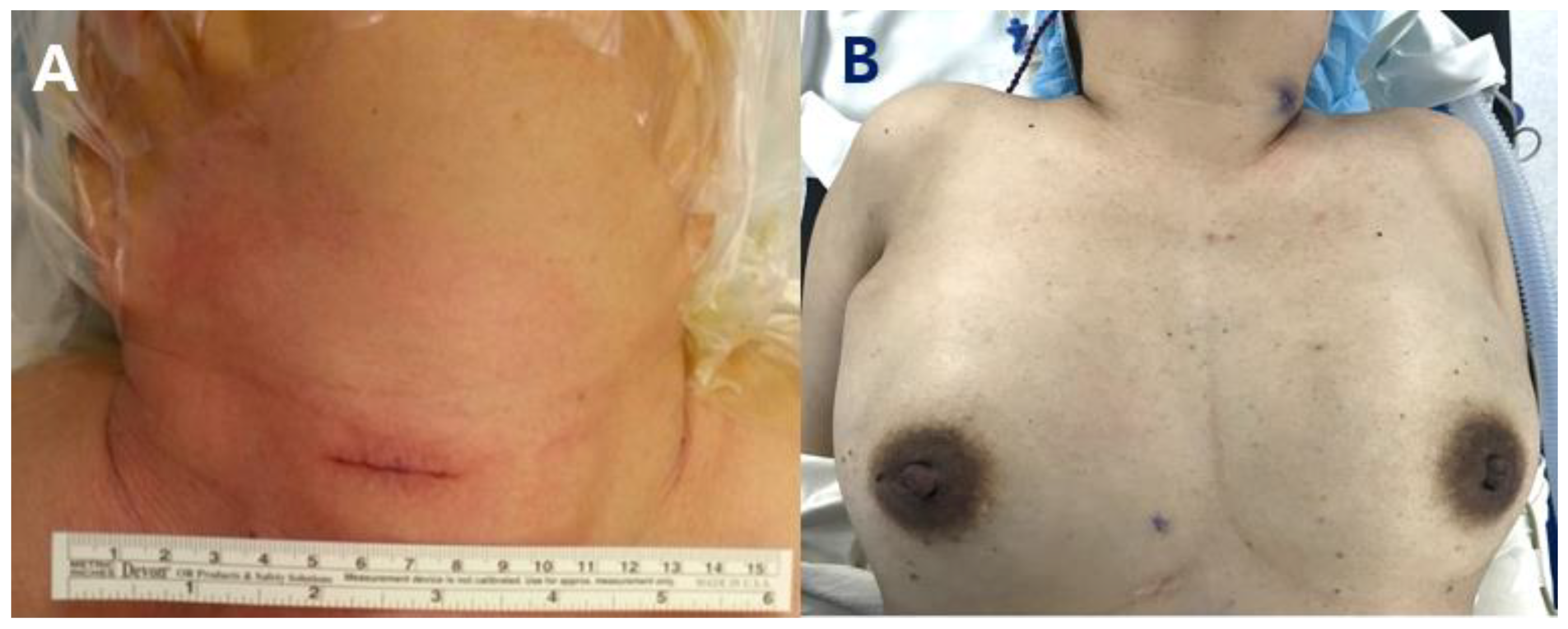
2.3. Surgical Methods
2.4. ICG Angiography
2.5. IOPTH Monitoring
2.6. Statistics and Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sociedade Brasileira de Endocrinologia e Metabolismo; Bandeira, F.; Griz, L.; Chaves, N.; Carvalho, N.C.; Borges, L.M.; Lazaretti-Castro, M.; Borba, V.; Castro, L.C.; Borges, J.L.; et al. Diagnosis and management of primary hyperparathyroidism—A scientific statement from the Department of Bone Metabolism, the Brazilian Society for Endocrinology and Metabolism. Arq. Bras. Endocrinol. Metabol. 2013, 57, 406–424. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Donnan, P.T.; Murphy, M.J.; Leese, G.P. Epidemiology of primary hyperparathyroidism in Tayside, Scotland, UK. Clin. Endocrinol. 2009, 71, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.W. Clinical aspects of primary hyperparathyroidism: Clinical manifestations, diagnosis, and therapy. Wien Med. Wochenschr. 2013, 163, 397–402. [Google Scholar] [CrossRef]
- Yeh, M.W.; Ituarte, P.H.; Zhou, H.C.; Nishimoto, S.; Liu, I.L.; Harari, A.; Haigh, P.I.; Adams, A.L. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J. Clin. Endocrinol. Metab. 2013, 98, 1122–1129. [Google Scholar] [CrossRef] [Green Version]
- Abood, A.; Vestergaard, P. Increasing incidence of primary hyperparathyroidism in Denmark. Dan Med. J. 2013, 60, A4567. [Google Scholar]
- Darba, J.; Marsa, A. Epidemiology and management of parathyroid gland disorders in Spain over 15 years: A retrospective multicentre analysis. PLoS ONE 2020, 15, e0230130. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Livolsi, V.A. The Parathyroids: Basic and Clinical Concepts, 3rd ed.; Elsevier: San Diego, CA, USA, 2015; pp. 25–28. [Google Scholar]
- Bandeira, L.; Bilezikian, J. Primary Hyperparathyroidism. F1000Res 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, S.J. Hyperparathyroid and hypoparathyroid disorders. N. Engl. J. Med. 2000, 343, 1863–1875. [Google Scholar] [CrossRef]
- Suh, Y.J.; Choi, J.Y.; Chai, Y.J.; Kwon, H.; Woo, J.W.; Kim, S.J.; Kim, K.H.; Lee, K.E.; Lim, Y.T.; Youn, Y.K. Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg. Endosc. 2015, 29, 2811–2817. [Google Scholar] [CrossRef]
- Yu, H.W.; Chung, J.W.; Yi, J.W.; Song, R.Y.; Lee, J.H.; Kwon, H.; Kim, S.J.; Chai, Y.J.; Choi, J.Y.; Lee, K.E. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly(R) technology during BABA robotic thyroidectomy. Surg. Endosc. 2017, 31, 3020–3027. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Kim, W.W. Intraoperative assessment of parathyroid perfusion using indocyanine green angiography in robotic thyroidectomy. J. Minim. Invasive Surg. 2022, 25, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Ahn, J.; Kim, J.H.; Yi, J.W.; Hur, M.H. Initial Experience of BABA Robotic Thyroidectomy Using the Da Vinci Xi System in Incheon, Korea. J. Endocr. Surg. 2019, 19, 59–67. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.B.; Ahn, J.; Kim, J.H.; Choi, S.W.; Choi, Y.S.; Yi, J.W. Harmonic versus LigaSure for Flap Creation in Bilateral Axillary Breast Approach Thyroid Surgery. J. Endocr Surg. 2020, 20, 69–77. [Google Scholar] [CrossRef]
- Terris, D.J.; Duke, W.S.; Pasieka, J.L. Parathyroid Surgery: Fundamental and Advanced Concepts, 1st ed.; Plural Publishing, Inc.: San Diego, CA, USA, 2014. [Google Scholar]
- Ferlin, G.; Borsato, N.; Camerani, M.; Conte, N.; Zotti, D. New perspectives in localizing enlarged parathyroids by technetium-thallium subtraction scan. J. Nucl. Med. 1983, 24, 438–441. [Google Scholar] [PubMed]
- Haber, R.S.; Kim, C.K.; Inabnet, W.B. Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: Comparison with (99m)technetium sestamibi scintigraphy. Clin. Endocrinol. 2002, 57, 241–249. [Google Scholar] [CrossRef]
- Arici, C.; Cheah, W.K.; Ituarte, P.H.; Morita, E.; Lynch, T.C.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Can localization studies be used to direct focused parathyroid operations? Surgery 2001, 129, 720–729. [Google Scholar] [CrossRef]
- Lumachi, F.; Zucchetta, P.; Marzola, M.C.; Boccagni, P.; Angelini, F.; Bui, F.; D’Amico, D.F.; Favia, G. Advantages of combined technetium-99m-sestamibi scintigraphy and high-resolution ultrasonography in parathyroid localization: Comparative study in 91 patients with primary hyperparathyroidism. Eur. J. Endocrinol. 2000, 143, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, S.R.; Thompson, A.R.; Hutcheson, K.A.; Gaz, R.D.; Wang, C.A. Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism. Surgery 1988, 104, 1121–1127. [Google Scholar]
- Haciyanli, M.; Lal, G.; Morita, E.; Duh, Q.Y.; Kebebew, E.; Clark, O.H. Accuracy of preoperative localization studies and intraoperative parathyroid hormone assay in patients with primary hyperparathyroidism and double adenoma. J. Am. Coll. Surg. 2003, 197, 739–746. [Google Scholar] [CrossRef]
- Fraker, D.L.; Harsono, H.; Lewis, R. Minimally invasive parathyroidectomy: Benefits and requirements of localization, diagnosis, and intraoperative PTH monitoring. long-term results. World J. Surg. 2009, 33, 2256–2265. [Google Scholar] [CrossRef]
- Ruda, J.M.; Hollenbeak, C.S.; Stack, B.C., Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol. Head Neck. Surg. 2005, 132, 359–372. [Google Scholar] [CrossRef]
- He, Q.; Zhu, J.; Zhuang, D.; Fan, Z. Robotic total parathyroidectomy by the axillo-bilateral-breast approach for secondary hyperparathyroidism: A feasibility study. J. Laparoendosc. Adv. Surg. Tech. A 2015, 25, 311–313. [Google Scholar] [CrossRef]
- Mohsin, K.; Alzahrani, H.; Bu Ali, D.; Kang, S.W.; Kandil, E. Robotic transaxillary parathyroidectomy. Gland Surg. 2017, 6, 410–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landry, C.S.; Grubbs, E.G.; Morris, G.S.; Turner, N.S.; Holsinger, F.C.; Lee, J.E.; Perrier, N.D. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery 2011, 149, 549–555. [Google Scholar] [CrossRef]
- Foley, C.S.; Agcaoglu, O.; Siperstein, A.E.; Berber, E. Robotic transaxillary endocrine surgery: A comparison with conventional open technique. Surg. Endosc. 2012, 26, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Boccara, G.; Guenoun, T.; Aidan, P. Anesthetic implications for robot-assisted transaxillary thyroid and parathyroid surgery: A report of twenty cases. J. Clin. Anesth. 2013, 25, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Noureldine, S.I.; Lewing, N.; Tufano, R.P.; Kandil, E. The role of the robotic-assisted transaxillary gasless approach for the removal of parathyroid adenomas. ORL J. Otorhinolaryngol. Relat. Spec. 2014, 76, 19–24. [Google Scholar] [CrossRef]
- Al Kadah, B.; Siemer, S.; Schick, B. First experience in the thyroid and parathyroid surgery using the da Vinci(R) system. Laryngorhinootologie 2014, 93, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Karagkounis, G.; Uzun, D.D.; Mason, D.P.; Murthy, S.C.; Berber, E. Robotic surgery for primary hyperparathyroidism. Surg. Endosc. 2014, 28, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Tolley, N.; Garas, G.; Palazzo, F.; Prichard, A.; Chaidas, K.; Cox, J.; Darzi, A.; Arora, A. Long-term prospective evaluation comparing robotic parathyroidectomy with minimally invasive open parathyroidectomy for primary hyperparathyroidism. Head Neck. 2016, 38 (Suppl. S1), E300–E306. [Google Scholar] [CrossRef]
- Alshehri, M.; Mohamed, H.E.; Moulthrop, T.; Kandil, E. Robotic thyroidectomy and parathyroidectomy: An initial experience with retroauricular approach. Head Neck 2017, 39, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.L.; Garstka, M.E.; Kang, S.W.; Kandil, E. Robotic Neck Surgery in the Pediatric Population. JSLS 2018, 22, e2018.00012. [Google Scholar] [CrossRef] [PubMed]
- Ozdenkaya, Y.; Ersavas, C.; Arslan, N.C. Robotic transoral vestibular parathyroidectomy: Two case reports and review of literature. World J. Clin. Cases 2018, 6, 542–547. [Google Scholar] [CrossRef]
- Van Slycke, S.; Van Den Heede, K.; Magamadov, K.; Brusselaers, N.; Vermeersch, H. Robotic-assisted parathyroidectomy through lateral cervical approach: First results in Belgium. Acta Chir. Belg. 2021, 121, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.; Hadedeya, D.; Shalaby, M.; Toraih, E.; Aparicio, D.; Garstka, M.; Munshi, R.; Elnahla, A.; Russell, J.O.; Aidan, P. Robotic-assisted parathyroidectomy via transaxillary approach: Feasibility and learning curves. Gland Surg. 2021, 10, 953–960. [Google Scholar] [CrossRef]
- Gioux, S.; Choi, H.S.; Frangioni, J.V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 237–255. [Google Scholar]
- Sound, S.; Okoh, A.; Yigitbas, H.; Yazici, P.; Berber, E. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg. Innov. 2019, 26, 774–779. [Google Scholar] [CrossRef]
- Zaidi, N.; Bucak, E.; Okoh, A.; Yazici, P.; Yigitbas, H.; Berber, E. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J. Surg. Oncol. 2016, 113, 771–774. [Google Scholar] [CrossRef]
- Zaidi, N.; Bucak, E.; Yazici, P.; Soundararajan, S.; Okoh, A.; Yigitbas, H.; Dural, C.; Berber, E. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J. Surg. Oncol. 2016, 113, 775–778. [Google Scholar] [CrossRef]
- Vidal Fortuny, J.; Belfontali, V.; Sadowski, S.M.; Karenovics, W.; Guigard, S.; Triponez, F. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br. J. Surg. 2016, 103, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahramangil, B.; Berber, E. Comparison of indocyanine green fluorescence and parathyroid autofluorescence imaging in the identification of parathyroid glands during thyroidectomy. Gland Surg. 2017, 6, 644–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Bos, J.; van Kooten, L.; Engelen, S.M.E.; Lubbers, T.; Stassen, L.P.S.; Bouvy, N.D. Feasibility of indocyanine green fluorescence imaging for intraoperative identification of parathyroid glands during thyroid surgery. Head Neck. 2019, 41, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.H.; Wong, C.K.; Hung, H.T.; Wong, K.P.; Mak, K.L.; Au, K.B. Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 2017, 161, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Alesina, P.F.; Meier, B.; Hinrichs, J.; Mohmand, W.; Walz, M.K. Enhanced visualization of parathyroid glands during video-assisted neck surgery. Langenbecks Arch Surg. 2018, 403, 395–401. [Google Scholar] [CrossRef]
- Vidal Fortuny, J.; Sadowski, S.M.; Belfontali, V.; Guigard, S.; Poncet, A.; Ris, F.; Karenovics, W.; Triponez, F. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br. J. Surg. 2018, 105, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Fan, J.; Yang, J.; Liao, K.; He, Z.; Cui, M. Application of indocyanine green in the parathyroid detection and protection: Report of 3 cases. Am. J. Otolaryngol. 2019, 40, 323–330. [Google Scholar] [CrossRef]
- Jin, H.; Dong, Q.; He, Z.; Fan, J.; Liao, K.; Cui, M. Research on indocyanine green angiography for predicting postoperative hypoparathyroidism. Clin. Endocrinol. 2019, 90, 487–493. [Google Scholar] [CrossRef]
- Rudin, A.V.; McKenzie, T.J.; Thompson, G.B.; Farley, D.R.; Lyden, M.L. Evaluation of Parathyroid Glands with Indocyanine Green Fluorescence Angiography After Thyroidectomy. World J. Surg. 2019, 43, 1538–1543. [Google Scholar] [CrossRef]

| Variables | Number of Patients |
|---|---|
| Age (years, mean ± sd) | 56.1 ± 12.6 |
| Sex | |
| Female | 29 |
| Male | 8 |
| BMI (kg/m2, mean ± sd) | 23.9 ± 4.2 |
| Parathyroidectomy extent | |
| One-gland parathyroidectomy | 34 |
| Two-gland parathyroidectomy | 2 |
| Bilateral exploration | 1 |
| Pathologic gland location | |
| Right upper | 6 |
| Right lower | 15 |
| Left upper | 6 |
| Left lower | 12 |
| Pathologic diagnosis | |
| Parathyroid adenoma | 28 |
| Parathyroid hyperplasia | 11 |
| Pre-operative imaging | |
| SPECT-CT | 36 |
| US | 37 |
| Comorbidity | |
| Hypertension | 12 |
| Chronic renal failure | 4 |
| Arrhythmia | 2 |
| Coronary artery disease | 2 |
| Osteoporosis | 9 |
| Fracture history | 1 |
| Ureter or renal stone | 14 |
| Variables | Open (n = 31) | Robot (n = 6) | p Value |
|---|---|---|---|
| Age (years) | 57.3 ± 12.6 | 49.7 ± 11.2 | 0.178 |
| Sex | 1.000 | ||
| Female | 24 | 5 | |
| Male | 7 | 1 | |
| BMI (kg/m2, mean ± sd) | 24.0 ± 4.4 | 23.7 ± 3.6 | 0.862 |
| Operation time (min) | 91.1 ± 69.1 | 152.5 ± 23.6 | 0.001 |
| Estimated blood loss (mL) | 46.1 ± 178.3 | 8.3 ± 20.4 | 0.262 |
| Largest gland size (cm) | 1.8 ± 0.9 | 1.7 ± 1.0 | 0.725 |
| Postoperative complications (n) | 1.000 | ||
| Transient vocal cord palsy | 1 | 0 | |
| Permanent vocal cord palsy | 0 | 0 | |
| Hypertrophic scar or keloid | 1 | 0 | |
| Hospital stay days after surgery (days) | 1.8 ± 1.2 | 3.0 ± 0.0 | <0.001 |
| PTH, pre-operative (pg/mL) | 167.5 ± 95.7 | 443.8 ± 459.4 | 0.201 |
| PTH, pre-incision (pg/mL) | 207.9 ± 170.5 | 542.5 ± 400.9 | 0.097 |
| PTH, pre-excision (pg/mL) | 153.1 ± 81.4 | 720.5 ± 626.8 | 0.077 |
| PTH, 5 min after excision (pg/mL) | 76.6 ± 52.8 | 228.5 ± 207.5 | 0.133 |
| PTH, 10 min after excision (pg/mL) | 44.7 ± 26.1 | 160.3 ± 138.9 | 0.097 |
| PTH, 6 months after surgery (pg/mL) | 40.2 ± 18.5 | 29.7 ± 7.7 | 0.278 |
| PTH, 12 months after surgery (pg/mL) | 46.7 ± 27.5 | 30.0 ± 14.4 | 0.337 |
| Calcium, pre-operative (mg/dL) | 10.8 ± 0.7 | 12.7 ± 1.8 | 0.071 |
| Calcium, postoperative (mg/dL) | 8.8 ± 0.7 | 10.3 ± 0.7 | <0.001 |
| Calcium, 6 months after surgery (mg/dL) | 9.3 ± 0.4 | 9.4 ± 0.3 | 0.673 |
| Calcium, 12 months after surgery (mg/dL) | 9.2 ± 0.5 | 9.2 ± 0.4 | 0.824 |
| Ionized calcium, pre-operative (mmol/L) | 1.4 ± 0.1 | 1.6 ± 0.3 | 0.095 |
| Ionized calcium, postoperative (mmol/L) | 1.1 ± 0.1 | 1.4 ± 0.2 | <0.001 |
| Ionized calcium, 6 months after surgery (mmol/L) | 1.2 ± 0.1 | 1.2 ± 0.2 | 0.455 |
| Ionized calcium, 12 months after surgery (mmol/L) | 1.3 ± 0.2 | 1.3 ± 0.0 | 0.976 |
| Year | Author | Study Design | Type of Approach | Patients (n) | Operation Time (min) | IOPTH | ICG | Complication | Conversion |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | Landry [27] | Case series | Trans -axillary | 2 | 108.5 | Yes | No | No | No |
| 2012 | Foley [28] | Comparative | Trans -axillary | 4 | 186 | Yes | No | Yes (1 wound infection, 1 seroma) | No |
| 2013 | Boccara [29] | Case series | Trans -axillary | 2 | 150 | No | No | No | Yes (n = 1) |
| 2014 | Noureldine [30] | Case series | Trans -axillary | 9 | 119 | Yes | No | No | Yes (n = 1) |
| 2014 | Al Kadah [31] | Case series | Trans -axillary | 2 | N/A | No | No | No | No |
| 2014 | Karagkounis [32] | Case series | Trans -axillary | 8 | 184 | Yes | No | Yes (1 seroma) | No |
| 2015 | Tolley [33] | Comparative | Axillary and anterior chest | 15 | 119 | No | No | No | Yes (n = 1) |
| 2015 | He [25] | Case series | BABA | 6 | 156 | Yes | No | No | No |
| 2017 | Alshehri [34] | Case series | Retro -auricular | 3 | 167.1 | No | No | N/A | No |
| 2017 | Mohsin [26] | Case report | Trans -axillary | 1 | N/A | Yes | Yes | No | No |
| 2018 | Wu [35] | Case series | Trans -axillary | 2 | 122.5 | No | No | Yes (1 transient hypocalcemia) | No |
| 2018 | Ozdenkaya [36] | Case series | Transoral | 4 | 184.7 | Yes | No | No | Yes (n = 2) |
| 2019 | Slycke [37] | Case series | Lateral cervical | 23 | 88 | Yes | No | No | Yes (n = 9) |
| 2021 | Kandil [38] | Case series | Trans -axillary | 102 | 116 | Yes | No | Yes (1 wound infection, 1 seroma) | Yes (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Choi, Y.S.; Hwang, Y.M.; Yi, J.W. Robot-Assisted Parathyroidectomy Using Indocyanine Green (ICG) Fluorescence in Primary Hyperparathyroidism. Medicina 2023, 59, 1456. https://doi.org/10.3390/medicina59081456
Park S-Y, Choi YS, Hwang YM, Yi JW. Robot-Assisted Parathyroidectomy Using Indocyanine Green (ICG) Fluorescence in Primary Hyperparathyroidism. Medicina. 2023; 59(8):1456. https://doi.org/10.3390/medicina59081456
Chicago/Turabian StylePark, Shin-Young, Yun Suk Choi, Young Mi Hwang, and Jin Wook Yi. 2023. "Robot-Assisted Parathyroidectomy Using Indocyanine Green (ICG) Fluorescence in Primary Hyperparathyroidism" Medicina 59, no. 8: 1456. https://doi.org/10.3390/medicina59081456





