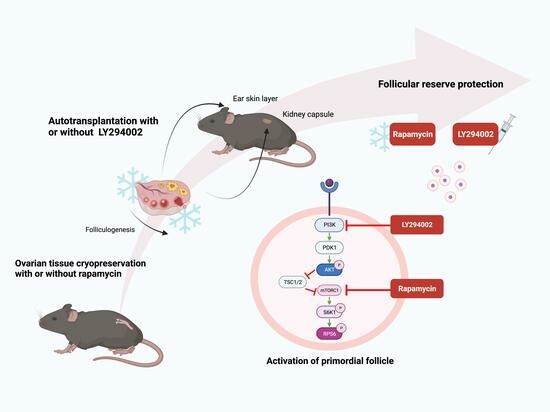
The mTOR Inhibitor Rapamycin Counteracts Follicle Activation Induced by Ovarian Cryopreservation in Murine Transplantation Models
Abstract
:1. Introduction
2. Materials and Methods
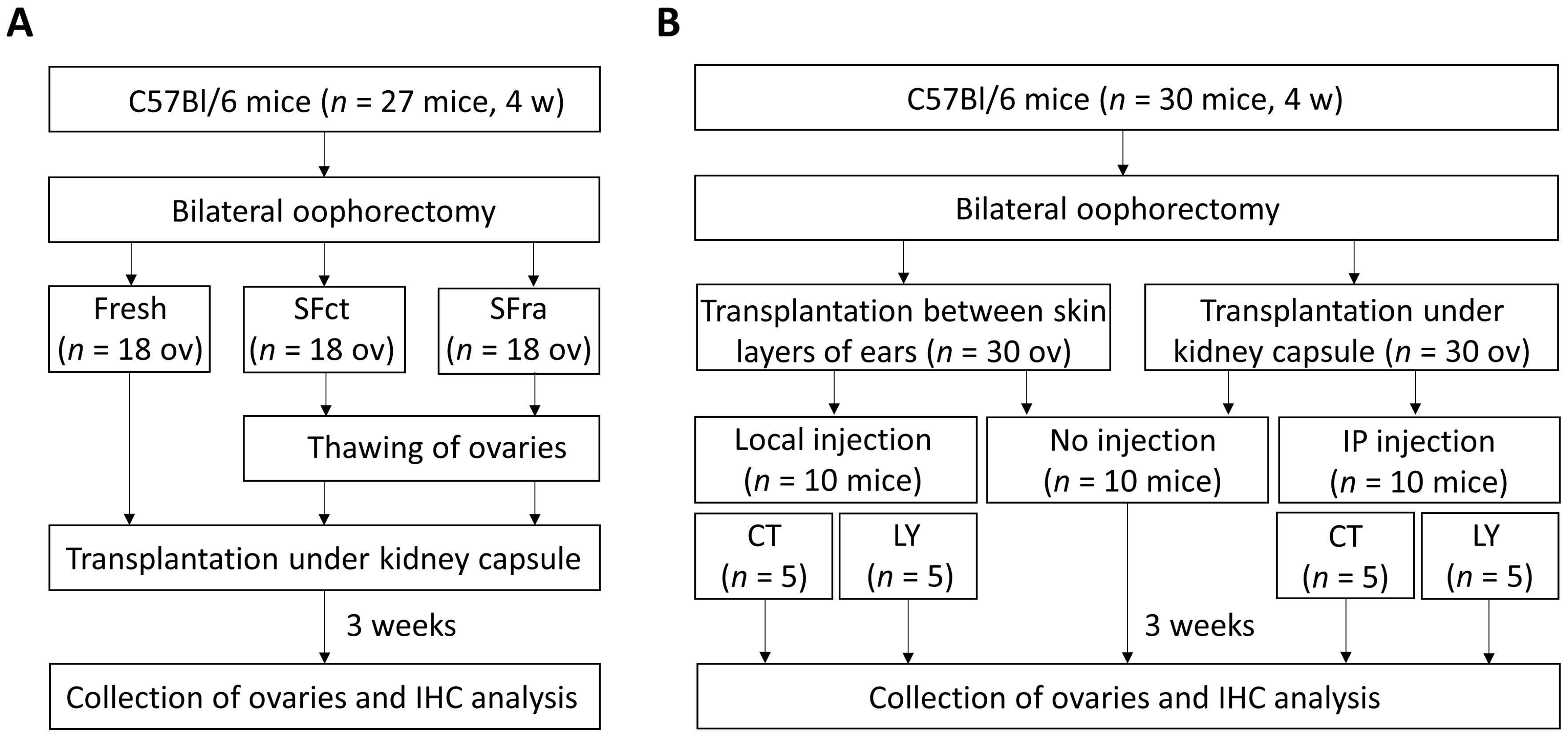
2.1. Experimental Design
2.2. Oophorectomy and Preparation of Ovaries
2.3. Ovarian Slow-Freezing and Thawing Method
2.4. Autotransplantation Procedures
2.4.1. Transplantation under the Kidney Capsule
2.4.2. Transplantation between Skin Layers of the Ears
2.5. Histological Assessment
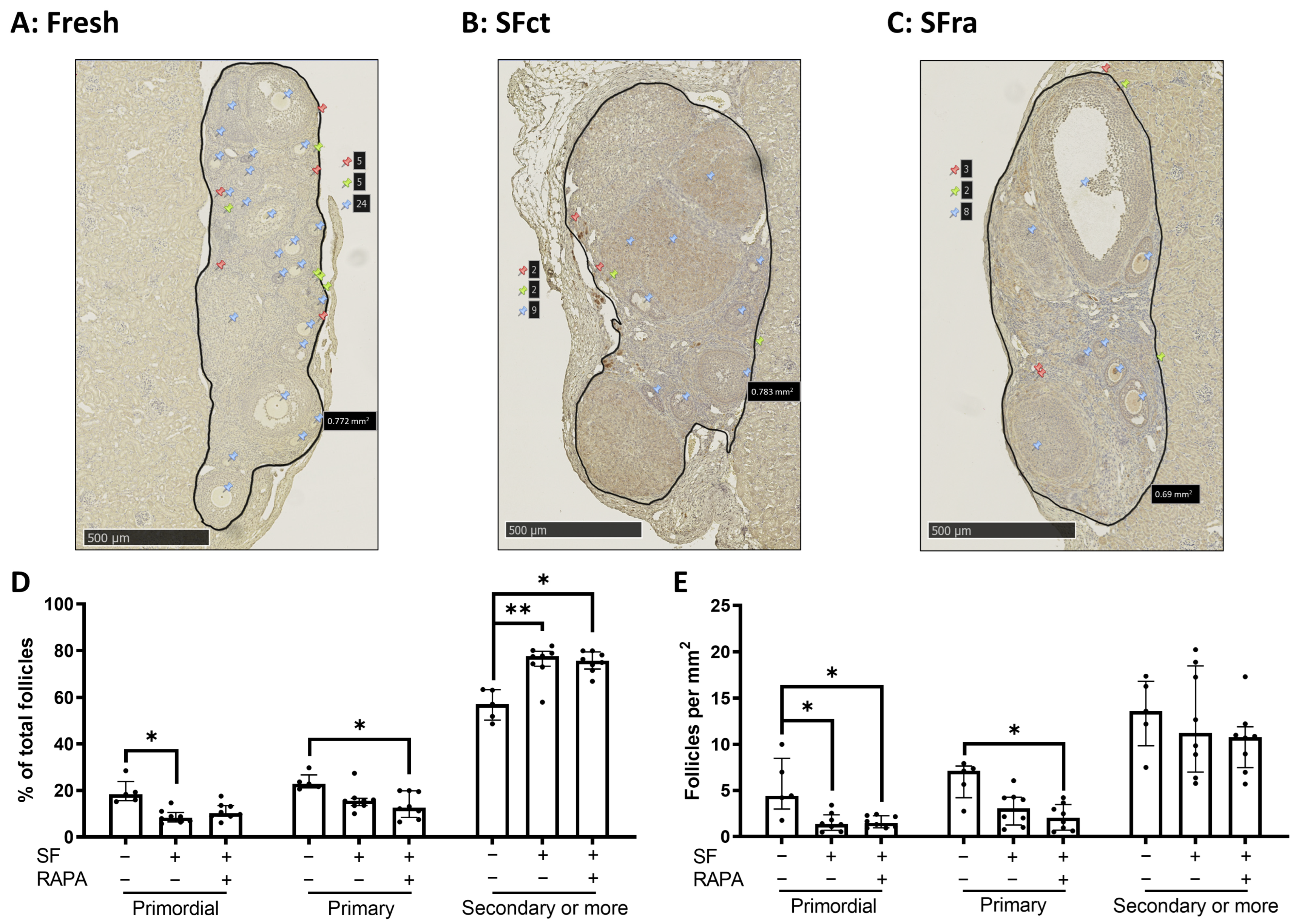
2.6. Follicle Quantification
2.7. Statistical Analysis
3. Results
3.1. In Vivo Effects of Adding Rapamycin to the Freezing Medium in Ovaries Heterotopically Transplanted under the Kidney Capsule of Mice
3.1.1. The Primordial Follicle Pool Is Decreased by Slow-Freezing with or without Rapamycin Compared to Fresh Ovaries
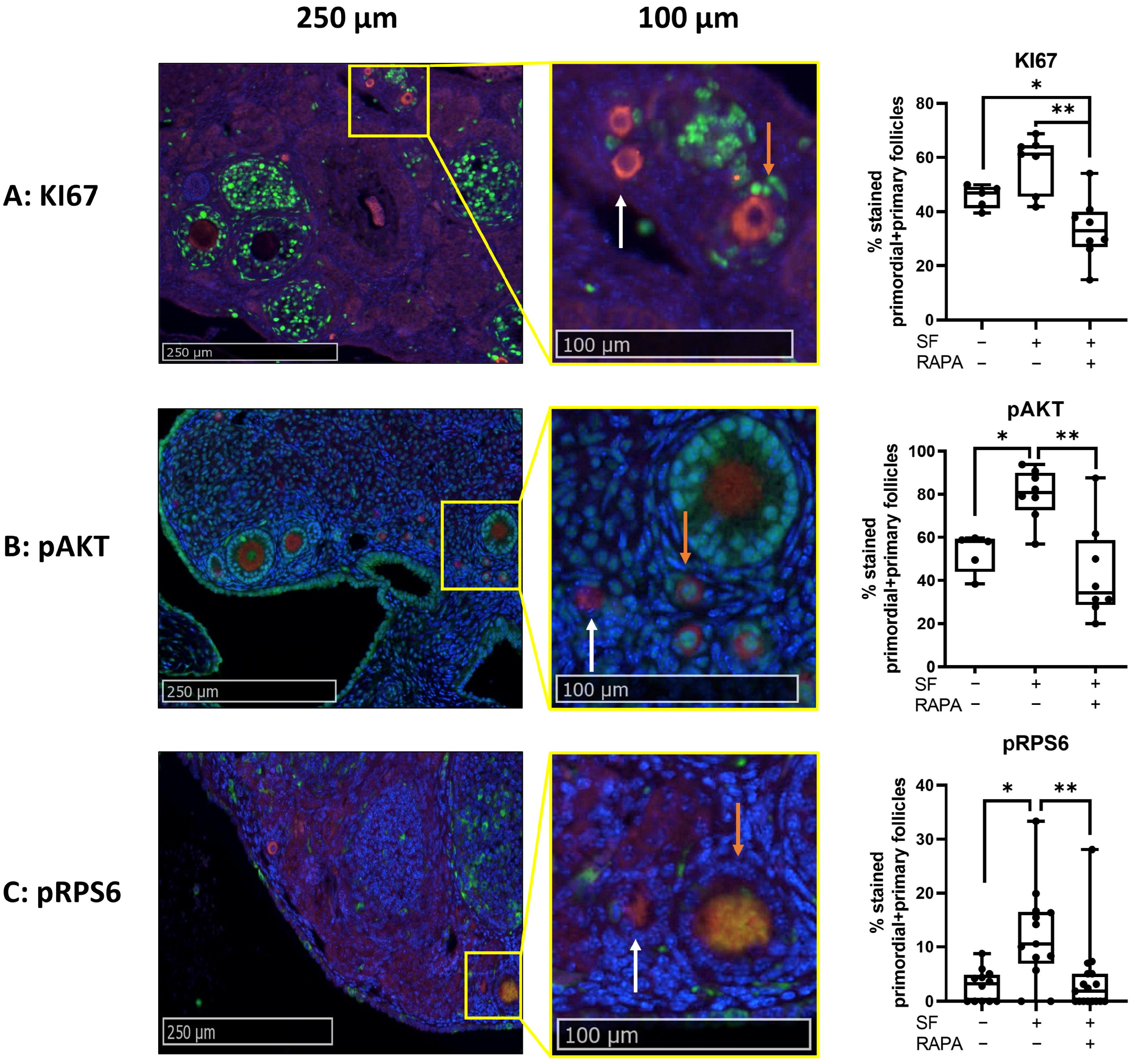
3.1.2. Addition of Rapamycin to the Freezing Medium Counteracts Follicle Proliferation and Activation Induced by Slow-Freezing and/or Transplantation In Vivo
3.1.3. No Difference in Apoptosis, Vascular Endothelial Cells, and Fibrosis Was Observed between Fresh, SF, or SF Ovaries, with Rapamycin
3.2. Comparison of Two Different Ovarian Tissue Transplantation Models
3.2.1. Graft Vascularization and Follicle Reserve Are Similar between Transplantation Sites, with No Observation of Apoptosis
3.2.2. Behavioral Score of Mice after LY294002 Injection in Both Transplantation Models
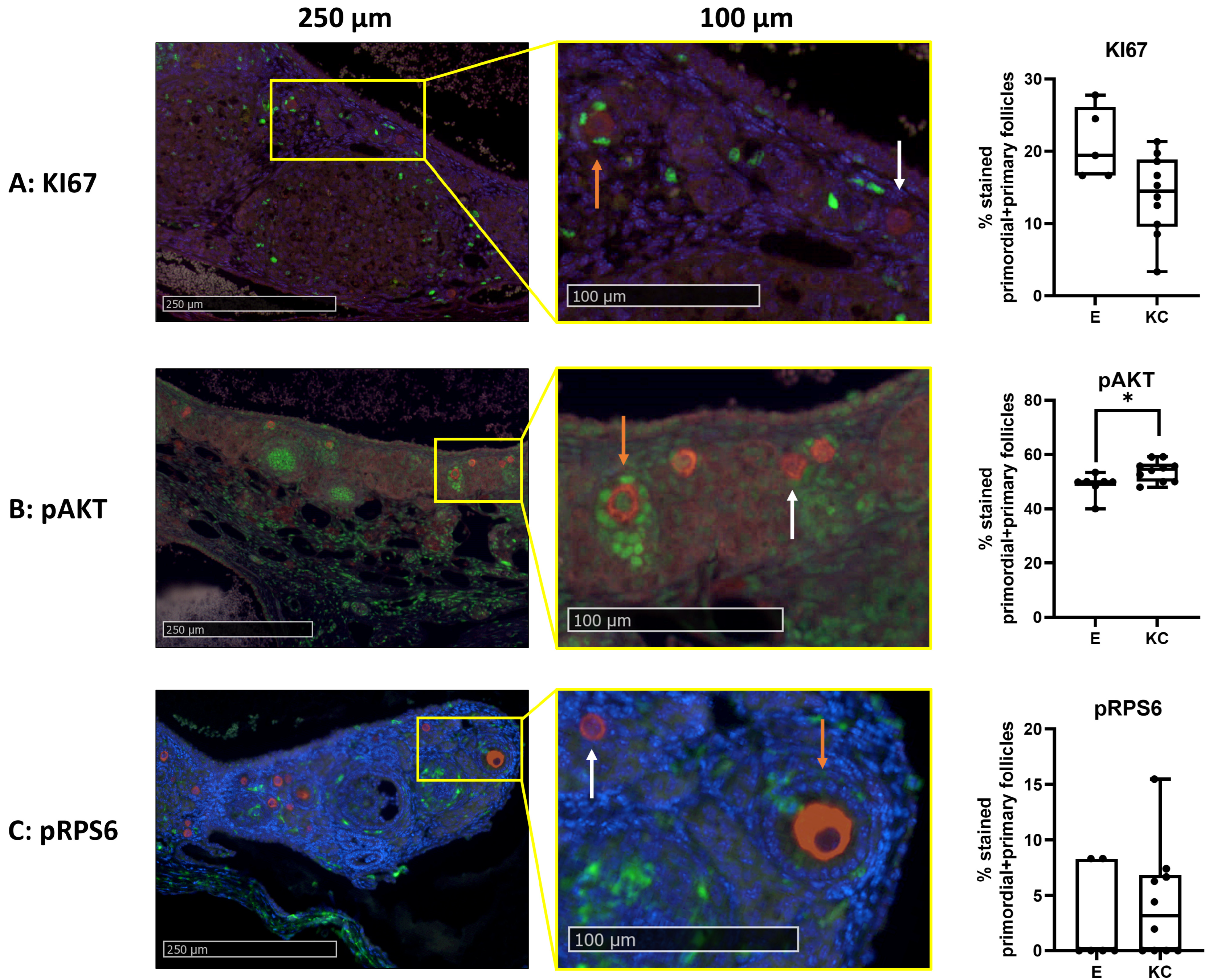
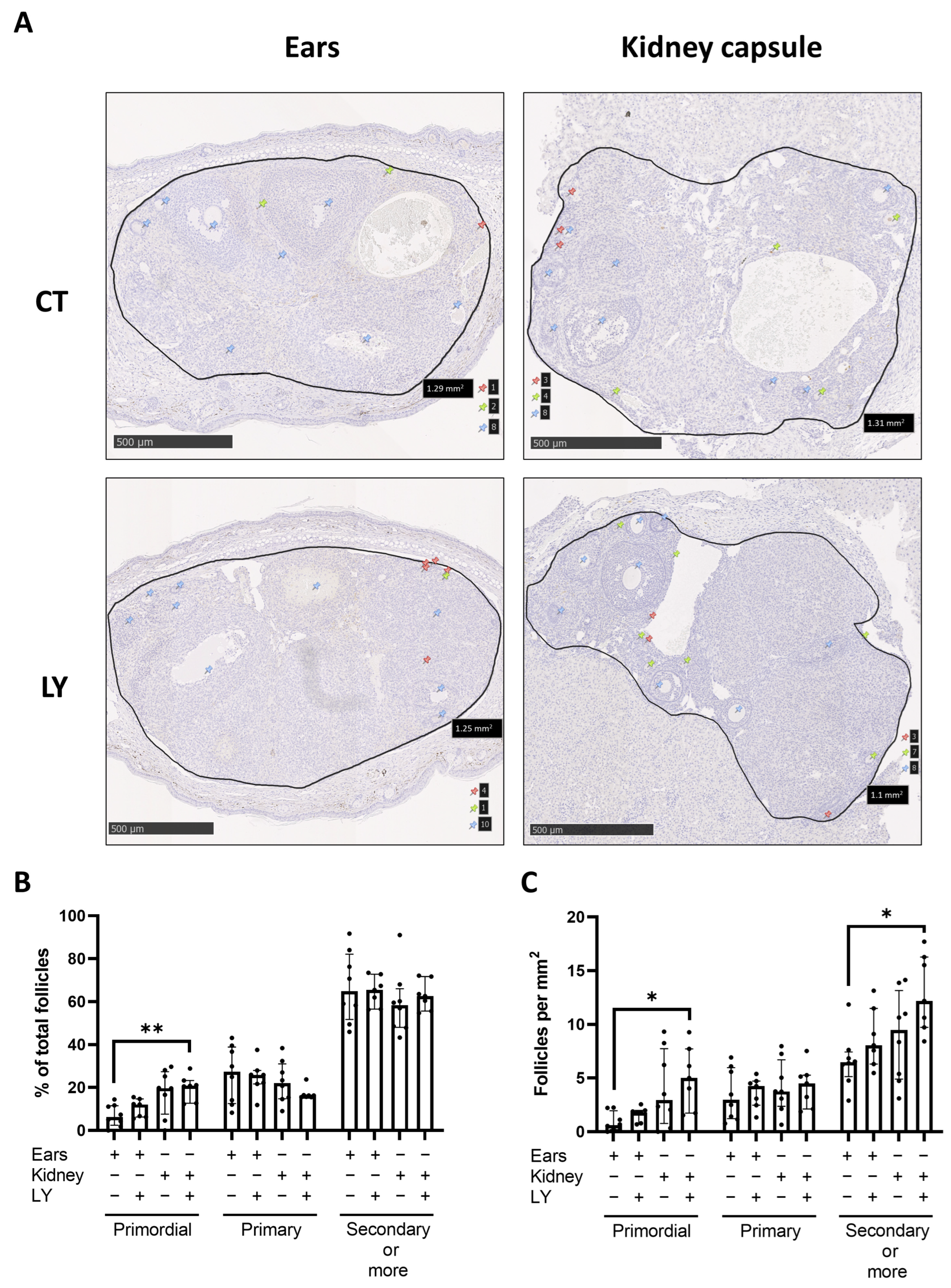
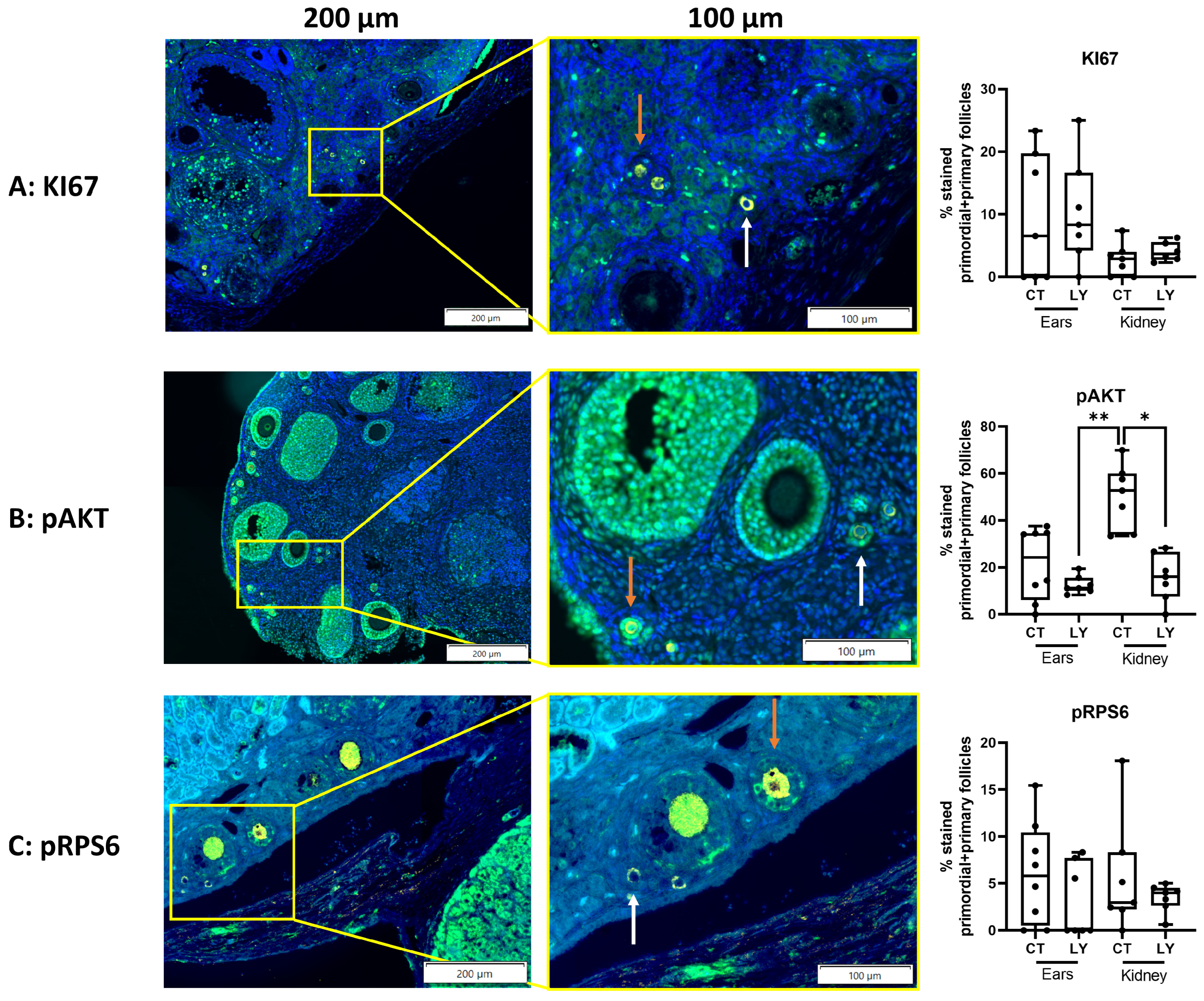
3.2.3. Follicle Density, Activation, and Proliferation Are Similar in Both Transplantation Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poorvu, P.D.; Frazier, A.L.; Feraco, A.M.; Manley, P.E.; Ginsburg, E.S.; Laufer, M.R.; LaCasce, A.S.; Diller, L.R.; Partridge, A.H. Cancer Treatment-Related Infertility: A Critical Review of the Evidence. JNCI Cancer Spectr. 2019, 3, pkz008. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Kelvin, J.F.; Quinn, G.P.; Gracia, C.R. Infertility in reproductive-age female cancer survivors. Cancer 2015, 121, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet. Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M.; Dolmans, M.M.; Silber, S.; Meirow, D. Evaluation of ovarian tissue transplantation: Results from three clinical centers. Fertil. Steril. 2020, 114, 388–397. [Google Scholar] [CrossRef]
- Marin, L.; Bedoschi, G.; Kawahara, T.; Oktay, K.H. History, Evolution and Current State of Ovarian Tissue Auto-Transplantation with Cryopreserved Tissue: A Successful Translational Research Journey from 1999 to 2020. Reprod. Sci. 2020, 27, 955–962. [Google Scholar] [CrossRef]
- Fraison, E.; Huberlant, S.; Labrune, E.; Cavalieri, M.; Montagut, M.; Brugnon, F.; Courbiere, B. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: A systematic review and meta-analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum. Reprod. 2023, 38, 489–502. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Donnez, J. Fertility preservation in women for medical and social reasons: Oocytes vs ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 63–80. [Google Scholar] [CrossRef]
- Lotz, L.; Dittrich, R.; Hoffmann, I.; Beckmann, M.W. Ovarian Tissue Transplantation: Experience from Germany and Worldwide Efficacy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119867357. [Google Scholar] [CrossRef]
- Salama, M.; Isachenko, V.; Isachenko, E.; Rahimi, G.; Mallmann, P. Updates in preserving reproductive potential of prepubertal girls with cancer: Systematic review. Crit. Rev. Oncol. Hematol. 2016, 103, 10–21. [Google Scholar] [CrossRef]
- Khattak, H.; Malhas, R.; Craciunas, L.; Afifi, Y.; Amorim, C.A.; Fishel, S.; Silber, S.; Gook, D.; Demeestere, I.; Bystrova, O.; et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: A systematic review and individual patient data meta-analysis. Hum. Reprod. Update 2022, 28, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Gavish, Z.; Spector, I.; Peer, G.; Schlatt, S.; Wistuba, J.; Roness, H.; Meirow, D. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J. Assist. Reprod. Genet. 2018, 35, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Martinez-Madrid, B.; Gadisseux, E.; Guiot, Y.; Yuan, W.Y.; Torre, A.; Camboni, A.; Van Langendonckt, A.; Donnez, J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction 2007, 134, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; David, A.; Dolmans, M.M.; Camboni, A.; Donnez, J.; Van Langendonckt, A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J. Assist. Reprod. Genet. 2011, 28, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Terren, C.; Munaut, C. Molecular Basis Associated with the Control of Primordial Follicle Activation During Transplantation of Cryopreserved Ovarian Tissue. Reprod. Sci. 2021, 28, 1257–1266. [Google Scholar] [CrossRef]
- Labied, S.; Delforge, Y.; Munaut, C.; Blacher, S.; Colige, A.; Delcombel, R.; Henry, L.; Fransolet, M.; Jouan, C.; Perrier d’Hauterive, S.; et al. Isoform 111 of vascular endothelial growth factor (VEGF111) improves angiogenesis of ovarian tissue xenotransplantation. Transplantation 2013, 95, 426–433. [Google Scholar] [CrossRef]
- Henry, L.; Fransolet, M.; Labied, S.; Blacher, S.; Masereel, M.C.; Foidart, J.M.; Noel, A.; Nisolle, M.; Munaut, C. Supplementation of transport and freezing media with anti-apoptotic drugs improves ovarian cortex survival. J. Ovarian Res. 2016, 9, 4. [Google Scholar] [CrossRef]
- Henry, L.; Labied, S.; Fransolet, M.; Kirschvink, N.; Blacher, S.; Noel, A.; Foidart, J.M.; Nisolle, M.; Munaut, C. Isoform 165 of vascular endothelial growth factor in collagen matrix improves ovine cryopreserved ovarian tissue revascularisation after xenotransplantation in mice. Reprod. Biol. Endocrinol. 2015, 13, 12. [Google Scholar] [CrossRef]
- Tavana, S.; Valojerdi, M.R.; Azarnia, M.; Shahverdi, A. Restoration of ovarian tissue function and estrous cycle in rat after autotransplantation using hyaluronic acid hydrogel scaffold containing VEGF and bFGF. Growth Factors 2016, 34, 97–106. [Google Scholar] [CrossRef]
- Kang, B.J.; Wang, Y.; Zhang, L.; Xiao, Z.; Li, S.W. bFGF and VEGF improve the quality of vitrified-thawed human ovarian tissues after xenotransplantation to SCID mice. J. Assist. Reprod. Genet. 2016, 33, 281–289. [Google Scholar] [CrossRef]
- Arapaki, A.; Christopoulos, P.; Kalampokas, E.; Triantafyllidou, O.; Matsas, A.; Vlahos, N.F. Ovarian Tissue Cryopreservation in Children and Adolescents. Children 2022, 9, 1256. [Google Scholar] [CrossRef]
- Dolmans, M.M. Recent advances in fertility preservation and counseling for female cancer patients. Expert. Rev. Anticancer Ther. 2018, 18, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Manavella, D.D.; Cacciottola, L.; Pomme, S.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum. Reprod. 2018, 33, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Manavella, D.D.; Cacciottola, L.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: A potential way to improve ovarian tissue transplantation. Hum. Reprod. 2018, 33, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Nguyen, T.Y.T.; Chiti, M.C.; Camboni, A.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Long-Term Advantages of Ovarian Reserve Maintenance and Follicle Development Using Adipose Tissue-Derived Stem Cells in Ovarian Tissue Transplantation. J. Clin. Med. 2020, 9, 2980. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Courtoy, G.E.; Nguyen, T.Y.T.; Hossay, C.; Donnez, J.; Dolmans, M.M. Adipose tissue-derived stem cells protect the primordial follicle pool from both direct follicle death and abnormal activation after ovarian tissue transplantation. J. Assist. Reprod. Genet. 2021, 38, 151–161. [Google Scholar] [CrossRef]
- Adib, S.; Valojerdi, M.R.; Alikhani, M. Dose optimisation of PTEN inhibitor, bpV (HOpic), and SCF for the in-vitro activation of sheep primordial follicles. Growth Factors 2019, 37, 178–189. [Google Scholar] [CrossRef]
- Hu, L.L.; Su, T.; Luo, R.C.; Zheng, Y.H.; Huang, J.; Zhong, Z.S.; Nie, J.; Zheng, L.P. Hippo pathway functions as a downstream effector of AKT signaling to regulate the activation of primordial follicles in mice. J. Cell. Physiol. 2019, 234, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Roness, H.; Gavish, Z.; Cohen, Y.; Meirow, D. Ovarian follicle burnout: A universal phenomenon? Cell Cycle 2013, 12, 3245–3246. [Google Scholar] [CrossRef]
- Masciangelo, R.; Hossay, C.; Donnez, J.; Dolmans, M.M. Does the Akt pathway play a role in follicle activation after grafting of human ovarian tissue? Reprod. Biomed. Online 2019, 39, 196–198. [Google Scholar] [CrossRef]
- Hu, L.; Zaloudek, C.; Mills, G.B.; Gray, J.; Jaffe, R.B. In Vivo and in Vitro Ovarian Carcinoma Growth Inhibition by a Phosphatidylinositol 3-Kinase Inhibitor (LY294002)1. Clin. Cancer Res. 2000, 6, 880–886. [Google Scholar]
- Kong, H.S.; Kim, S.K.; Lee, J.; Youm, H.W.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effect of Exogenous Anti-Mullerian Hormone Treatment on Cryopreserved and Transplanted Mouse Ovaries. Reprod. Sci. 2016, 23, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Detti, L.; Fletcher, N.M.; Saed, G.M.; Sweatman, T.W.; Uhlmann, R.A.; Pappo, A.; Peregrin-Alvarez, I. Xenotransplantation of pre-pubertal ovarian cortex and prevention of follicle depletion with anti-Mullerian hormone (AMH). J. Assist. Reprod. Genet. 2018, 35, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.; Ozkavukcu, S.; Celik-Ozenci, C. Recombinant anti-Mullerian hormone treatment attenuates primordial follicle loss after ovarian cryopreservation and transplantation. J. Assist. Reprod. Genet. 2023, 40, 1117–1134. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhou, L.; Lin, H.; Jiao, X.; Qiu, Q.; Liang, Y.; Zhang, Q. Rapamycin preserves the primordial follicle pool during cisplatin treatment in vitro and in vivo. Mol. Reprod. Dev. 2020, 87, 442–453. [Google Scholar] [CrossRef]
- Jeon, H.J.; Lee, H.E.; Yang, J. Safety and efficacy of Rapamune(R) (Sirolimus) in kidney transplant recipients: Results of a prospective post-marketing surveillance study in Korea. BMC Nephrol. 2018, 19, 201. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, L.; Xu, J.J.; Wang, N.; Liu, W.J.; Lin, X.H.; Fu, Y.C.; Luo, L.L. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 2013, 523, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yorino, S.; Kawamura, K. Rapamycin treatment maintains developmental potential of oocytes in mice and follicle reserve in human cortical fragments grafted into immune-deficient mice. Mol. Cell. Endocrinol. 2020, 504, 110694. [Google Scholar] [CrossRef] [PubMed]
- The Practice Committee of the American Society for Reproductive Medicine. Ovarian tissue cryopreservation: A committee opinion. Fertil. Steril. 2014, 101, 1237–1243. [Google Scholar] [CrossRef]
- Terren, C.; Nisolle, M.; Munaut, C. Pharmacological inhibition of the PI3K/PTEN/Akt and mTOR signalling pathways limits follicle activation induced by ovarian cryopreservation and in vitro culture. J. Ovarian Res. 2021, 14, 95. [Google Scholar] [CrossRef]
- Cheng, Y.; Kim, J.; Li, X.X.; Hsueh, A.J. Promotion of ovarian follicle growth following mTOR activation: Synergistic effects of AKT stimulators. PLoS ONE 2015, 10, e0117769. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Xiao, W.; Xiao-Hui, D.; Chun-Yan, H.; Hong-Ling, Y. Tissue culture before transplantation of frozen-thawed human fetal ovarian tissue into immunodeficient mice. Fertil. Steril. 2010, 93, 913–919. [Google Scholar] [CrossRef]
- Van Eyck, A.S.; Jordan, B.F.; Gallez, B.; Heilier, J.F.; Van Langendonckt, A.; Donnez, J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil. Steril. 2009, 92, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Masciangelo, R.; Hossay, C.; Chiti, M.C.; Manavella, D.D.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J. Assist. Reprod. Genet. 2020, 37, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Behringer, R. Mouse Ovary Transplantation. Cold Spring Harb. Protoc. 2017, 2017, 3. [Google Scholar] [CrossRef]
- Soleimani, R.; Van der Elst, J.; Heytens, E.; Van den Broecke, R.; Gerris, J.; Dhont, M.; Cuvelier, C.; De Sutter, P. Back muscle as a promising site for ovarian tissue transplantation, an animal model. Hum. Reprod. 2008, 23, 619–626. [Google Scholar] [CrossRef]
- Ayuandari, S.; Winkler-Crepaz, K.; Paulitsch, M.; Wagner, C.; Zavadil, C.; Manzl, C.; Ziehr, S.C.; Wildt, L.; Hofer-Tollinger, S. Follicular growth after xenotransplantation of cryopreserved/thawed human ovarian tissue in SCID mice: Dynamics and molecular aspects. J. Assist. Reprod. Genet. 2016, 33, 1585–1593. [Google Scholar] [CrossRef]
- Ruan, X.; Cui, Y.; Du, J.; Jin, J.; Gu, M.; Chen, S.; Mueck, A.O. Randomized study to prove the quality of human ovarian tissue cryopreservation by xenotransplantation into mice. J. Ovarian Res. 2019, 12, 46. [Google Scholar] [CrossRef]
- Garcia-Caballero, M.; Van de Velde, M.; Blacher, S.; Lambert, V.; Balsat, C.; Erpicum, C.; Durre, T.; Kridelka, F.; Noel, A. Modeling pre-metastatic lymphvascular niche in the mouse ear sponge assay. Sci. Rep. 2017, 7, 41494. [Google Scholar] [CrossRef]
- Van de Velde, M.; Garcia-Caballero, M.; Durre, T.; Kridelka, F.; Noel, A. Ear Sponge Assay: A Method to Investigate Angiogenesis and Lymphangiogenesis in Mice. Methods Mol. Biol. 2018, 1731, 223–233. [Google Scholar] [CrossRef]
- Herrmann, K.; Flecknell, P. Severity Classification of Surgical Procedures and Application of Health Monitoring Strategies in Animal Research Proposals: A Retrospective Review. Altern. Lab. Anim. 2020, 46, 273–289. [Google Scholar] [CrossRef]
- Terren, C.; Fransolet, M.; Ancion, M.; Nisolle, M.; Munaut, C. Slow Freezing Versus Vitrification of Mouse Ovaries: From Ex Vivo Analyses to Successful Pregnancies after Auto-Transplantation. Sci. Rep. 2019, 9, 19668. [Google Scholar] [CrossRef] [PubMed]
- Gosden, R.G.; Baird, D.T.; Wade, J.C.; Webb, R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum. Reprod. 1994, 9, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.M.; Uchtmann, K.S.; Valdez, C.D.; Theberge, A.B.; Miralem, T.; Ricke, W.A. Renal capsule xenografting and subcutaneous pellet implantation for the evaluation of prostate carcinogenesis and benign prostatic hyperplasia. J. Vis. Exp. 2013, 78, e50574. [Google Scholar] [CrossRef]
- Terren, C.; Bindels, J.; Nisolle, M.; Noel, A.; Munaut, C. Evaluation of an alternative heterotopic transplantation model for ovarian tissue to test pharmaceuticals improvements for fertility restoration. Reprod. Biol. Endocrinol. 2022, 20, 35. [Google Scholar] [CrossRef]
- Myers, M.; Britt, K.L.; Wreford, N.G.; Ebling, F.J.; Kerr, J.B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004, 127, 569–580. [Google Scholar] [CrossRef]
- Grabinski, N.; Ewald, F.; Hofmann, B.T.; Staufer, K.; Schumacher, U.; Nashan, B.; Jucker, M. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol. Cancer 2012, 11, 85. [Google Scholar] [CrossRef]
- Chen, X.G.; Liu, F.; Song, X.F.; Wang, Z.H.; Dong, Z.Q.; Hu, Z.Q.; Lan, R.Z.; Guan, W.; Zhou, T.G.; Xu, X.M.; et al. Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol. Carcinog. 2010, 49, 603–610. [Google Scholar] [CrossRef]
- Sulaimanov, N.; Klose, M.; Busch, H.; Boerries, M. Understanding the mTOR signaling pathway via mathematical modeling. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1379. [Google Scholar] [CrossRef]
- Celik, S.; Ozkavukcu, S.; Celik-Ozenci, C. Altered expression of activator proteins that control follicle reserve after ovarian tissue cryopreservation/transplantation and primordial follicle loss prevention by rapamycin. J. Assist. Reprod. Genet. 2020, 37, 2119–2136. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, L.; Liang, S.; Xu, B.; Ying, X.; Li, J. The protective effects of rapamycin pretreatment on ovarian damage during ovarian tissue cryopreservation and transplantation. Biochem. Biophys. Res. Commun. 2021, 534, 780–786. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Guan, H.; Xia, H.; Gu, C.; Xu, Y.; Li, B.; Zhang, W. Rapamycin maintains the primordial follicle pool and protects ovarian reserve against cyclophosphamide-induced damage. J. Reprod. Dev. 2022, 68, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Ma, A.G.; Duthie, S.J. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 1995, 336, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Elvis-Offiah, U.B.; Isuman, S.; Johnson, M.O.; Ikeh, V.G.; Agbontaen, S. Our Clear-Cut Improvement to the Impact of Mouse and Rat Models in the Research Involving Female Reproduction. In Animal Models and Experimental Research in Medicine; InTechOpen: London, UK, 2023. [Google Scholar]
- Lee, H.N.; Chang, E.M. Primordial follicle activation as new treatment for primary ovarian insufficiency. Clin. Exp. Reprod. Med. 2019, 46, 43–49. [Google Scholar] [CrossRef]
- Fujiwara, M.; Izuishi, K.; Sano, T.; Hossain, M.A.; Kimura, S.; Masaki, T.; Suzuki, Y. Modulating effect of the PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2008, 27, 76. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]



| Behavioral Score | Score Condition |
|---|---|
| 0 | No activity; breathing issues; death anticipated |
| 1 | No activity; no food intake |
| 2 | Low active state and sleepy; unreactive to human interaction |
| 3 | Low to normal active state; reactive to human interaction |
| 4 | Normal active state and fast; tries to escape when scruffed |
| 5 | Very active state, strong and fast; agitated when scruffed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindels, J.; Squatrito, M.; Bernet, L.; Nisolle, M.; Henry, L.; Munaut, C. The mTOR Inhibitor Rapamycin Counteracts Follicle Activation Induced by Ovarian Cryopreservation in Murine Transplantation Models. Medicina 2023, 59, 1474. https://doi.org/10.3390/medicina59081474
Bindels J, Squatrito M, Bernet L, Nisolle M, Henry L, Munaut C. The mTOR Inhibitor Rapamycin Counteracts Follicle Activation Induced by Ovarian Cryopreservation in Murine Transplantation Models. Medicina. 2023; 59(8):1474. https://doi.org/10.3390/medicina59081474
Chicago/Turabian StyleBindels, Jules, Marlyne Squatrito, Laëtitia Bernet, Michelle Nisolle, Laurie Henry, and Carine Munaut. 2023. "The mTOR Inhibitor Rapamycin Counteracts Follicle Activation Induced by Ovarian Cryopreservation in Murine Transplantation Models" Medicina 59, no. 8: 1474. https://doi.org/10.3390/medicina59081474