Assessing Continuous Epidural Infusion and Programmed Intermittent Epidural Bolus for Their Effectiveness in Providing Labor Analgesia: A Mono-Centric Retrospective Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
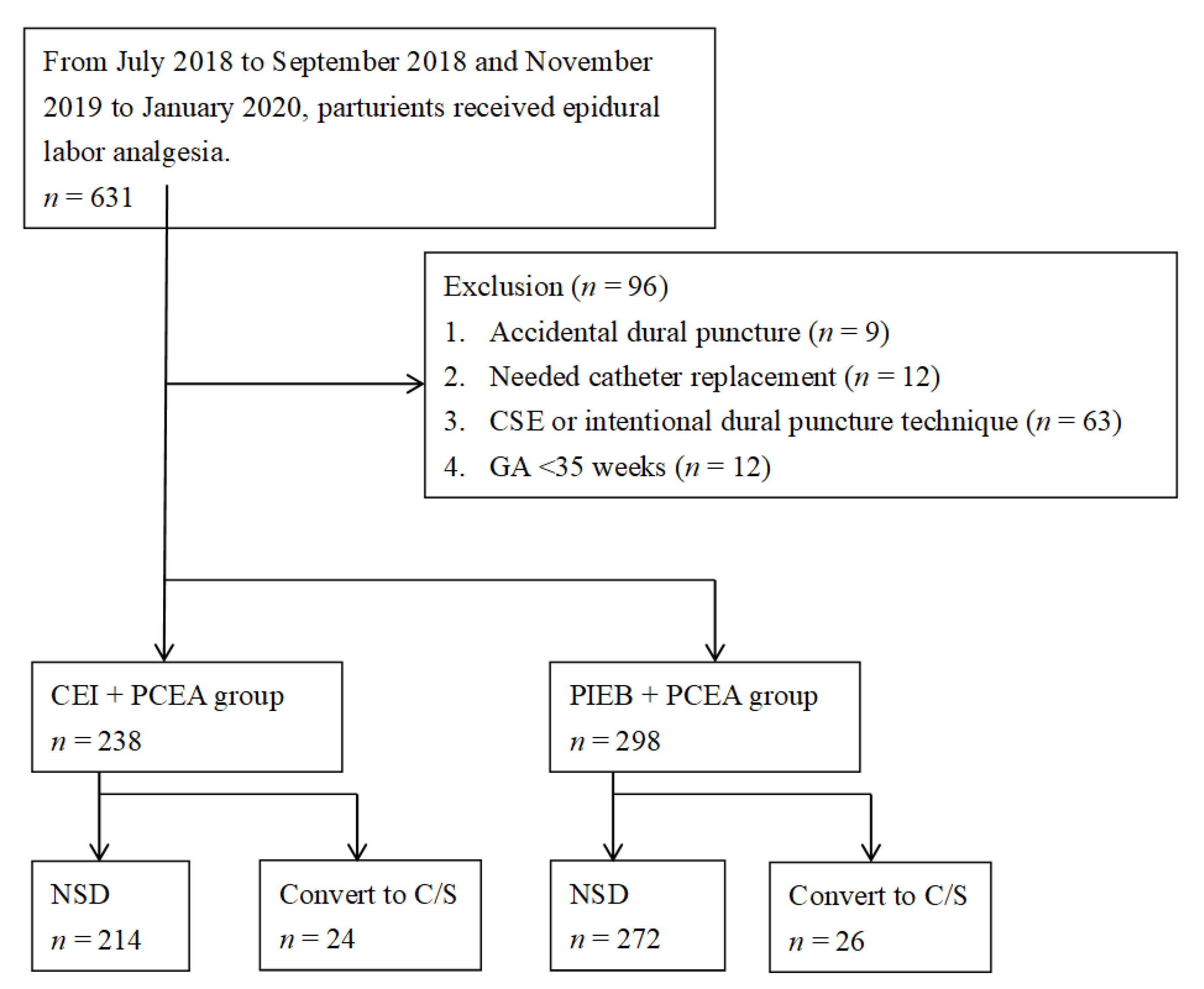
2.2. Study Group and Outcome Measurement
2.3. Plans of Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Comparative Analysis of Epidural Analgesia Techniques
3.3. Analysis for Perinatal Pain Management between PIEB and CEI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sng, B.L.; Kwok, S.C.; Sia, A.T. Modern neuraxial labour analgesia. Curr. Opin. Anaesthesiol. 2015, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.L.; Turnbull, D.; Cyna, A.M.; Adelson, P.; Wilkinson, C. Pain relief for childbirth: The preferences of pregnant women, midwives and obstetricians. Women Birth 2013, 26, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.; Hauck, Y.L.; Bayes, S.; Butt, J. Exploring midwives’ perception of confidence around facilitating water birth in Western Australia: A qualitative descriptive study. Midwifery 2016, 33, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tanvisut, R.; Traisrisilp, K.; Tongsong, T. Efficacy of aromatherapy for reducing pain during labor: A randomized controlled trial. Arch. Gynecol. Obstet. 2018, 297, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Laflamme, E.; Komanecky, C. Pain management in labor. Am. Fam. Physician 2021, 103, 355–364. [Google Scholar]
- Schrock, S.D.; Harraway-Smith, C. Labor analgesia. Am. Fam. Physician 2012, 85, 447–454. [Google Scholar]
- Chau, A.; Bibbo, C.; Huang, C.C.; Elterman, K.G.; Cappiello, E.C.; Robinson, J.N.; Tsen, L.C. Dural puncture epidural technique improves labor analgesia quality with fewer side effects compared with epidural and combined spinal epidural techniques: A randomized clinical trial. Anesth. Analg. 2017, 124, 560–569. [Google Scholar] [CrossRef]
- Sng, B.L.; Sia, A.T.H. Maintenance of epidural labour analgesia: The old, the new and the future. Best Pr. Res. Clin. Anaesthesiol. 2017, 31, 15–22. [Google Scholar] [CrossRef]
- Ojo, O.A.; Mehdiratta, J.E.; Gamez, B.H.; Hunting, J.; Habib, A.S. Comparison of programmed intermittent epidural boluses with continuous epidural infusion for the maintenance of labor analgesia: A randomized, controlled, double-blind study. Anesth. Analg. 2020, 130, 426–435. [Google Scholar] [CrossRef]
- Gambling, D.R.; Cole, P.Y.C.; McMorland, G.H.; Palmer, L. A comparative study of patient controlled epidural analgesia (PCEA) and continuous infusion epidural analgesia (CIEA) during labour. Can. J. Anaesth. 1988, 35, 249–254. [Google Scholar] [CrossRef]
- Wong, C.A.; Ratliff, J.T.; Sullivan, J.T.; Scavone, B.M.; Toledo, P.; McCarthy, R.J. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth. Analg. 2006, 102, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.A.; McCarthy, R.J.; Hewlett, B. The effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: A randomized controlled trial. Anesth. Analg. 2011, 112, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, B.; Skogvoll, E.; Jónsdóttir, I.H.; Røislien, J.; Smárason, A.K. On predicting time to completion for the first stage of spontaneous labor at term in multiparous women. BMC Pregnancy Childbirth 2017, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, L.; Cao, W.; Wang, H.; Zhu, C.; Zhou, R. Factors affecting labor duration in Chinese pregnant women. Medicine 2018, 97, e13901. [Google Scholar] [CrossRef]
- Fettes, P.D.W.; Moore, C.S.; Whiteside, J.B.; Mcleod, G.A.; Wildsmith, J.A. Intermittent vs. continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. Br. J. Anaesth. 2006, 97, 359–364. [Google Scholar] [CrossRef]
- Capogna, G.; Camorcia, M.; Stirparo, S.; Farcomeni, A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: The effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth. Analg. 2011, 113, 826–831. [Google Scholar] [CrossRef]
- Kanczuk, M.E.; Barrett, N.M.; Arzola, C.; Downey, K.; Ye, X.Y.; Carvalho, J.C. Programmed intermittent epidural bolus for labor analgesia during first stage of labor: A biased-coin up-and-down sequential allocation trial to determine the optimum interval time between boluses of a fixed volume of 10 mL of bupivacaine 0.0625% with fentanyl 2 μg/mL. Anesth. Analg. 2017, 124, 537–541. [Google Scholar] [CrossRef]
- Tien, M.; Allen, T.K.; Mauritz, A.; Habib, A.S. A retrospective comparison of programmed intermittent epidural bolus with continuous epidural infusion for maintenance of labor analgesia. Curr. Med. Res. Opin. 2016, 32, 1435–1440. [Google Scholar] [CrossRef]
- Cox, C.R.; Faccenda, K.A.; Gilhooly, C.; Bannister, J.; Scott, N.B.; Morrison, L.M. Extradural S(-)-bupivacaine: Comparison with racemic RS-bupivacaine. Br. J. Anaesth. 1998, 80, 289–293. [Google Scholar] [CrossRef]
- McKenzie, C.; Cobb, B.; Riley, E.T.; Carvalho, B. Programmed intermittent epidural boluses for maintenance of labor analgesia: An impact study. Int. J. Obstet. Anesth. 2016, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Nageotte, M.P.; Larson, D.; Rumney, P.J.; Sidhu, M.; Hollenbach, K. Epidural analgesia compared with combined spinal-epidural analgesia during labor in nulliparous women. N. Engl. J. Med. 1997, 337, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Afshan, G.; Chohan, U.; Khan, F.A.; Chaudhry, N.; Khan, Z.E.; Khan, A.A. Appropriate length of epidural catheter in the epidural space for postoperative analgesia: Evaluation by epidurography. Anaesthesia 2011, 66, 913–918. [Google Scholar] [CrossRef]
- Bullingham, A.; Liang, S.; Edmonds, E.; Mathur, S.; Sharma, S. Continuous epidural infusion vs. programmed intermittent epidural bolus for labour analgesia: A prospective, controlled, before-and-after cohort study of labour outcomes. Br. J. Anaesth. 2018, 121, 432–437. [Google Scholar] [CrossRef] [PubMed]




| CEI | PIEB | t | pa,b | |
|---|---|---|---|---|
| Sample size, n | 214 | 272 | ||
| Age (yrs) | 33.3 ± 4.6 | 33.0 ± 4.6 | 0.55 | 0.579 |
| Weight (kg) | 68.3 ± 9.5 | 68.6 ± 9.2 | −0.48 | 0.630 |
| BMI c (kg/m2) | 26.4 ± 3.2 | 26.6 ± 3.2 | −0.55 | 0.582 |
| Gestational age (wks) | 38.5 ± 1.2 | 38.5 ± 1.2 | −0.32 | 0.752 |
| Parity, n (%) | 0.054 | |||
| Primiparity | 146 (68) | 178 (65) | ||
| Muliparity | 68 (32) | 94 (35) | ||
| Cervical os (cm) | 2.4 ± 1.5 | 2.5 ± 1.6 | −0.19 | 0.847 |
| Puncture level, n (%) | 0.052 | |||
| Lumbar 2–3 | 9 (4) | 27 (10) | ||
| Lumbar 3–4 | 198 (93) | 234 (86) | ||
| Lumbar 4–5 | 7 (3) | 11 (4) | ||
| Depth from the skin to epidural (cm) | 4.8 ± 0.9 | 5.0 ± 1.1 | −1.90 | 0.06 |
| Depth of catheter insertion (cm) | 6.2 ± 0.5 | 6.0 ± 0.7 | 2.84 | 0.01 |
| Catheter fixation mark (cm) | 11.0 ± 0.9 | 11.0 ± 1.2 | 0.11 | 0.92 |
| CEI | PIEB | t | p | |
|---|---|---|---|---|
| Total time to delivery (mins) | 820 ± 531 | 814 ± 559 | 0.13 | 0.894 |
| Total amount of medication (mL) | 142 ± 92 | 122 ± 81 | 2.42 | 0.014 |
| Average hourly dosage (mL/h) | 11.0 ± 3.2 | 9.6 ± 2.2 | 5.71 | <0.001 |
| Shift from NSD to CS, n (%) | 24 (10.1) | 26 (8.7) | 0.541 | |
| Satisfaction score (1–5) | 3.9 ± 0.4 | 3.9 ± 0.4 | 0.327 |
| Time | Groups | SS | df | MS | F | p | |||
|---|---|---|---|---|---|---|---|---|---|
| CEI | PIEB | ||||||||
| n | Mean (sd) | n | Mean (sd) | ||||||
| <4 | 14 | 14.31 (3.57) | 25 | 12.00 (1.98) | 33.14 | 1 | 33.14 | 5.36 | 0.02 |
| 4–8 | 60 | 11.78 (2.76) | 55 | 10.66 (2.26) | 35.52 | 1 | 35.52 | 5.74 | 0.02 |
| 8–16 | 68 | 10.98 (3.71) | 108 | 9.14 (2.04) | 141.08 | 1 | 141.08 | 22.81 | <0.001 |
| 16–24 | 40 | 10.10 (2.10) | 49 | 8.87 (1.37) | 47.83 | 1 | 47.83 | 7.73 | <0.01 |
| >24 | 32 | 9.60 (2.45) | 35 | 8.53 (2.03) | 19.01 | 1 | 19.01 | 3.07 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, S.-L.; Li, W.-T.; Chang, L.-Y.; Yeh, P.-H.; Yeh, L.-T.; Liu, L.-J.; Yeh, C.-B. Assessing Continuous Epidural Infusion and Programmed Intermittent Epidural Bolus for Their Effectiveness in Providing Labor Analgesia: A Mono-Centric Retrospective Comparative Study. Medicina 2023, 59, 1579. https://doi.org/10.3390/medicina59091579
Tsao S-L, Li W-T, Chang L-Y, Yeh P-H, Yeh L-T, Liu L-J, Yeh C-B. Assessing Continuous Epidural Infusion and Programmed Intermittent Epidural Bolus for Their Effectiveness in Providing Labor Analgesia: A Mono-Centric Retrospective Comparative Study. Medicina. 2023; 59(9):1579. https://doi.org/10.3390/medicina59091579
Chicago/Turabian StyleTsao, Shao-Lun, Wen-Tyng Li, Li-Yun Chang, Pin-Hung Yeh, Liang-Tsai Yeh, Ling-Jun Liu, and Chao-Bin Yeh. 2023. "Assessing Continuous Epidural Infusion and Programmed Intermittent Epidural Bolus for Their Effectiveness in Providing Labor Analgesia: A Mono-Centric Retrospective Comparative Study" Medicina 59, no. 9: 1579. https://doi.org/10.3390/medicina59091579





