The Interplay of Raloxifene and Sonochemical Bio-Oss in Early Maxillary Sinus Bone Regeneration: A Histological and Immunohistochemical Analysis in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Number of Samples
2.2. Biomaterial Preparation
2.3. Surgical Procedures
- Group 1: 8 maxillary sinuses grafted with Bio-Oss® (BO)
- Group 2: 8 maxillary sinuses grafted with sonicated Bio-Oss® (BS)
- Group 3: 8 maxillary sinuses grafted with sonicated Bio-Oss® with Raloxifene (4:1) (BR)
2.4. Histomorphometric Analysis
2.5. Immunohistochemistry Analysis
2.6. Collagen Fibers Maturation
2.7. Statistical Analysis
3. Results
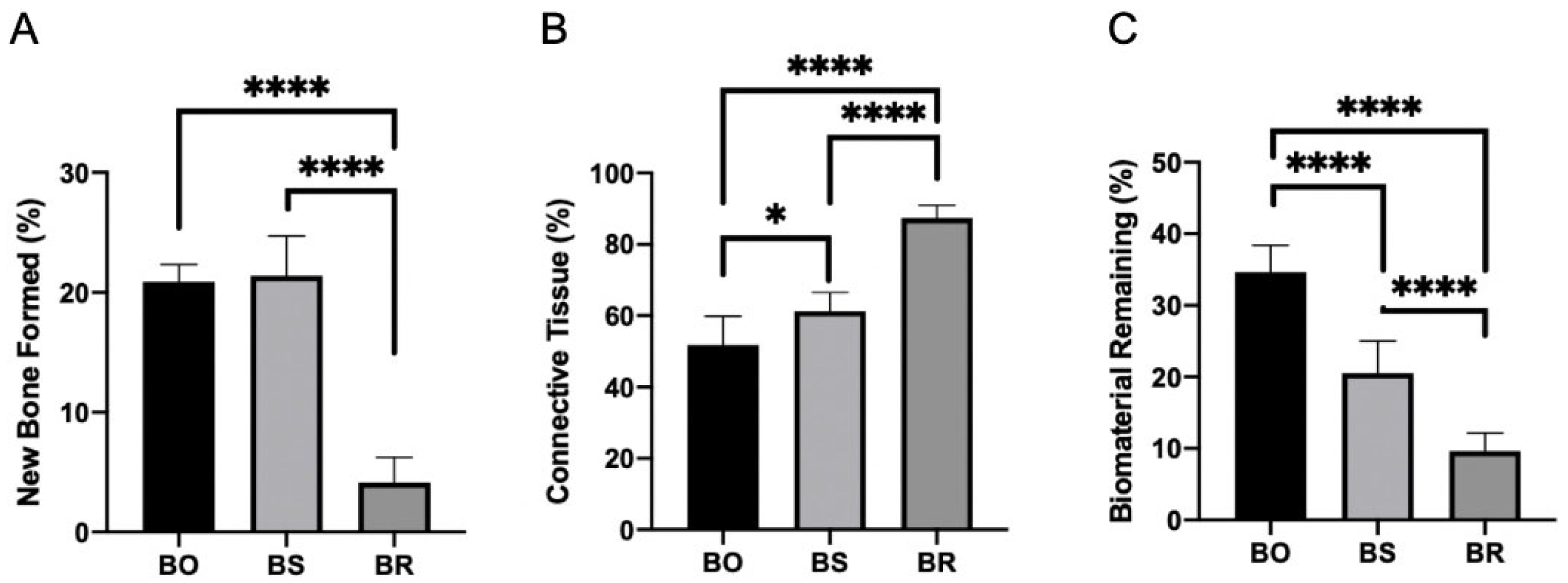
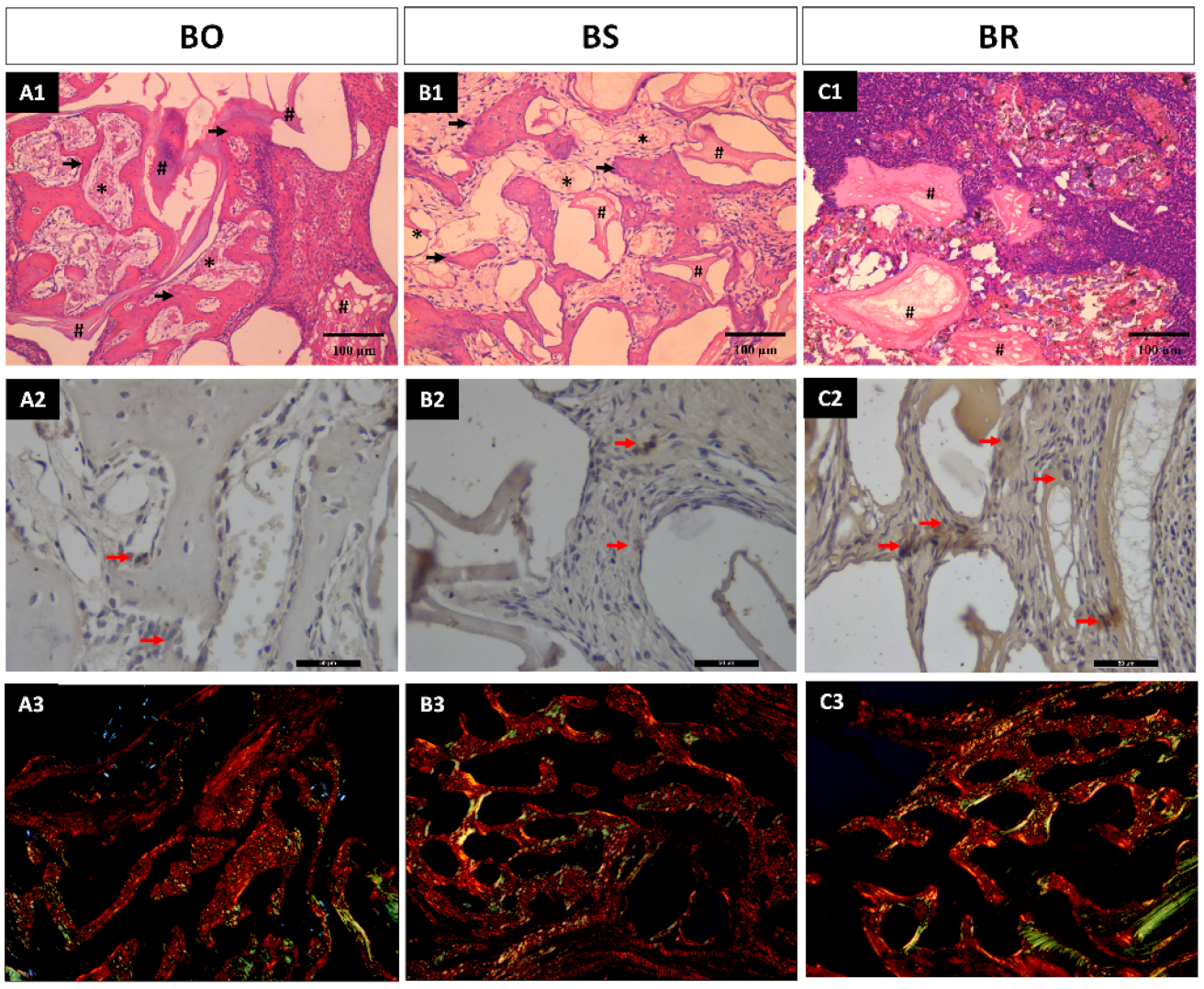
3.1. Histomorphometric Analysis Results
3.2. Immunohistochemistry Analysis
3.3. Collagen Fibers Maturation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar] [PubMed]
- Tatum, H., Jr. Maxillary and sinus implant reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarim, H.H.; Miley, D.D.; McLeod, D.E.; Garcia, M.N. Short-term evaluation of bioactive glass using the modified osteotome sinus elevation technique. Implant. Dent. 2013, 22, 491–498. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Dietsh, F. Endosteal implants and iliac crest grafts to restore severely resorbed totally edentulous maxillae—A retrospective study. J. Oral Implant. 1994, 20, 100–110. [Google Scholar]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft in animals: A systematic review. Int. J. Oral Maxillofac. Surg. 2012, 41, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Manfro, R.; Fonseca, F.S.; Bortoluzzi, M.C.; Sendyk, W.R. Comparative, Histological and Histomorphometric Analysis of Three Anorganic Bovine Xenogenous Bone Substitutes: Bio-Oss, Bone-Fill and Gen-Ox Anorganic. J. Maxillofac. Oral Surg. 2014, 13, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Lutz, R.; Doering, H.; Lell, M.; Ratky, J.; Schlegel, K.A. Bio-Oss® blocks combined with BMP-2 and VEGF for the regeneration of bony defects and vertical augmentation. Clin. Oral Implant. Res. 2013, 24, 450–460. [Google Scholar] [CrossRef]
- Kao, D.W.; Kubota, A.; Nevins, M.; Fiorellini, J.P. The negative effect of combining rhBMP-2 and Bio-Oss on bone formation for maxillary sinus augmentation. Int. J. Periodontics Restor. Dent. 2012, 32, 61–67. [Google Scholar]
- Lisboa-Filho, P.N.; Gomes-Ferreira, P.H.S.; Batista, F.R.S.; Momesso, G.A.C.; Faverani, L.P.; Okamoto, R. Bone repair with raloxifene and bioglass nanoceramic composite in animal experiment. Connect. Tissue Res. 2018, 59, 97–101. [Google Scholar] [CrossRef]
- Kavas, A.; Keskin, D.; Altunbas, K.; Tezcaner, A. Raloxifene-/raloxifene-poly(ethylene glycol) conjugate-loaded microspheres: A novel strategy for drug delivery to bone forming cells. Int. J. Pharm. 2016, 510, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Maximov, P.Y.; Lee, T.M.; Jordan, V.C. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr. Clin. Pharmacol. 2013, 8, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Yogui, F.C.; Momesso, G.A.C.; Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Okamoto, R. A SERM increasing the expression of the osteoblastogenesis and mineralization-related proteins and improving quality of bone tissue in an experimental model of osteoporosis. J. Appl. Oral Sci. 2018, 26, e20170329. [Google Scholar] [CrossRef]
- Hermansen, L.L.; Sørensen, M.; Barckman, J.; Bechtold, J.E.; Søballe, K.; Baas, J. Incorporation of raloxifene-impregnated allograft around orthopedic titanium implants impairs early fixation but improves new bone formation. Acta Orthop. 2015, 86, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Momesso, G.A.C.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Luvizuto, E.R.; Gruber, R.; et al. Raloxifene but not alendronate can compensate the impaired osseointegration in osteoporotic rats. Clin. Oral Investig. 2018, 22, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Heo, H.A.; Min, J.S.; Pyo, S.W. Effect of Raloxifene on Bone Formation Around Implants in the Osteoporotic Rat Maxilla: Histomorphometric and Microcomputed Tomographic Analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Szurkowska, K.; Kazimierczak, P.; Kolmas, J. Mg, Si-Co-Substituted Hydroxyapatite/Alginate Composite Beads Loaded with Raloxifene for Potential Use in Bone Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 2933. [Google Scholar] [CrossRef] [PubMed]
- Adomaityte, J.; Farooq, M.; Qayyum, R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb. Haemost. 2008, 99, 338–342. [Google Scholar] [CrossRef]
- Lagari, V.; Gavcovich, T.; Levis, S. The Good and the Bad About the 2017 American College of Physicians Osteoporosis Guidelines. Clin. Ther. 2018, 40, 168–176. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Lisboa-Filho, P.N.; da Silva, A.C.; Bim-Junior, O.; de Souza Batista, F.R.; Ervolino-Silva, A.C.; Garcia-Junior, I.R.; Okamoto, R. Sonochemical time standardization for bioactive materials used in periimplantar defects filling. Ultrason. Sonochem. 2019, 56, 437–446. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Lang, N.P.; Garcia Rangel, I.J.; Ferreira, S.; Caneva, M.; De Santis, E.; Botticelli, D. Sinus mucosa elevation using Bio-Oss® or Gingistat® collagen sponge: An experimental study in rabbits. Clin. Oral Implant. Res. 2017, 28, e21–e30. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Shimizu, Y.; Ooya, K. Maxillary sinus augmentation model in rabbits: Effect of occluded nasal ostium on new bone formation. Clin. Oral Implant. Res. 2002, 13, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Merz, W.A. Die Streckenmessung an gerichteten Strukturen im Mikroskop und ihre Anwendung zur Bestimmung von Oberflachen-Volumen-Relationen im Knochengewebe. Mikroskopie 1967, 22, 132–142. [Google Scholar]
- Franceschi, R.T.; Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Yang, S.; Reith, E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect. Tissue Res. 2003, 44 (Suppl. 1), 109–116. [Google Scholar] [CrossRef]
- Dos Santos Pereira, R.; Boos, F.B.; Gorla, L.F.; Garcia, I.R., Jr.; Okamoto, R.; Hochuli-Vieira, E. Maxillary Sinus Elevation Surgery with ChronOS and Autogenous Bone Graft: Immunohistochemical Assessment of RUNX2, VEGF, TRAP, and Osteocalcin. Int. J. Periodontics Restor. Dent. 2017, 37, e321–e327. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; Bueno, C.R.E.; Reis-Prado, A.H.D.; Souza, M.T.; Goto, J.; Camargo, J.M.P.; Duarte, M.A.H.; Dezan-Júnior, E.; Zanotto, E.D.; Cintra, L.T.A. Biocompatibility, Biomineralization, and Maturation of Collagen by RTR®, Bioglass and DM Bone® Materials. Braz. Dent. J. 2020, 31, 477–484. [Google Scholar] [CrossRef]
- Kangwannarongkul, T.; Subbalekha, K.; Vivatbutsiri, P.; Suwanwela, J. Gene Expression and Microcomputed Tomography Analysis of Grafted Bone Using Deproteinized Bovine Bone and Freeze-Dried Human Bone. Int. J. Oral Maxillofac. Implant. 2018, 33, 541–548. [Google Scholar] [CrossRef]
- Suwanwela, J.; Puangchaipruk, D.; Wattanasirmkit, K.; Kamolratanakul, P.; Jansisyanont, P. Maxillary Sinus Floor Augmentation Using Xenograft: Gene Expression and Histologic Analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, 611–616. [Google Scholar] [CrossRef]
- de Molon, R.S.; Magalhaes-Tunes, F.S.; Semedo, C.V.; Furlan, R.G.; de Souza, L.G.L.; de Souza Faloni, A.P.; Marcantonio, E., Jr.; Faeda, R.S. A randomized clinical trial evaluating maxillary sinus augmentation with different particle sizes of demineralized bovine bone mineral: Histological and immunohistochemical analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 810–823. [Google Scholar] [CrossRef]
- Matheus, H.R.; Ervolino, E.; Gusman, D.J.R.; Alves, B.E.S.; Fiorin, L.G.; Pereira, P.A.; de Almeida, J.M. Association of hyaluronic acid with a deproteinized bovine graft improves bone repair and increases bone formation in critical-size bone defects. J. Periodontol. 2021, 92, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Arıcı, G.; Şençimen, M.; Coşkun, A.T.; Altuğ, H.A.; Güreşci, S.; Çelik, H.H.; Uzuner, M.B.; Ocak, M. Evaluation of the Efficiency of the Graft Material Combined with Ozonized Blood in Maxillary Sinus Lifting Applications in Rabbits. J. Maxillofac. Oral Surg. 2022, 21, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; He, Q.; Xin, L.; Li, Y.; Yuan, S.; Zou, H.; Shu, L.; Song, J.; Huang, Y.; Chen, T. Effects of injectable platelet rich fibrin on bone remodeling in combination with DBBM in maxillary sinus elevation: A randomized preclinical study. Am. J. Transl. Res. 2020, 12, 7312–7325. [Google Scholar] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, N.B.; da Silva, A.C.E.; dos Santos Pereira, R.; Hochuli-Vieira, E.; Lisboa-Filho, P.N.; de Deus, C.B.D.; Okamoto, R. Different responses of heterogeneous graft presentations in bone reconstructions during sinus lift elevation surgery: An immunolabeling and histomorphometric study performed in rabbits. Front. Oral Maxillofac. Med. 2022, 4, 1. [Google Scholar] [CrossRef]
| BO | BS | BR | |
|---|---|---|---|
| Bone Formed (%) | 20.87 ± 1.34 A | 21.37 ± 3.33 AB | 4.12 ± 2.10 C |
| Connective tissue (%) | 51.75 ± 8.69 A | 61.25 ± 5.23 B | 87.37 ± 3.54 C |
| Biomaterial Remining (%) | 34.62 ± 3.54 A | 20.50 ± 4.50 B | 9.62 ± 2.55 C |
| Mature Collagen Fibers (pixels) | 14.83 ± 8.61 A | 19.95 ± 8.06 A | 26.85 ± 13.59 A |
| Immature Collagen Fibers (pixels) | 0.84 A | 2.29 A | 4.21 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Santos, A.M.; dos Santos Pereira, R.; Montemezzi, P.; Mello-Machado, R.C.; Okamoto, R.; Sacco, R.; Noronha Lisboa-Filho, P.; Messora, M.R.; Mourão, C.F.; Hochuli-Vieira, E. The Interplay of Raloxifene and Sonochemical Bio-Oss in Early Maxillary Sinus Bone Regeneration: A Histological and Immunohistochemical Analysis in Rabbits. Medicina 2023, 59, 1521. https://doi.org/10.3390/medicina59091521
de Souza Santos AM, dos Santos Pereira R, Montemezzi P, Mello-Machado RC, Okamoto R, Sacco R, Noronha Lisboa-Filho P, Messora MR, Mourão CF, Hochuli-Vieira E. The Interplay of Raloxifene and Sonochemical Bio-Oss in Early Maxillary Sinus Bone Regeneration: A Histological and Immunohistochemical Analysis in Rabbits. Medicina. 2023; 59(9):1521. https://doi.org/10.3390/medicina59091521
Chicago/Turabian Stylede Souza Santos, Anderson Maikon, Rodrigo dos Santos Pereira, Pietro Montemezzi, Rafael Coutinho Mello-Machado, Roberta Okamoto, Roberto Sacco, Paulo Noronha Lisboa-Filho, Michel Reis Messora, Carlos Fernando Mourão, and Eduardo Hochuli-Vieira. 2023. "The Interplay of Raloxifene and Sonochemical Bio-Oss in Early Maxillary Sinus Bone Regeneration: A Histological and Immunohistochemical Analysis in Rabbits" Medicina 59, no. 9: 1521. https://doi.org/10.3390/medicina59091521
APA Stylede Souza Santos, A. M., dos Santos Pereira, R., Montemezzi, P., Mello-Machado, R. C., Okamoto, R., Sacco, R., Noronha Lisboa-Filho, P., Messora, M. R., Mourão, C. F., & Hochuli-Vieira, E. (2023). The Interplay of Raloxifene and Sonochemical Bio-Oss in Early Maxillary Sinus Bone Regeneration: A Histological and Immunohistochemical Analysis in Rabbits. Medicina, 59(9), 1521. https://doi.org/10.3390/medicina59091521









