Assessment of Microvascular Disease in Heart and Brain by MRI: Application in Heart Failure with Preserved Ejection Fraction and Cerebral Small Vessel Disease
Abstract
:1. Introduction
2. Background
2.1. HFpEF: Epidemiology and Clinical Presentation
2.2. HFpEF: Pathophysiology
2.3. HFpEF: Non-MRI Assessment of Microvascular Disease
2.4. CSVD: Epidemiology and Clinical Presentation
2.5. CSVD: Pathophysiology
2.6. CSVD: Non-MRI Assessment of Microvascular Disease
2.7. Similarities and Differences of HFpEF and CSVD
2.8. MRI Techniques for Assessing Microvascular Disease
2.9. CMR Assessment of HFpEF
2.9.1. Cardiac Structure
2.9.2. Cardiac Function
2.10. Myocardial Perfusion CMR
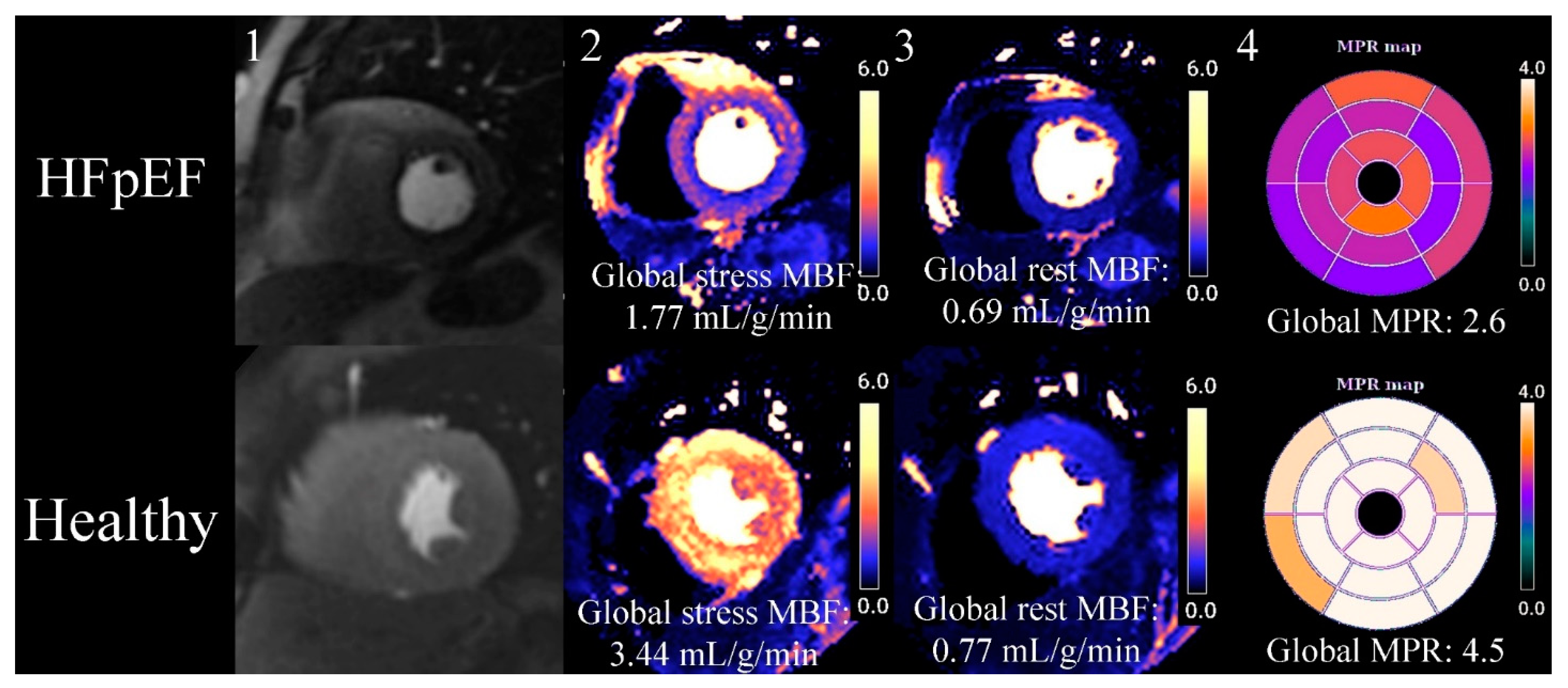
2.11. Tissue Characterisation
2.12. MRI Assessment of CSVD
2.13. Cerebral Perfusion
2.14. Vessel Size Imaging
2.15. Blood–Brain Barrier Integrity
2.16. Diffusion Tensor Imaging
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| BBB | Blood–brain barrier |
| CBF | Cerebral blood flow |
| CMD | Coronary microvascular dysfunction |
| CMR | Cardiovascular magnetic resonance |
| CSVD | Cerebral small vessel disease |
| CT | Computed tomography |
| CVR | Cerebral vascular reserve |
| DCE | Dynamic contrast enhanced MRI |
| DTI | Diffusion tensor imaging |
| ECV | Extracellular volume fraction |
| GBCA | Gadolinium-based contrast agents |
| HFpEF | Heart failure with preserved ejection fraction |
| LGE | Late gadolinium enhancement |
| LVEF | Left ventricular ejection fraction |
| LVH | Left ventricular hypertrophy |
| LVH | Left ventricular |
| MACE | Major adverse cardiovascular events |
| MBF | Myocardial blood flow |
| MPR | Myocardial perfusion reserve |
| MRI | Magnetic resonance imaging |
| PET | Positron emission tomography |
| STRIVE | Standards for reporting vascular changes on neuroimaging |
| WMH | White matter hyperintensity |
References
- Rothwell, P.; Coull, A.; Giles, M.; Howard, S.; Silver, L.; Bull, L.; Gutnikov, S.; Edwards, P.; Mant, D.; Sackley, C.; et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004, 363, 1925–1933. [Google Scholar] [CrossRef]
- Yeh, R.W.; Sidney, S.; Chandra, M.; Sorel, M.; Selby, J.V.; Go, A.S. Population Trends in the Incidence and Outcomes of Acute Myocardial Infarction. N. Engl. J. Med. 2010, 362, 2155–2165. [Google Scholar] [CrossRef]
- Cook, C.; Cole, G.; Asaria, P.; Jabbour, R.; Francis, D.P. The annual global economic burden of heart failure. Int. J. Cardiol. 2014, 171, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Lewis, G.A.; Schelbert, E.B.; Williams, S.G.; Cunnington, C.; Ahmed, F.; McDonagh, T.A.; Miller, C.A. Biological Phenotypes of Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 2186–2200. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.W.; Terrera, G.M.; Quinn, T.J. Dementia trials and dementia tribulations: Methodological and analytical challenges in dementia research. Alzheimer’s Res. Ther. 2015, 7, 31. [Google Scholar] [CrossRef]
- Dhingra, A.; Garg, A.; Kaur, S.; Chopra, S.; Batra, J.S.; Pandey, A.; Chaanine, A.H.; Agarwal, S.K. Epidemiology of heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2014, 11, 354–365. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Rosch, S.; Kresoja, K.-P.; Besler, C.; Fengler, K.; Schöber, A.R.; von Roeder, M.; Lücke, C.; Gutberlet, M.; Klingel, K.; Thiele, H.; et al. Characteristics of Heart Failure with Preserved Ejection Fraction Across the Range of Left Ventricular Ejection Fraction. Circulation 2022, 146, 506–518. [Google Scholar] [CrossRef]
- Kanagala, P.; Cheng, A.S.H.; Singh, A.; McAdam, J.; Marsh, A.-M.; Arnold, J.R.; Squire, I.B.; Ng, L.L.; McCann, G.P. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in heart failure with preserved ejection fraction—Implications for clinical trials. J. Cardiovasc. Magn. Reson. 2018, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.S.; Yanek, L.R.; Vaishnav, J.; Ying, W.; Vaidya, D.; Lee, Y.Z.J.; Riley, S.J.; Subramanya, V.; Brown, E.E.; Hopkins, C.D.; et al. Endomyocardial Biopsy Characterization of Heart Failure with Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail. 2020, 8, 712–724. [Google Scholar] [CrossRef]
- Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. Eur. Heart J. 2012, 33, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients with Heart Failure and Normal Ejection Fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Inflammatory Markers and Incident Heart Failure Risk in Older Adults. J. Am. Coll. Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative Stress Drives Heart Failure with Preserved Ejection Fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Okruhlicova, L.; Tribulova, N.; Weismann, P.; Sotnikova, R. Ultrastructure and histochemistry of rat myocardial capillary endothelial cells in response to diabetes and hypertension. Cell Res. 2005, 15, 532–538. [Google Scholar] [CrossRef]
- Van de Wouw, J.; Sorop, O.; van Drie, R.W.A.; van Duin, R.W.B.; Nguyen, I.T.N.; Joles, J.A.; Verhaar, M.C.; Merkus, D.; Duncker, D.J. Perturbations in myocardial perfusion and oxygen balance in swine with multiple risk factors: A novel model of ischemia and no obstructive coronary artery disease. Basic Res. Cardiol. 2020, 115, 21. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef]
- Tartière-Kesri, L.; Tartière, J.-M.; Logeart, D.; Beauvais, F.; Cohen Solal, A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2012, 59, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J. Appl. Physiol. 2008, 105, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Andersen, M.J.; Obokata, M.; Koepp, K.E.; Kane, G.C.; Melenovsky, V.; Olson, T.P.; Borlaug, B.A. Arterial Stiffening with Exercise in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Mottram, P.M.; Haluska, B.A.; Leano, R.; Carlier, S.; Case, C.; Marwick, T.H. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart 2005, 91, 1551–1556. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Faxén, U.L.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Srivaratharajah, K.; Coutinho, T.; deKemp, R.; Liu, P.; Haddad, H.; Stadnick, E.; Davies, R.A.; Chih, S.; Dwivedi, G.; Guo, A.; et al. Reduced Myocardial Flow in Heart Failure Patients with Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002562. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.R.; Kanagala, P.; Budgeon, C.A.; Jerosch-Herold, M.; Gulsin, G.S.; Singh, A.; Khan, J.N.; Chan, D.C.S.; Squire, I.B.; Ng, L.L.; et al. Prevalence and Prognostic Significance of Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2022, 15, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Shan, Y.; Cai, W.; Liu, S.; Hu, M.; Liao, S.; Huang, X.; Zhang, B.; Wang, Y.; et al. Cerebral small vessel disease: Neuroimaging markers and clinical implication. J. Neurol. 2019, 266, 2347–2362. [Google Scholar] [CrossRef]
- Nam, K.-W.; Kwon, H.-M.; Lim, J.-S.; Han, M.-K.; Nam, H.; Lee, Y.-S. The presence and severity of cerebral small vessel disease increases the frequency of stroke in a cohort of patients with large artery occlusive disease. PLoS ONE 2017, 12, e0184944. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.-E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging standards for research into small vessel disease—Advances since 2013. Lancet Neurol. 2023, 22, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Chabriat, H. Incident cerebral lacunes: A review. J. Cereb. Blood Flow Metab. 2020, 40, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Benveniste, H.; Black, S.E.; Charpak, S.; Dichgans, M.; Joutel, A.; Nedergaard, M.; Smith, K.J.; Zlokovic, B.V.; Wardlaw, J.M. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018, 114, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Weaver, N.A.; Kuijf, H.J.; Aben, H.P.; Abrigo, J.; Bae, H.-J.; Barbay, M.; Best, J.G.; Bordet, R.; Chappell, F.M.; Chen, C.P.L.H.; et al. Strategic infarct locations for post-stroke cognitive impairment: A pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol. 2021, 20, 448–459. [Google Scholar] [CrossRef]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS small vessel disease. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, H.J.; Jang, H.; Weiner, M.W.; DeCarli, C.; Na, D.L.; Seo, S.W. Interaction between Alzheimer’s Disease and Cerebral Small Vessel Disease: A Review Focused on Neuroimaging Markers. Int. J. Mol. Sci. 2022, 23, 10490. [Google Scholar] [CrossRef]
- Esiri, M.M.; Matthews, F.; Brayne, C.; Ince, P.G.; Matthews, F.E.; Xuereb, J.H.; Broome, J.C.; McKenzie, J.; Rossi, M.; McKeith, I.G.; et al. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001, 357, 169–175. [Google Scholar] [CrossRef]
- Van Dinther, M.; Voorter, P.H.; Jansen, J.F.; Jones, E.A.; van Oostenbrugge, R.J.; Staals, J.; Backes, W.H. Assessment of microvascular rarefaction in human brain disorders using physiological magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2022, 42, 718–737. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Engedal, T.S.; Moreton, F.; Hansen, M.B.; Wardlaw, J.M.; Dalkara, T.; Markus, H.S.; Muir, K.W. Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J. Cereb. Blood Flow Metab. 2016, 36, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R.; Moody, D.M.; Thore, C.R.; Anstrom, J.A.; Challa, V.R. Microvascular changes in the white mater in dementia. J. Neurol. Sci. 2009, 283, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tucsek, Z.; Sosnowska, D.; Toth, P.; Gautam, T.; Podlutsky, A.; Csiszar, A.; Losonczy, G.; Valcarcel-Ares, M.N.; Sonntag, W.E.; et al. Aging-Induced Dysregulation of Dicer1-Dependent MicroRNA Expression Impairs Angiogenic Capacity of Rat Cerebromicrovascular Endothelial Cells. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.E.; Taylor, J.L.; Smith, C.J.; Pritchard, H.A.T.; Greenstein, A.S.; Allan, S.M. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc. Res. 2021, 117, 2575–2588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Y.; Hong, F.-F.; Yang, S.-L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4540. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.-F.; Lin, G.; Chen, X.; Chen, L.; Zheng, W.; Raghow, R.; Zhou, F.-M.; Shih, A.Y.; Tan, X.-L. Endothelial Nitric Oxide Synthase–Deficient Mice: A Model of Spontaneous Cerebral Small-Vessel Disease. Am. J. Pathol. 2021, 191, 1932–1945. [Google Scholar] [CrossRef]
- Zang, J.; Shi, J.; Liang, J.; Zhang, X.; Wei, W.; Yao, C.; Zhuang, X.; Wu, G. Pulse Pressure, Cognition, and White Matter Lesions: A Mediation Analysis. Front. Cardiovasc. Med. 2021, 8, 654522. [Google Scholar] [CrossRef]
- Henskens, L.H.G.; Kroon, A.A.; van Oostenbrugge, R.J.; Gronenschild, E.H.B.M.; Fuss-Lejeune, M.M.J.J.; Hofman, P.A.M.; Lodder, J.; de Leeuw, P.W. Increased Aortic Pulse Wave Velocity Is Associated with Silent Cerebral Small-Vessel Disease in Hypertensive Patients. Hypertension 2008, 52, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Thrippleton, M.J.; Makin, S.D.; Marshall, I.; Geerlings, M.I.; de Craen, A.J.M.; van Buchem, M.A.; Wardlaw, J.M. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2016, 36, 1653–1667. [Google Scholar] [CrossRef]
- Thomas, M.A.; Hazany, S.; Ellingson, B.M.; Hu, P.; Nguyen, K.-L. Pathophysiology, classification, and MRI parallels in microvascular disease of the heart and brain. Microcirculation 2020, 27, e12648. [Google Scholar] [CrossRef]
- Heiss, W.-D. The Additional Value of PET in the Assessment of Cerebral Small Vessel Disease. J. Nucl. Med. 2018, 59, 1660–1664. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Berry, C.; Sidik, N.; Pereira, A.C.; Ford, T.J.; Touyz, R.M.; Kaski, J.-C.; Hainsworth, A.H. Small-Vessel Disease in the Heart and Brain: Current Knowledge, Unmet Therapeutic Need, and Future Directions. J. Am. Heart Assoc. 2019, 8, 11104. [Google Scholar] [CrossRef]
- Lohner, V.; Pehlivan, G.; Sanroma, G.; Miloschewski, A.; Schirmer, M.D.; Stöcker, T.; Reuter, M.; Breteler, M.M.B. Relation between Sex, Menopause, and White Matter Hyperintensities: The Rhineland Study. Neurology 2022, 99, e935–e943. [Google Scholar] [CrossRef]
- Thurston, R.C.; Aizenstein, H.J.; Derby, C.A.; Sejdić, E.; Maki, P.M. Menopausal hot flashes and white matter hyperintensities. Menopause 2016, 23, 27–32. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, L.; Hamilton, O.K.L.; Clancy, U.; Backhouse, E.V.; Stewart, C.R.; Stringer, M.S.; Doubal, F.N.; Wardlaw, J.M. Sex Differences in Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 756887. [Google Scholar] [CrossRef]
- Lau, C.; Elshibly, M.M.M.; Kanagala, P.; Khoo, J.P.; Arnold, J.R.; Hothi, S.S. The role of cardiac magnetic resonance imaging in the assessment of heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2022, 9, 922398. [Google Scholar] [CrossRef]
- Camici, P.G.; Olivotto, I.; Rimoldi, O.E. The coronary circulation and blood flow in left ventricular hypertrophy. J. Mol. Cell. Cardiol. 2012, 52, 857–864. [Google Scholar] [CrossRef]
- Heinzel, F.R.; Hohendanner, F.; Jin, G.; Sedej, S.; Edelmann, F. Myocardial Hypertrophy and Its Role in Heart Failure with Preserved Ejection Fraction. J. Appl. Physiol. 2015, 119, 1233–1242. [Google Scholar] [CrossRef]
- Davies, R.H.; Augusto, J.B.; Bhuva, A.; Xue, H.; Treibel, T.A.; Ye, Y.; Hughes, R.K.; Bai, W.; Lau, C.; Shiwani, H.; et al. Precision measurement of cardiac structure and function in cardiovascular magnetic resonance using machine learning. J. Cardiovasc. Magn. Reson. 2022, 24, 16. [Google Scholar] [CrossRef]
- Zile, M.R.; Gottdiener, J.S.; Hetzel, S.J.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Baicu, C.F.; Massie, B.M.; Carson, P.E. Prevalence and Significance of Alterations in Cardiac Structure and Function in Patients with Heart Failure and a Preserved Ejection Fraction. Circulation 2011, 124, 2491–2501. [Google Scholar] [CrossRef]
- Nerlerkar, N.; Moir, S. Would adding two left atrial piloted images to a cardiac magnetic resonance protocol enable rapid, accurate calculation of left atrial volume? Use of 320 slice cardiac CT as proof of concept. J. Cardiovasc. Magn. Reson. 2016, 18 (Suppl. 1), Q51. [Google Scholar] [CrossRef]
- Kammerlander Andreas, A.; Kraiger Jakob, A.; Nitsche, C.; Donà, C.; Duca, F.; Zotter-Tufaro, C.; Binder, C.; Aschauer, S.; Loewe, C.; Hengstenberg, C.; et al. Global Longitudinal Strain by CMR Feature Tracking Is Associated with Outcome in HFPEF. JACC Cardiovasc. Imaging 2019, 12, 1585–1587. [Google Scholar] [CrossRef]
- Tagliamonte, E.; Sperlongano, S.; Montuori, C.; Riegler, L.; Scarafile, R.; Carbone, A.; Forni, A.; Radmilovic, J.; Di Vilio, A.; Astarita, R.; et al. Coronary microvascular dysfunction affects left ventricular global longitudinal strain response to dipyridamole stress echocardiography: A pilot study. Heart Vessel. 2023, 38, 470–477. [Google Scholar] [CrossRef]
- Tamarappoo, B.; Samuel, T.J.; Elboudwarej, O.; Thomson, L.E.J.; Aldiwani, H.; Wei, J.; Mehta, P.; Cheng, S.; Sharif, B.; AlBadri, A.; et al. Left ventricular circumferential strain and coronary microvascular dysfunction: A report from the Women’s Ischemia Syndrome Evaluation Coronary Vascular Dysfunction (WISE-CVD) Project. Int. J. Cardiol. 2021, 327, 25–30. [Google Scholar] [CrossRef]
- Jin, C.; Weber, J.; Singh, H.; Gliganic, K.; Cao, J.J. The association of reduced left ventricular strains with increased extracellular volume and their collective impact on clinical outcomes. J. Cardiovasc. Magn. Reson. 2021, 23, 93. [Google Scholar] [CrossRef]
- Heinke, R.; Pathan, F.; Le, M.; D’Angelo, T.; Winau, L.; Arendt, C.; Vogl, T.J.; Zeiher, A.; Nagel, E.; Puntmann, V.O. Towards standardized postprocessing of global longitudinal strain by feature tracking—OptiStrain CMR-FT study. BMC Cardiovasc. Disord. 2019, 19, 267. [Google Scholar] [CrossRef]
- Chemla, D.; Nitenberg, A.; Teboul, J.-L.; Richard, C.; Monnet, X.; Le Clesiau, H.; Valensi, P.; Brahimi, M. Subendocardial Viability Index Is Related to the Diastolic/Systolic Time Ratio and Left Ventricular Filling Pressure, Not to Aortic Pressure: An Invasive Study in Resting Humans. Clin. Exp. Pharmacol. Physiol. 2009, 36, 413–418. [Google Scholar] [CrossRef]
- Manny, J.; Justice, R.; Hechtman, H.B. Left Ventricular Filling Pressure as a Determinant of Subendocardial Blood Flow. Ann. Thorac. Surg. 1979, 27, 451–459. [Google Scholar] [CrossRef]
- Markley, R.; Del Buono, M.G.; Mihalick, V.; Pandelidis, A.; Trankle, C.; Jordan, J.H.; Decamp, K.; Winston, C.; Carbone, S.; Billingsley, H.; et al. Abnormal left ventricular subendocardial perfusion and diastolic function in women with obesity and heart failure and preserved ejection fraction. Int. J. Cardiovasc. Imaging 2023, 39, 811–819. [Google Scholar] [CrossRef]
- Rathi, V.K.; Doyle, M.; Yamrozik, J.; Williams, R.B.; Caruppannan, K.; Truman, C.; Vido, D.; Biederman, R.W. Routine evaluation of left ventricular diastolic function by cardiovascular magnetic resonance: A practical approach. J. Cardiovasc. Magn. Reson. 2008, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Singh, A.; McCann, G.P. Cardiovascular magnetic resonance in the evaluation of heart valve disease. BMC Med. Imaging 2017, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Krautz, B.; Schnackenburg, B.; Abdel-Aty, H.; Santos, M.F.B.; Andre, F.; Maertens, M.J.; Mereles, D.; Korosoglou, G.; Giannitsis, E.; et al. Classification of diastolic function with phase-contrast cardiac magnetic resonance imaging: Validation with echocardiography and age-related reference values. Clin. Res. Cardiol. 2014, 103, 441–450. [Google Scholar] [CrossRef]
- Xue, H.; Artico, J.; Fontana, M.; Moon, J.C.; Davies, R.H.; Kellman, P. Landmark Detection in Cardiac MRI by Using a Convolutional Neural Network. Radiol. Artif. Intell. 2021, 3, e200197. [Google Scholar] [CrossRef]
- Assadi, H.; Li, R.; Grafton-Clarke, C.; Uthayachandran, B.; Alabed, S.; Maiter, A.; Archer, G.; Swoboda, P.P.; Sawh, C.; Ryding, A.; et al. Automated 4D flow cardiac MRI pipeline to derive peak mitral inflow diastolic velocities using short-axis cine stack: Two centre validation study against echocardiographic pulse-wave doppler. BMC Cardiovasc. Disord. 2023, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Sharrack, N.; Chiribiri, A.; Schwitter, J.; Plein, S. How to do quantitative myocardial perfusion cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 315–318. [Google Scholar] [CrossRef]
- Kellman, P.; Hansen, M.S.; Nielles-Vallespin, S.; Nickander, J.; Themudo, R.; Ugander, M.; Xue, H. Myocardial perfusion cardiovascular magnetic resonance: Optimized dual sequence and reconstruction for quantification. J. Cardiovasc. Magn. Reson. 2017, 19, 43. [Google Scholar] [CrossRef]
- Scannell, C.M.; Alskaf, E.; Sharrack, N.; Razavi, R.; Ourselin, S.; Young, A.A.; Plein, S.; Chiribiri, A. AI-AIF: Artificial intelligence-based arterial input function for quantitative stress perfusion cardiac magnetic resonance. Eur. Heart J.-Digit. Health 2023, 4, 12–21. [Google Scholar] [CrossRef]
- Kato, S.; Saito, N.; Kirigaya, H.; Gyotoku, D.; Iinuma, N.; Kusakawa, Y.; Iguchi, K.; Nakachi, T.; Fukui, K.; Futaki, M.; et al. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients with Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2016, 5, e002649. [Google Scholar] [CrossRef]
- Löffler, A.I.; Pan, J.A.; Balfour, P.C.; Shaw, P.W.; Yang, Y.; Nasir, M.; Auger, D.A.; Epstein, F.H.; Kramer, C.M.; Gan, L.-M.; et al. Frequency of Coronary Microvascular Dysfunction and Diffuse Myocardial Fibrosis (Measured by Cardiovascular Magnetic Resonance) in Patients with Heart Failure and Preserved Left Ventricular Ejection Fraction. Am. J. Cardiol. 2019, 124, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Hovasse, T.; Sanguineti, F.; Kinnel, M.; Garot, P.; Champagne, S.; Toupin, S.; Unterseeh, T.; Garot, J. Long-Term Prognostic Value of Stress CMR in Patients with Heart Failure and Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2021, 14, 2319–2333. [Google Scholar] [CrossRef] [PubMed]
- Manisty, C.; Ripley, D.P.; Herrey, A.S.; Captur, G.; Wong, T.C.; Petersen, S.E.; Plein, S.; Peebles, C.; Schelbert, E.B.; Greenwood, J.P.; et al. Splenic Switch-off: A Tool to Assess Stress Adequacy in Adenosine Perfusion Cardiac MR Imaging. Radiology 2015, 276, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef]
- Treibel, T.A.; Kozor, R.; Schofield, R.; Benedetti, G.; Fontana, M.; Bhuva, A.N.; Sheikh, A.; López, B.; González, A.; Manisty, C.; et al. Reverse Myocardial Remodeling Following Valve Replacement in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 860–871. [Google Scholar] [CrossRef]
- Khalique, Z.; Ferreira, P.F.; Scott, A.D.; Nielles-Vallespin, S.; Firmin, D.N.; Pennell, D.J. Diffusion Tensor Cardiovascular Magnetic Resonance Imaging: A Clinical Perspective. JACC Cardiovasc. Imaging 2020, 13, 1235–1255. [Google Scholar] [CrossRef]
- Popescu, I.A.; Werys, K.; Zhang, Q.; Puchta, H.; Hann, E.; Lukaschuk, E.; Ferreira, V.M.; Piechnik, S.K. Standardization of T1-mapping in cardiovascular magnetic resonance using clustered structuring for benchmarking normal ranges. Int. J. Cardiol. 2021, 326, 220–225. [Google Scholar] [CrossRef]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef]
- Lynch, K.M.; Sepehrband, F.; Toga, A.W.; Choupan, J. Brain perivascular space imaging across the human lifespan. Neuroimage 2023, 271, 120009. [Google Scholar] [CrossRef]
- Guerrero, R.; Qin, C.; Oktay, O.; Bowles, C.; Chen, L.; Joules, R.; Wolz, R.; Valdés-Hernández, M.C.; Dickie, D.A.; Wardlaw, J.; et al. White matter hyperintensity and stroke lesion segmentation and differentiation using convolutional neural networks. NeuroImage Clin. 2018, 17, 918–934. [Google Scholar] [CrossRef]
- Hundley, W.G.; Bluemke, D.A.; Bogaert, J.; Flamm, S.D.; Fontana, M.; Friedrich, M.G.; Grosse-Wortmann, L.; Karamitsos, T.D.; Kramer, C.M.; Kwong, R.Y.; et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidelines for reporting cardiovascular magnetic resonance examinations. J. Cardiovasc. Magn. Reson. 2022, 24, 29. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Hazany, S.; Nguyen, K.-L.; Lee, M.; Zhang, A.; Mokhtar, P.; Crossley, A.; Luthra, S.; Butani, P.; Dergalust, S.; Ellingson, B.; et al. Regional Cerebral Small Vessel Disease (rCSVD) Score: A clinical MRI grading system validated in a stroke cohort. J. Clin. Neurosci. 2022, 105, 131–136. [Google Scholar] [CrossRef]
- Amin Al Olama, A.; Wason, J.M.S.; Tuladhar, A.M.; van Leijsen, E.M.C.; Koini, M.; Hofer, E.; Morris, R.G.; Schmidt, R.; de Leeuw, F.-E.; Markus, H.S. Simple MRI score aids prediction of dementia in cerebral small vessel disease. Neurology 2020, 94, e1294–e1302. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, M.; Maclaren, J.; Herbst, M. Motion Artefacts in MRI: A Complex Problem with Many Partial Solutions. J. Magn. Reson. Imaging 2015, 42, 887–901. [Google Scholar] [CrossRef]
- Van Den Brink, H.; Doubal, F.N.; Duering, M. Advanced MRI in cerebral small vessel disease. Int. J. Stroke 2023, 18, 28–35. [Google Scholar] [CrossRef]
- Paschoal, A.M.; Secchinatto, K.F.; da Silva, P.H.R.; Zotin, M.C.Z.; dos Santos, A.C.; Viswanathan, A.; Pontes-Neto, O.M.; Leoni, R.F. Contrast-agent-free state-of-the-art MRI on cerebral small vessel disease—Part 1. ASL, IVIM, and CVR. NMR Biomed. 2022, 35, e4742. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Lythgoe, D.J.; Pereira, A.C.; Summers, P.E.; Jarosz, J.M.; Williams, S.C.R.; Markus, H.S. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 2002, 59, 321–326. [Google Scholar] [CrossRef]
- Joutel, A.; Monet-Leprêtre, M.; Gosele, C.; Baron-Menguy, C.; Hammes, A.; Schmidt, S.; Lemaire-Carrette, B.; Domenga, V.; Schedl, A.; Lacombe, P.; et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Investig. 2010, 120, 433–445. [Google Scholar] [CrossRef]
- Willats, L.; Calamante, F. The 39 steps: Evading error and deciphering the secrets for accurate dynamic susceptibility contrast MRI. NMR Biomed. 2013, 26, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Bolar, D.S.; Achten, E.; Barkhof, F.; Bastos-Leite, A.J.; Detre, J.A.; Golay, X.; Günther, M.; Wang, D.J.J.; Haller, S.; et al. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn. Reson. Med. 2023, 89, 2024–2047. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Krámská, L.; Brožová, H.; Růžička, E.; Rulseh, A.M. Does serial administration of gadolinium-based contrast agents affect patient neurological and neuropsychological status? Fourteen-year follow-up of patients receiving more than fifty contrast administrations. J. Magn. Reson. Imaging 2020, 51, 1912–1913. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Huuskonen, M.T.; Rajagopal, G.; Sweeney, M.D.; Nation, D.A.; Sepehrband, F.; D’Orazio, L.M.; Harrington, M.G.; Chui, H.C.; Law, M.; et al. Undetectable gadolinium brain retention in individuals with an age-dependent blood-brain barrier breakdown in the hippocampus and mild cognitive impairment. Alzheimer’s Dement. 2019, 15, 1568–1575. [Google Scholar] [CrossRef]
- Visser, V.L.; Rusinek, H.; Weickenmeier, J. Peak ependymal cell stretch overlaps with the onset locations of periventricular white matter lesions. Sci. Rep. 2021, 11, 21956. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, X.; Deng, B.; Chang, Z.; Jin, D.; Huang, Y.; Zhang, J.H.; Yenari, M.A.; Jin, K.; Wang, Q. Cerebral small vessel disease alters neurovascular unit regulation of microcirculation integrity involved in vascular cognitive impairment. Neurobiol. Dis. 2022, 170, 105750. [Google Scholar] [CrossRef]
- Thrippleton, M.J.; Shi, Y.; Blair, G.; Hamilton, I.; Waiter, G.; Schwarzbauer, C.; Pernet, C.; Andrews, P.J.; Marshall, I.; Doubal, F.; et al. Cerebrovascular reactivity measurement in cerebral small vessel disease: Rationale and reproducibility of a protocol for MRI acquisition and image processing. Int. J. Stroke 2018, 13, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.W.; Thrippleton, M.J.; Shi, Y.; Hamilton, I.; Stringer, M.; Chappell, F.; Dickie, D.A.; Andrews, P.; Marshall, I.; Doubal, F.N.; et al. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology 2020, 94, e2258–e2269. [Google Scholar] [CrossRef] [PubMed]
- Sam, K.; Conklin, J.; Holmes, K.R.; Sobczyk, O.; Poublanc, J.; Crawley, A.P.; Mandell, D.M.; Venkatraghavan, L.; Duffin, J.; Fisher, J.A.; et al. Impaired dynamic cerebrovascular response to hypercapnia predicts development of white matter hyperintensities. NeuroImage Clin. 2016, 11, 796–801. [Google Scholar] [CrossRef]
- Foda, A.; Kellner, E.; Gunawardana, A.; Gao, X.; Janz, M.; Kufner, A.; Khalil, A.A.; Geran, R.; Mekle, R.; Fiebach, J.B.; et al. Differentiation of Cerebral Neoplasms with Vessel Size Imaging (VSI). Clin. Neuroradiol. 2022, 32, 239–248. [Google Scholar] [CrossRef]
- Choi, H.-I.; Ryu, C.-W.; Kim, S.; Rhee, H.Y.; Jahng, G.-H. Changes in Microvascular Morphology in Subcortical Vascular Dementia: A Study of Vessel Size Magnetic Resonance Imaging. Front. Neurol. 2020, 11, 545450. [Google Scholar] [CrossRef]
- Freeze, W.M.; Jacobs, H.I.L.; de Jong, J.J.; Verheggen, I.C.M.; Gronenschild, E.H.B.M.; Palm, W.M.; Hoff, E.I.; Wardlaw, J.M.; Jansen, J.F.A.; Verhey, F.R.; et al. White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol. Aging 2020, 85, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.E.; Wong, S.M.; van de Haar, H.J.; Staals, J.; Jansen, J.F.A.; Jeukens, C.R.L.P.N.; Hofman, P.A.M.; van Oostenbrugge, R.J.; Backes, W.H. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology 2017, 88, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ohene, Y.; Harrison, I.F.; Evans, P.G.; Thomas, D.L.; Lythgoe, M.F.; Wells, J.A. Increased blood–brain barrier permeability to water in the aging brain detected using noninvasive multi-TE ASL MRI. Magn. Reson. Med. 2021, 85, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.; Ohene, Y.; Battiston, M.; Dickie, B.R.; Parkes, L.M.; Parker, G.J.M. Blood-brain barrier water exchange measurements using FEXI: Impact of modeling paradigm and relaxation time effects. Magn. Reson. Med. 2023, 90, 34–50. [Google Scholar] [CrossRef]
- D’Souza, M.M.; Gorthi, S.; Vadwala, K.; Trivedi, R.; Vijayakumar, C.; Kaur, P.; Khushu, S. Diffusion tensor tractography in cerebral small vessel disease: Correlation with cognitive function. Neuroradiol. J. 2018, 31, 83–89. [Google Scholar] [CrossRef]
- Pasi, M.; van Uden, I.W.M.; Tuladhar, A.M.; de Leeuw, F.-E.; Pantoni, L. White Matter Microstructural Damage on Diffusion Tensor Imaging in Cerebral Small Vessel Disease. Stroke 2016, 47, 1679–1684. [Google Scholar] [CrossRef]
- Tini, G.; Cannatà, A.; Canepa, M.; Masci, P.G.; Pardini, M.; Giacca, M.; Sinagra, G.; Marchionni, N.; Del Monte, F.; Udelson, J.E.; et al. Is heart failure with preserved ejection fraction a “dementia” of the heart? Heart Fail. Rev. 2022, 27, 587–594. [Google Scholar] [CrossRef]
- Van Dinther, M.; Schram, M.T.; Jansen, J.F.A.; Backes, W.H.; Houben, A.J.H.M.; Berendschot, T.T.J.M.; Schalkwijk, C.G.; Stehouwer, C.D.A.; van Oostenbrugge, R.J.; Staals, J. Extracerebral microvascular dysfunction is related to brain MRI markers of cerebral small vessel disease: The Maastricht Study. GeroScience 2022, 44, 147–157. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Woodhouse, L.J.; Mhlanga, I.I.; Oatey, K.; Heye, A.K.; Bamford, J.; Cvoro, V.; Doubal, F.N.; England, T.; Hassan, A.; et al. Isosorbide Mononitrate and Cilostazol Treatment in Patients with Symptomatic Cerebral Small Vessel Disease: The Lacunar Intervention Trial-2 (LACI-2) Randomized Clinical Trial. JAMA Neurol. 2023, 80, 682–692. [Google Scholar] [CrossRef]
- Blair, G.W.; Stringer, M.S.; Thrippleton, M.J.; Chappell, F.M.; Shuler, K.; Hamilton, I.; Garcia, D.J.; Doubal, F.N.; Kopczak, A.; Duering, M.; et al. Imaging neurovascular, endothelial and structural integrity in preparation to treat small vessel diseases. The INVESTIGATE-SVDs study protocol. Part of the SVDs@Target project. Cereb. Circ. Cogn. Behav. 2021, 2, 100020. [Google Scholar] [CrossRef] [PubMed]
- van Dinther, M.; Bennett, J.; Thornton, G.D.; Voorter, P.H.M.; Ezponda Casajús, A.; Hughes, A.; Captur, G.; Holtackers, R.J.; Staals, J.; Backes, W.H.; et al. Evaluation of miCRovascular rarefaction in vascUlar Cognitive Impairment and Heart Failure (CRUCIAL): Study protocol for an observational study. Cerebrovasc. Dis. Extra 2023, 13, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pinto, A.; Ravikumar, N.; Attar, R.; Suinesiaputra, A.; Zhao, Y.; Levelt, E.; Dall’armellina, E.; Lorenzi, M.; Chen, Q.; Keenan, T.D.L.; et al. Predicting myocardial infarction through retinal scans and minimal personal information. Nat. Mach. Intell. 2022, 4, 55–61. [Google Scholar] [CrossRef]
- Nelis, P.; Kleffner, I.; Burg, M.C.; Clemens, C.R.; Alnawaiseh, M.; Motte, J.; Marziniak, M.; Eter, N.; Alten, F. OCT-Angiography reveals reduced vessel density in the deep retinal plexus of CADASIL patients. Sci. Rep. 2018, 8, 8148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Gregori, G.; Zheng, F.; Vanner, E.A.; Lam, B.L.; Rundek, T.; et al. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J. Neuroophthalmol. 2018, 38, 292–298. [Google Scholar] [CrossRef]
| Imaging Feature | Description and Pathophysiology | Clinical Image | MRI Findings |
|---|---|---|---|
| Recent small subcortical infarct (formerly lacunar infarct): | Occlusion of perforating artery causing distal infarction of brain parenchyma [28]. Makes up ~25% of acute ischaemic strokes. |  | ≤20 mm Best identified on DWI Hyperintense: T2, FLAIR, DWI Hypointense: T1 Isointense: T2*-GRE |
| White matter hyperintensity: | White matter demyelination resulting from multiple pathological insults including: chronic hypoperfusion, blood–brain barrier leakage, impaired amyloid clearance, and iron deposition [28] | 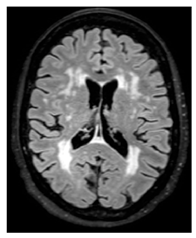 | Variable in size Hyperintense: FLAIR, T2, and T2*-GRE Hypointense: T1 Isointense: T1 |
| Lacune: | CSF-filled cavity within the basal ganglia or white matter that is presumed to arise from prior small deep brain infarction [32] |  | 3–15 mm Best distinguished on FLAIR with hypointense centre and hyperintense rim Hyperintense: T2 Hypointense: T1, DWI Isointense: DWI, T2*-GRE |
| Enlarged perivascular spaces: | Fluid-filled compartments surrounding the small blood vessels in the brain that allow clearance of waste metabolites from the brain. Enlargement possibly arises from blockage of the perivascular space leading to accumulation of waste products [33]. |  | <2 mm Hyperintense: T2 Hypointense: T1, FLAIR Isointense: DWI, T2*-GRE |
| Cerebral microbleeds: | Perivascular haemosiderin deposits that leak from capillaries, implying the breakdown of the blood–brain barrier and endothelial dysfunction [28] | 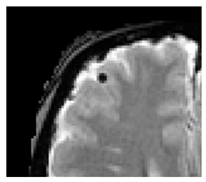 | <10 mm Hypointense: T2*-GRE, SWI Isointense: DWI, FLAIR, T2, T1 |
| Cortical cerebral microinfarct: | New additions with STRIVE-2 that are typically visible on microscopic neuropathological examination or high-field (7T) MRI. Some larger cortical cerebral microinfarcts (0.5–4 mm) can be seen on conventional MRI strength. |  | <4 mm Hyperintense: T2, DWI (if acute) Hypointense: T1 |
| Study | Population | Modality | Perfusion Parameters | Tissue Characteristics | Outcome | ||
|---|---|---|---|---|---|---|---|
| Kato et al. 2015 [80] | HFpEF (n = 25) Controls (n = 19) Hypertensives (n = 13) | Phase-contrast CMR | Stress MBF | ↓ | No tissue characterisation | Not linked to outcomes | |
| Rest MBF | ↑ | ||||||
| MPR | ↓ | ||||||
| Löffler et al. 2019 [81] | HFpEF (n = 19) Controls (n = 15) | Quantitative perfusion CMR | Stress MBF | ↓ | ECV | ↑ | Not linked to outcomes |
| Rest MBF | ↑ | LGE presence | Not compared to controls | ||||
| MPR | ↓ | ||||||
| Arnold et al. 2022 [27] | HFpEF (n = 101) Controls (n = 42) | Quantitative perfusion CMR | Stress MBF | ↓ | ECV: | ↑ | Reduced MPR independently associated with MACE. Increased ECV associated with MACE. |
| Rest MBF | ↔ | LGE presence: | ↑ | ||||
| MPR | ↓ | ||||||
| Pezel et al. 2021 [82] | HFpEF (n = 1203) No control group | Semi-quantitative visually assessed perfusion for segments of ischaemia on CMR | N/A (no control group) | N/A (no control group) | Moderate (3–5 segments) and severe (≥6) segments of ischaemia associated with MACE. Presence of LGE associated with MACE on multivariate regression. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennett, J.; van Dinther, M.; Voorter, P.; Backes, W.; Barnes, J.; Barkhof, F.; Captur, G.; Hughes, A.D.; Sudre, C.; Treibel, T.A. Assessment of Microvascular Disease in Heart and Brain by MRI: Application in Heart Failure with Preserved Ejection Fraction and Cerebral Small Vessel Disease. Medicina 2023, 59, 1596. https://doi.org/10.3390/medicina59091596
Bennett J, van Dinther M, Voorter P, Backes W, Barnes J, Barkhof F, Captur G, Hughes AD, Sudre C, Treibel TA. Assessment of Microvascular Disease in Heart and Brain by MRI: Application in Heart Failure with Preserved Ejection Fraction and Cerebral Small Vessel Disease. Medicina. 2023; 59(9):1596. https://doi.org/10.3390/medicina59091596
Chicago/Turabian StyleBennett, Jonathan, Maud van Dinther, Paulien Voorter, Walter Backes, Josephine Barnes, Frederick Barkhof, Gabriella Captur, Alun D. Hughes, Carole Sudre, and Thomas A. Treibel. 2023. "Assessment of Microvascular Disease in Heart and Brain by MRI: Application in Heart Failure with Preserved Ejection Fraction and Cerebral Small Vessel Disease" Medicina 59, no. 9: 1596. https://doi.org/10.3390/medicina59091596
APA StyleBennett, J., van Dinther, M., Voorter, P., Backes, W., Barnes, J., Barkhof, F., Captur, G., Hughes, A. D., Sudre, C., & Treibel, T. A. (2023). Assessment of Microvascular Disease in Heart and Brain by MRI: Application in Heart Failure with Preserved Ejection Fraction and Cerebral Small Vessel Disease. Medicina, 59(9), 1596. https://doi.org/10.3390/medicina59091596






