Resection of Intramedullary Hemangioblastoma: Timing of Surgery and Its Impact on Neurological Outcome and Quality of Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
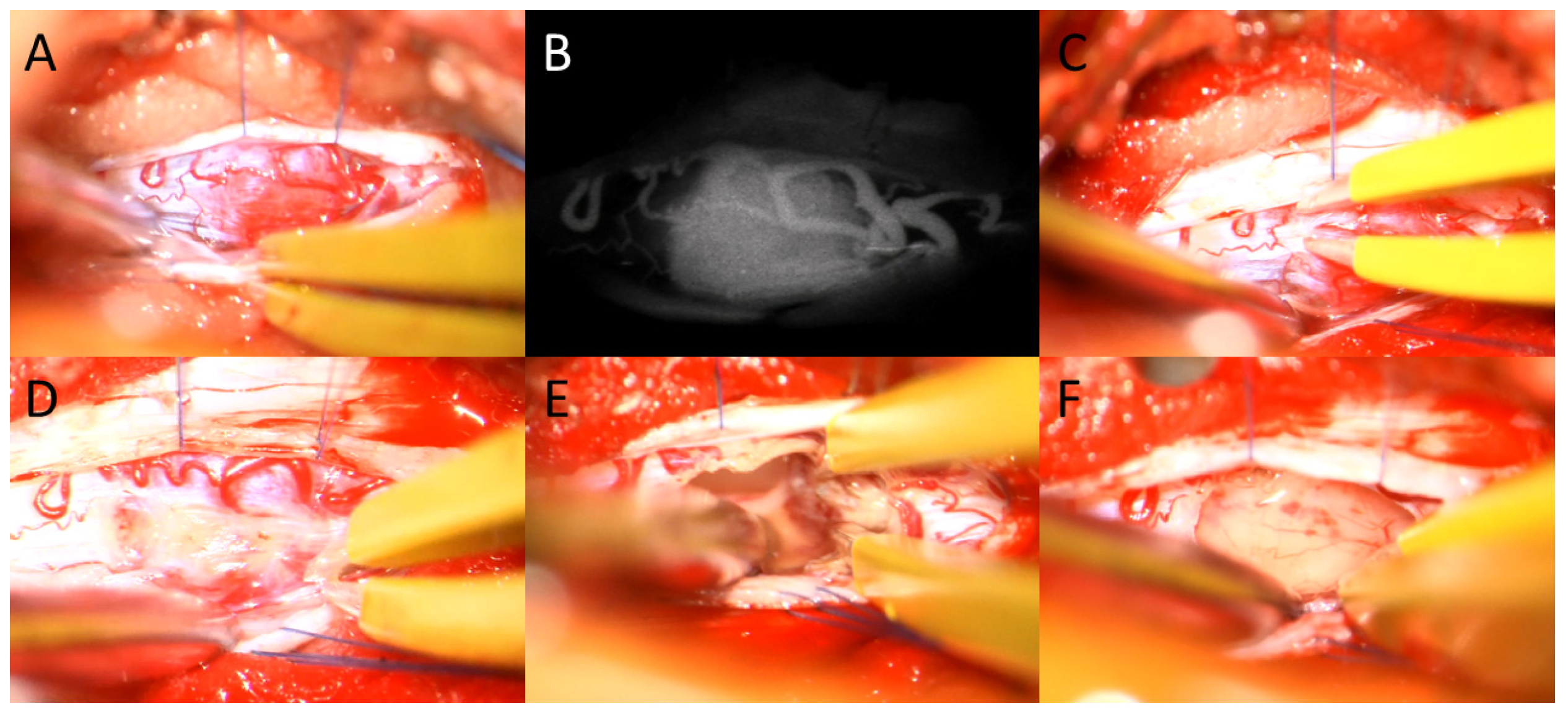
2.2. Surgical Intervention
2.3. Outcome and Assessment of QoL
2.4. Statistical Analysis
3. Results
3.1. Population
3.2. Surgical Intervention
3.3. Neurological Outcome and QI
3.4. Quality of Life
4. Discussion
4.1. Favorable Postoperative Outcome May Depend on the Preoperative Neurological Status
4.2. A Good Postoperative Outcome Is Associated with a Higher Quality of Life
4.3. An Interdisciplinary Approach Prevents Complications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoda, R.A.; Cimino, P.J. Neuropathologic features of central nervous system hemangioblastoma. J. Pathol. Transl. Med. 2022, 56, 115–125. [Google Scholar] [CrossRef]
- Lonser, R.R.; Glenn, G.M.; Walther, M.; Chew, E.Y.; Libutti, S.K.; Linehan, W.M.; Oldfield, E.H. von Hippel-Lindau disease. Lancet 2003, 361, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.R.; Yates, J.R.W.; Harries, R.; Benjamin, C.; Moore, A.T.; Ferguson-Smith, M.A. Clinical features and natural history of von Hippel-Lindau disease. Q. J. Med. 1990, 77, 1151–1163. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Na, J.H.; Kim, H.S.; Eoh, W.; Kim, J.H.; Kim, J.S.; Kim, E.-S. Spinal cord hemangioblastoma: Diagnosis and clinical outcome after surgical treatment. J. Korean Neurosurg. Soc. 2007, 42, 436. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Tucker, W.S.; Bilbao, J.M.; Gladstone, R.M.; Bass, A.G. Subarachnoid hemorrhage due to a spinal cord hemangioblastoma: Case report. Neurosurgery 1980, 6, 657–660. [Google Scholar] [CrossRef]
- Koeller, K.K.; Rosenblum, R.S.; Morrison, A.L. Neoplasms of the spinal cord and filum terminale: Radiologic-pathologic correlation. Radiographics 2000, 20, 1721–1749. [Google Scholar] [CrossRef]
- Westwick, H.J.; Giguère, J.F.; Shamji, M.F. Incidence and Prognosis of Spinal Hemangioblastoma: A Surveillance Epidemiology and End Results Study. Neuroepidemiology 2016, 46, 14–23. [Google Scholar] [CrossRef]
- Lee, D.K.; Choe, W.J.; Chung, C.K.; Kim, H.J. Spinal cord hemangioblastoma: Surgical strategy and clinical outcome. J. Neurooncol. 2003, 61, 27–34. [Google Scholar] [CrossRef]
- Boström, A.; Hans, F.-J.; Reinacher, P.C.; Krings, T.; Bürgel, U.; Gilsbach, J.M.; Reinges, M.H.T. Intramedullary hemangioblastomas: Timing of surgery, microsurgical technique and follow-up in 23 patients. Eur. Spine J. 2008, 17, 882–886. [Google Scholar] [CrossRef]
- Harati, A.; Mahler, L.; Elsharkawy, A.; Hernesniemi, J.; Satopää, J.; Billon-Grand, R.; Niemelä, M. Early microsurgical treatment for spinal hemangioblastomas improves outcome in patients with von Hippel-Lindau disease. Surg. Neurol. Int. 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Molina, E.S.; Windheuser, J.; Doods, J.; Schwake, M.; Wilbers, E.; Alsofy, S.Z.; Warneke, N.; Stummer, W. The 30-day readmission rate in neurosurgery—A useful indicator for quality assessment? Acta Neurochir. 2020, 162, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Menke, C.; Lohmann, S.; Baehr, A.; Grauer, O.; Holling, M.; Brokinkel, B.; Schwake, M.; Stummer, W.; Schipmann, S. Classical and disease-specific quality indicators in glioma surgery-Development of a quality checklist to improve treatment quality in glioma patients. Neurooncol. Pract. 2022, 9, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Spille, D.C.; Lohmann, S.; Brokinkel, B.; Schipmann, S.; Schwake, M.; Spille, J.H.; Alsofy, S.Z.; Stummer, W. Can currently suggested quality indicators be transferred to meningioma surgery?—A single-centre pilot study. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2022, 84, 409–418. [Google Scholar] [CrossRef]
- Schipmann, S.; Schwake, M.; Molina, E.S.; Roeder, N.; Steudel, W.-I.; Warneke, N.; Stummer, W. Quality Indicators in Cranial Neurosurgery: Which Are Presently Substantiated? A Systematic Review. World Neurosurg. 2017, 104, 104–112. [Google Scholar] [CrossRef]
- Pettersson-Segerlind, J.; Fletcher-Sandersjöö, A.; Tatter, C.; Burström, G.; Persson, O.; Förander, P.; Mathiesen, T.; Bartek, J.; Edström, E.; Elmi-Terander, A. Long-Term Follow-Up and Predictors of Functional Outcome after Surgery for Spinal Meningiomas: A Population-Based Cohort Study. Cancers 2021, 13, 3244. [Google Scholar] [CrossRef]
- Butenschoen, V.M.; Gloßner, T.; Hostettler, I.C.; Meyer, B.; Wostrack, M. Quality of life and return to work and sports after spinal ependymoma resection. Sci. Rep. 2022, 12, 4926. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-SD: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [CrossRef]
- Barzilai, O.; Versteeg, A.L.; Goodwin, C.R.; Sahgal, A.; Rhines, L.D.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Lazary, A.; Fehlings, M.G.; et al. Association of neurologic deficits with surgical outcomes and health-related quality of life after treatment for metastatic epidural spinal cord compression. Cancer 2019, 125, 4224–4231. [Google Scholar] [CrossRef]
- Schwake, M.; Adeli, A.; Sporns, P.; Ewelt, C.; Schmitz, T.; Sicking, J.; Hess, K.; Spille, D.C.; Paulus, W.; Stummer, W.; et al. Spinal meningiomas—Risks and potential of an increasing age at the time of surgery. J. Clin. Neurosci. 2018, 57, 86–92. [Google Scholar] [CrossRef]
- Butenschoen, V.M.; Schwendner, M.; Hubertus, V.; Onken, J.; Koegl, N.; Mohme, T.; Maurer, S.; Boeckh-Behrens, T.; Eicker, S.O.; Thomé, C.; et al. Preoperative angiographic considerations and neurological outcome after surgical treatment of intradural spinal hemangioblastoma: A multicenter retrospective case series. J. Neurooncol. 2023, 161, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Feletti, A.; Boaro, A.; Giampiccolo, D.; Casoli, G.; Moscolo, F.; Ferrara, M.; Sala, F.; Pavesi, G. Spinal hemangioblastomas: Analysis of surgical outcome and prognostic factors. Neurosurg. Rev. 2022, 45, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Skrap, B.; Kothbauer, K.F.; Deletis, V. Intraoperative neurophysiology in intramedullary spinal cord tumor surgery. Handb. Clin. Neurol. 2022, 186, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Bricolo, A.; Faccioli, F.; Lanteri, P.; Gerosa, M. Surgery for intramedullary spinal cord tumors: The role of intraoperative (neurophysiological) monitoring. Eur. Spine J. 2007, 16 (Suppl. 2), 130–139. [Google Scholar] [CrossRef]
- Guarino, A.; Polini, C.; Forte, G.; Favieri, F.; Boncompagni, I.; Casagrande, M. The effectiveness of psychological treatments in women with breast cancer: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 209. [Google Scholar] [CrossRef]
- Rick, O.; Dauelsberg, T.; Kalusche-Bontemps, E.M. Oncological Rehabilitation. Oncol. Res. Treat. 2017, 40, 772–777. [Google Scholar] [CrossRef]
- Boersma, I.; Miyasaki, J.; Kutner, J.; Kluger, B. Palliative care and neurology: Time for a paradigm shift. Neurology 2014, 83, 561–567. [Google Scholar] [CrossRef]
- Provinciali, L.; Carlini, G.; Tarquini, D.; Defanti, C.A.; Veronese, S.; Pucci, E. Need for palliative care for neurological diseases. Neurol. Sci. 2016, 37, 1581–1587. [Google Scholar] [CrossRef]
- Schöller, K.; Alimi, M.; Cong, G.T.; Christos, P.; Härtl, R. Lumbar Spinal Stenosis Associated with Degenerative Lumbar Spondylolisthesis: A Systematic Review and Meta-analysis of Secondary Fusion Rates Following Open vs. Minimally Invasive Decompression. Neurosurgery 2017, 80, 355–367. [Google Scholar] [CrossRef]
- Dobran, M.; Paracino, R.; Nasi, D.; Aiudi, D.; Capece, M.; Carrassi, E.; Lattanzi, S.; DI Rienzo, A.; Iacoangeli, M. Laminectomy versus Unilateral Hemilaminectomy for the Removal of Intraspinal Schwannoma: Experience of a Single Institution and Review of Literature. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Krahwinkel, S.; Schipmann, S.; Spille, D.; Maragno, E.; Al Barim, B.; Warneke, N.; Stummer, W.; Gallus, M.; Schwake, M. The Role of Prolonged Bed Rest in Postoperative Cerebrospinal Fluid Leakage After Surgery of Intradural Pathology-A Retrospective Cohort Study. Neurosurgery 2023, 93, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.T.; Steiert, C.; Gläsker, S.; Klingler, J.H. Minimally invasive resection of spinal hemangioblastoma: Feasibility and clinical results in a series of 18 patients. J. Neurosurg. Spine 2019, 31, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, D.; Ma, G.; Yang, J.; Wang, G. Application of intraoperative indocyanine green videoangiography for resection of spinal cord hemangioblastoma: Advantages and limitations. J. Clin. Neurosci. 2013, 20, 1269–1275. [Google Scholar] [CrossRef]
- Hojo, M.; Arakawa, Y.; Funaki, T.; Yoshida, K.; Kikuchi, T.; Takagi, Y.; Araki, Y.; Ishii, A.; Kunieda, T.; Takahashi, J.C.; et al. Usefulness of tumor blood flow imaging by intraoperative indocyanine green videoangiography in hemangioblastoma surgery. World Neurosurg. 2014, 82, E495–E501. [Google Scholar] [CrossRef]
- Tamura, Y.; Hirota, Y.; Miyata, S.; Yamada, Y.; Tucker, A.; Kuroiwa, T. The use of intraoperative near-infrared indocyanine green videoangiography in the microscopic resection of hemangioblastomas. Acta Neurochir. 2012, 154, 1407–1412. [Google Scholar] [CrossRef]



| Variable | Value |
|---|---|
| Age (Mean±SD) | 38.20 ± 13.12 |
| Sex female/male (N/%) | 11 (40.74%)/16 (59.26%) |
| Localization | |
| Cervical (N/%) | 13 (48.15%) |
| Thoracic | 13 (48.15%) |
| Lumbar | 1 (3.70%) |
| Tumor volume (cm3, Mean±SD) | 0.60 ± 0.93 |
| Preoperative neurological status | |
| McCormick I (N/%) | 10 (37.04%) |
| McCormick II (N/%) | 9 (33.33%) |
| McCormick III (N/%) | 2 (7.41%) |
| Mc Cormick IV (N/%) | 6 (22.22%) |
| Syringomyelia (N/%) | 20 (74.07%) |
| Von Hippel-Lindau mutation (N/%) | 22 (81.48%) |
| Variable | Value |
|---|---|
| Surgical approach | |
| Laminoplasty (N/%) | 8 (29.63%) |
| Unilateral laminotomy | 18 (66.7%) |
| Laminectomy | 1 (3.7%) |
| Dura closure | |
| Direct closure | 23 (85.19%) |
| Extension duroplasty | 4 (14.81%) |
| Extent of resection (EOR) | |
| Gros total resection (GTR) | 22 (81.48%) |
| Subtotal resection (STR) | 5 (18.52%) |
| Blood loss (ml, mean,±SD) | 345.19 ± 562.87 |
| Preoperative embolization (N/%) | 3 (11.11%) |
| Indocyanine green (ICG) angiography (N/%) | 22 (81.48%) |
| Intraoperative monitoring (N/%) | 27 (100%) |
| Duration of surgery (Minutes, mean,±SD) | 332.3 ± 159.98 |
| Postoperative neurological status | |
| McCormick I (N/%) | 13 (48.15%) |
| McCormick II (N/%) | 7 (25.93%) |
| McCormick III (N/%) | 2 (7.41%) |
| McCormick IV (N/%) | 5 (18.52%) |
| Length of hospital stay (days, mean± SD) | 7.11 ± 3.61 |
| Nosocomial infection | |
| Urinary tract infection | 2 (7.41%) |
| 90 days readmission (N/%) | 3 (11.11%) |
| 90 days re-operation (N/%) | 2 (7.41%) |
| Favorable Outcome (N = 13) | Unfavorable Outcome (N = 14) | p | |
|---|---|---|---|
| Sex (females, N/%) | 5 (38.46%) | 6 (42.86%) | 0.816 |
| Age (years, mean, ± SD) | 35.46 ±10.10 | 42.00 ±15.39 | 0.302 |
| Tumor volume (ml) | 0.46 ± 0.72 | 0.71 ± 1.08 | 0.720 |
| Localization | 0.568 | ||
| Cervical | 7 | 6 | |
| Thoraco-lumbar | 6 | 8 | |
| Von Hippel-Lindau mutation (N/%) | 8 (61.53%) | 14 (100%) | 0.010 |
| Syringomyelia (N/%) | 3 (23.08%) | 4 (28.57%) | 0.745 |
| Preoperative neurological status (N/%) | <0.001 | ||
| McCormick scale 1 | 10 (76.92%) | 0 | |
| McCormick scale 2 | 3 (23.08%) | 6 (42.86%) | |
| McCormick scale 3 | 0 | 2 (14.29%) | |
| McCormick scale 4 | 0 | 6 (42.86%) | |
| Preoperative embolization (N/%) | 1 (7.7.69%) | 2 (14.29%) | 0.586 |
| Surgical approach | 0.948 | ||
| Hemilaminectomy | 9 (69.23%) | 9 (64.29%) | |
| Laminectomy | 1 (7.69%) | 0 | |
| Laminoplasty | 3 (23.08%) | 5 (35.71%) | |
| Primary surgery (N/%) | 12 (92.3%) | 12 (85.71%) | 0.586 |
| Indocyanine green (ICG) angiography (N/%) | 12 (92.31%) | 10 (71.43%) | 0.163 |
| Intraoperative neuromonitoring | 13 (100%) | 14 (100%) | >0.999 |
| Duration of surgery (minutes, mean, ± SD) | 273.23 ± 76.42 | 387.14 ± 197.66 | 0.185 |
| Blood loss (ml, mean, ± SD) | 288.46 ± 445.42 | 430.00 ± 646.04 | 0.458 |
| Gross total resection (N/%) | 13 (100%) | 9 (64.29%) | 0.017 |
| Expansion duraplasty (N/%) | 1 (7.69%) | 3 (21.43%) | 0.315 |
| Bed rest after surgery (N/%) | 7 (53.85%) | 7 (50.00%) | 0.842 |
| Leakage of cerebrospinal fluid (N/%) | 0 | 1 (7.14%) | 0.326 |
| Length of hospital stay (days, mean, ± SD) | 5.85 ± 2.37 | 8.29 ± 4.21 | 0.022 |
| Nosocomial infections (N/%) | 0 | 2 (14.29%) | 0.157 |
| Readmission within 90 days (N/%) | 1 (7.69%) | 2 (14.29%) | 0.586 |
| Re-Surgery within 90 days (N/%) | 0 | 2 (14.29%) | 0.157 |
| Tumor recurrence (N/%) | 0 | 0 | >0.999 |
| Patient | Mobility | Self-Care | Activity | Pain | Anxiety | EQ_VAS | McCormick Scale Pre-Operative | McCormick Scale Post-Operative |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 2 | 85 | 1 | 1 |
| 2 | 1 | 1 | 1 | 2 | 1 | 85 | 2 | 2 |
| 3 | 2 | 1 | 2 | 2 | 1 | 75 | 3 | 2 |
| 4 | 2 | 2 | 2 | 2 | 2 | 40 | 4 | 4 |
| 5 | 1 | 1 | 2 | 2 | 1 | 70 | 3 | 4 |
| 6 | 1 | 1 | 2 | 2 | 2 | 85 | 2 | 2 |
| 7 | 2 | 1 | 2 | 2 | 2 | 45 | 2 | 1 |
| 8 | 1 | 1 | 1 | 1 | 2 | 90 | 2 | 2 |
| 9 | 2 | 1 | 2 | 3 | 3 | 30 | 1 | 1 |
| 10 | 2 | 2 | 2 | 3 | 2 | 25 | 4 | 4 |
| 11 | 1 | 1 | 1 | 2 | 1 | 90 | 2 | 1 |
| 12 | 1 | 1 | 1 | 1 | 2 | 90 | 1 | 1 |
| 13 | 1 | 1 | 2 | 2 | 1 | 90 | 1 | 1 |
| 14 | 1 | 1 | 1 | 2 | 1 | 92 | 1 | 1 |
| Median | 1 | 1 | 2 | 2 | 2 | 85 | ||
| IQR | 1–2 | 1 | 1–2 | 1.5–2 | 1–2 | 43.75–90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwake, M.; Ricchizzi, S.; Krahwinkel, S.; Maragno, E.; Schipmann, S.; Stummer, W.; Gallus, M.; Holling, M. Resection of Intramedullary Hemangioblastoma: Timing of Surgery and Its Impact on Neurological Outcome and Quality of Life. Medicina 2023, 59, 1611. https://doi.org/10.3390/medicina59091611
Schwake M, Ricchizzi S, Krahwinkel S, Maragno E, Schipmann S, Stummer W, Gallus M, Holling M. Resection of Intramedullary Hemangioblastoma: Timing of Surgery and Its Impact on Neurological Outcome and Quality of Life. Medicina. 2023; 59(9):1611. https://doi.org/10.3390/medicina59091611
Chicago/Turabian StyleSchwake, Michael, Sarah Ricchizzi, Sophia Krahwinkel, Emanuele Maragno, Stephanie Schipmann, Walter Stummer, Marco Gallus, and Markus Holling. 2023. "Resection of Intramedullary Hemangioblastoma: Timing of Surgery and Its Impact on Neurological Outcome and Quality of Life" Medicina 59, no. 9: 1611. https://doi.org/10.3390/medicina59091611
APA StyleSchwake, M., Ricchizzi, S., Krahwinkel, S., Maragno, E., Schipmann, S., Stummer, W., Gallus, M., & Holling, M. (2023). Resection of Intramedullary Hemangioblastoma: Timing of Surgery and Its Impact on Neurological Outcome and Quality of Life. Medicina, 59(9), 1611. https://doi.org/10.3390/medicina59091611






