Abstract
Background and Objectives: This study confirms the possibility of using Rhus verniciflua Stokes (RVS) extract as a natural treatment for oral candidiasis. Materials and Methods: RVS was extracted with 70% ethanol to examine the antioxidant activity through polyphenol, flavonoid content, and DPPH (1,1-diphenyl-2-picrylhydrazyl). To evaluate the antifungal effect against Candida albicans (C. albicans; KCTC 7965/ATCC 10231) and evaluate the stability of RVS, a water-soluble tetrazolium salt (WST-1) assay was performed in human keratinocytes (HaCaT). Results: The findings revealed that RVS extract has fairly high antioxidant activity. The clear zones of the RVS extract against C. albicans increased in diameter due to the inhibition of fungal growth at higher concentrations. Treatment with the 1.25 mg/mL RVS extract had a more than 99% antifungal effect against C. albicans, and the 20 mg/mL RVS extract had a 100% antifungal effect. The WST-1 assay showed that the RVS extract induced low cell viability in the HaCaT cells, which inhibited their proliferation, and the RVS extract is also toxic to normal cells. Conclusions: Although the RVS extract with high antioxidant activity showed clear antifungal activity against C. albicans, it exhibited a low survival rate. Therefore, the development of a safe natural antibiotic is necessary.
1. Introduction
The oral cavity is an important organ in the human body, exposed to many microorganisms because it communicates directly with the outside world and offers environmental conditions suitable for the growth of bacteria, resulting in various oral diseases [1]. Moreover, as the importance of oral health has increased, especially due to the rise in mask-wearing as a result of viral infections, interest in managing bacteria in the mouth has increased [2]. Candida accounts for 70–90% of fungal infections that can occur in humans and can have fatal consequences when acquired in intensive care units, with a mortality rate of 40% [3]. In cases of long-term use of antibiotics or reduced immunity, intra-oral resident bacteria cause opportunistic infections. Among them, oral candidiasis occurs when yeast-type fungi of the genus Candida overgrow in the oral cavity [4]. Types of Candida include C. albicans, C. tropicalis, C. glabrata, C. pseudotropicalis, C. guillierimondii, C. krusei, C. lusitaniae, C. parapsilosis, and C. stellatoidea [4]. More than 80% of Candida are isolated as C. albicans, C. glabrata, and C. tropicalis [5]. C. albicans usually exists in the oral cavity and normally does not cause problems in healthy people [4]. In ordinary people, the carriage rate without symptoms is known to be 20–75% [6], but the incidence rate of oral candidiasis induced by C. albicans has been reported to be 30–45% in healthy adults [7]. The incidence rate of oral candidiasis caused by C. albicans isolated from the oral cavity is 54% in two- to six-week-olds, 46% for one-year-olds, 39% for one- to six-year-olds [8], and 30–45% in healthy adults [7]. In recent years, higher incidences of the above-mentioned non-C. albicans Candida (NCAC) species have also been reported [9]. In particular, for all cancer treatments, the weighted prevalence of oral colonization with fungal organisms was 48.2% before treatment, 72.2% during treatment, and 70.1% after treatment [10,11].
The incidence of oral candidiasis was 50–65% in patients using removable dentures and 65–88% in patients receiving long-term treatment for acute diseases [7,12]. Local factors that induce candidiasis include denture use, salivary malfunction, steroid inhalation, and oral cancer. The most common contributing factors include age, smoking, diabetes, Cushing’s syndrome, decreased immunity, malignant tumors, nutritional deficiencies, and antibiotic use [13]. Symptoms of oral candidiasis cause problems with oral tissues, such as the mucous membrane and tongue in the oral cavity, leading to discomfort, burning pain, dysgeusia, and dysphagia during chewing [14]. The first line of defense against oral candidiasis infection is innate immunity, which relies on immune cells (e.g., macrophages, dendritic cells, neutrophils, natural killer cells, monocytes, etc.) to defend against invasion [15]. During fungal infection, innate immune cells can recognize fungal PAMPs via PRRs to inhibit, phagocytose, and kill the fungus while initiating the innate immune response against pathogens [16]. Oral candidiasis can be naturally cured if the causative factor can be removed, and innate immunity can be enhanced. However, in most cases, antifungal treatment is required. Representative drugs for antifungal treatment of oral candidiasis are polyene-based and azole-based drugs [17]. Polyene-based drugs are not absorbed into the gastrointestinal tract, so they are either applied to the oral mucosa or taken orally. However, these drugs are difficult to use due to the discomfort of frequently applying them and their unpleasant taste. In many cases, the drugs are not sufficiently applied to the lesion because of obstructing structures in the oral cavity, such as the tongue, palate, gum, and teeth, or because they are diluted by the saliva of the patients [17]. Moreover, natural ingredients are in the spotlight because they have fewer side effects than chemical substances and, unlike chemical substances, do not cause resistance, mutation, and cytotoxicity [18]. Promising antifungal agents extracted from natural compounds isolated from plants are currently attracting much attention as potential cures for various diseases and as sources of new drugs [19]. Therefore, studies are being actively conducted to develop natural products or food-derived natural antifungal agents that outperform synthetic antifungal agents in terms of safety and effectiveness [20]. Medicinal plants from orders such as Acorales Mart., Apiales Nakai, Asterales Link, Lamiales Bromhead, La-urales Juss. ex Bercht. and J. Presl, Myrtales Juss. ex Bercht. and J. Presl, Poales Small, and Sapindales Juss. ex Bercht. and J. Presl have exhibited strong anti-biofilm activity against Candida species [21]. Among them, Rhus verniciflua Stokes (RVS) is an Asian tree species in the Anacardiaceae family. It is used in East Asian countries such as Korea, Japan, and China as a traditional herbal medicine for gastrointestinal diseases and pain relief in various diseases such as cancer [22]. RVS contains several compounds, such as quercetin, fustin, fisetin, sulferetin, and butane [23], which are reported to have antioxidant, anti-proliferative, anti-inflammatory, and anti-tumor effects [24]. Specifically, RVS has anti-tumor effects in the stomach, breast, liver, lymph nodes, and bones [25]. The mechanism underlying the anti-tumor effect of RVS is known as the activation of AMP-activated protein kinase (AMPK) [26], cell cycle arrest [27], activation of manganese superoxidase or reduction in glutathione content [28], and activation of caspase [16]. Despite the pharmacological effect of RVS, its use is very limited because it contains an allergen called urushiol. Various detoxification methods for RVS have been developed to remove its urushiol content [29]. However, since continuous use of natural drugs may expose patients to potential dangers, they should be carefully applied after ensuring their stability [30]. Posadzki et al. [31] reported that 19 out of 50 natural drugs had moderate or severe adverse events. Other studies claimed that hepatotoxicity or renal toxicity occurred frequently due to the toxic effects of natural drugs [32]. The potential risks of using natural drugs can arise from their contamination, incorporation, misrecognition, interaction with other drugs, and inherent toxicity. Therefore, for the safe use of natural drugs, they must undergo safety evaluation and quality control [33]. This study was conducted to confirm the possibility of applying a natural plant with an antioxidant effect and evaluate the antifungal effect(s) of RVS extract against C. albicans and its effect on cell viability. This study also provides data on animal experiments to confirm the potential of RVS as an antifungal agent for patients with oral candidiasis.
2. Materials and Methods
2.1. Extract Preparation
RVS-producing areas are located in Gyeongsan, Gyeongsangbuk, Republic of Korea, and were purchased from Cheongmyeong Co., Ltd. (Chungju, Republic of Korea). Ethanol extract was obtained via a rotary vacuum evaporator (N-1300E.V.S., Tokyo Rikakikai Co., Ltd. (EYELA), Tokyo, Japan) after filtration by adding 70% ethanol to the pulverized RVS at 60 °C for 12 h. RVS powder samples were obtained by freeze-drying through dehydration.
2.2. Assay for Total Polyphenol Content
The total polyphenol content was determined using the modified Folin-Denis method [34]. This involved adding 0.15 mL of Foline-Ciocalteau’s phenol reagent (Sigma-Aldrich, St. Louis, MO, USA) and 0.3 mL of 20% Na2CO3 (Daejung chemicals & Metals Co., LTD., Gyeonggi, Republic of Korea) into 1 mg/mL RVS extract. The mixture was left to react for 2 h in a dark room at room temperature, after which the absorbance was measured at 725 nm using a spectrophotometer (Thermo Scientific Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA). Gallic acid (Sigma-Aldrich Co., St. Louis, MO, USA) was used as a reference material to calculate the total polyphenol content of RVS extract and was expressed in terms of gallic acid equivalents (mg GAE/g extract). Average values were calculated from the triplicate experiment.
2.3. Assay for Total Flavonoid Content
The total flavonoid content was determined using the modified Davis WB’s method [35]. This involved mixing 0.7 mL of diethylene glycol with 0.1 mL of RVS extract and adding 0.1 mL of 1 N NaOH solution. The mixture was left to react for 1 h in a dark room at room temperature, after which the absorbance was measured at 420 nm using a spectrophotometer (Thermo Scientific Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA). Catechin (Sigma-Aldrich Co., St. Louis, MO, USA) was used as a reference material to calculate the total flavonoid content of RVS extract, expressed in terms of catechin equivalents (mg CE/g extract). Average values were calculated from triplicate experiments.
2.4. Measurement of DPPH Radical Scavenging Activity
DPPH (1,1-diphenyl-2-picrylhydrazyl, Sigma Chemical Co., St. Louis, MO, USA) radical scavenging activity was evaluated using the modified Sharma and Bhat’s method [36]. This involved adding 0.2 mL of RVS extract to 0.8 mL of 0.2 mM DPPH solution. The mixture was left to react in a dark room for 30 min, after which the absorbance was measured at 517 nm using a spectrophotometer (Thermo Scientific Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA). Sterile distilled water was added as a control instead of the sample, gallic acid was used as a positive control, and the radical scavenging activity was expressed as a percentage (%). The antioxidant activity was expressed by obtaining the electron-donating ability (%) using the formula [1 − (sample absorbance/control absorbance)] × 100. Average values were calculated from the triplicate experiment.
2.5. Fungal Cultures
The C. albicans (KCTC 7965/ATCC 10231) were incubated in yeast mold broth (YM, Difco, MI, USA) and cultivated at 37 °C for 24 h. Then, it was inoculated with the C. albicans cultured with 1 × 105 colony-forming units per milliliter (CFU/mL).
2.6. Disc Diffusion Method
To confirm the antifungal effect of RVS, 100 μL of each concentration was dropped onto a paper disc (Ø8 mm; Advantec Toyo Kaisha, Ltd., Tokyo, Japan) of YM agar medium and aerobically incubated at 37 °C for 24 h. The diameter of the clear zone around was measured with the soaked filter paper discs using a ruler. Experiments were performed in triplicate.
2.7. Antifungal Activity
To measure the fungal proliferation, the extracts were prepared with RSV concentrations of 1.25, 2.5, 5, 10, and 20 mg/mL, which were inoculated with a 100 µL solution with C. albicans. To use an equal amount of C. albicans, the culture absorbance was maintained at 1.0 at a wavelength of 660 nm. The mixture was cultured aerobically at 37 °C for 24 h and diluted. A precise amount of it was spread onto a YM agar medium. The CFU was counted after incubation for 24 h, and the results of all colonies appearing on the YM agar medium were recorded. The data were based on triplicated experiments.
2.8. Cell Proliferation Assay
A water-soluble tetrazolium salt (WST-1) assay was performed [37] to quantify the effect of RVS on cell growth. HaCaT cells used in this experiment were purchased from the Department of Oral Anatomy, Pusan National University School of Dentistry (Yangsan, Republic of Korea). HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (USA), with 10% (v/v) heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (Gibco, Grand Island, NY, USA) at 37 °C and with 5% CO2 in a humidified chamber. The experiments were performed in 96-well plates that contained a final volume of 100 μL of the medium/well. The HaCaT cells were seeded at an initial density of 1 × 105 cells/cm2 and incubated at 37 °C for 24 h. The medium was changed to serum-free DMEM that contained the indicated concentrations of RVS (1.25, 2.5, 5, 10, and 20 mg/mL). After 3 h, the incubation medium was removed and replaced with the WST-1 solution. The plates were incubated at 37 °C for 2 h, and an ELISA reader (Multiskan FC, Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the 450 nm absorption. The cell viability was confirmed by optical density, and the same process was repeated thrice.
2.9. Statistical Analysis
Statistical analysis was performed using a statistical software (SPSS v. 24.0, SPSS Inc., Chicago, IL, USA) to evaluate the antifungal activity of RVS through one-way ANOVA and Duncan’s test at a significance level of 0.05.
3. Results
3.1. Antioxidant Activity Assays
Analysis of the total polyphenol content, total flavonoid content, and DPPH radical scavenging activity at RVS extract 1 mg/mL concentration showed that the total polyphenol content was 501.405 mg GAE/g, the total flavonoid content was 1634.153 mg CE/g, and the DPPH radical scavenging activity was 72.228% (Table 1).

Table 1.
Total phenolic content (mg GAE/g extract), flavonoid content (mg CE/g extract), and DPPH radical scavenging activity (%) of RVS extract. Values are the mean ± SD, which were triplicated (n = 3).
3.2. Antifungal Activity and Growth Inhibition of C. albicans
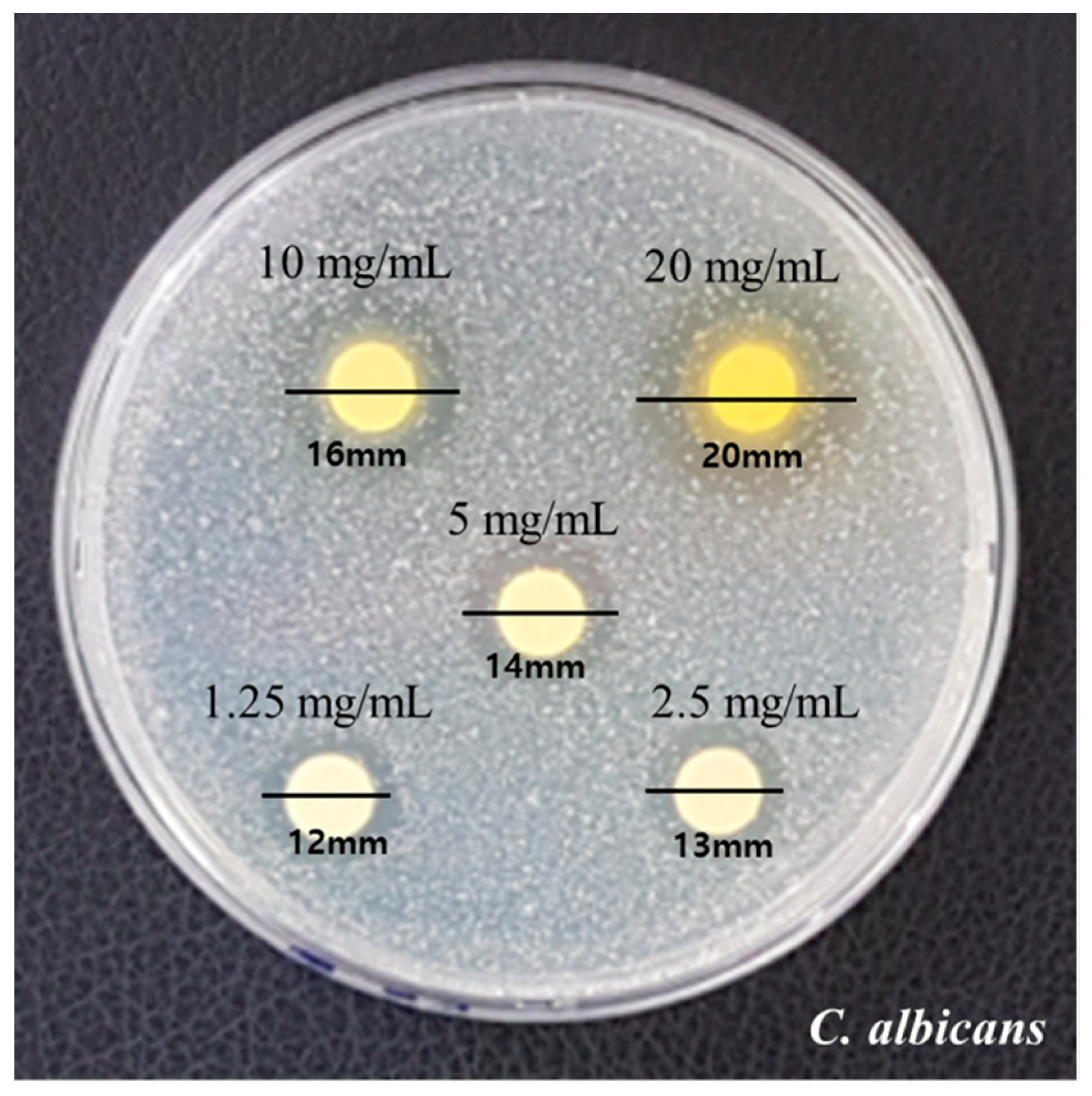
The measured diameters by the disk diffusion method are presented in Figure 1. Growth inhibition showed clear zones with diameters 12 mm, 13 mm, and 14 mm at concentrations of 1.25 mg/mL, 2.5 mg/mL, and 5 mg/mL, respectively. Diameters of 16 mm and 20 mm were observed at 10 mg/mL and 20 mg/mL, respectively. The fungal growth and inhibition area increased as the concentration of RVS extract increased.
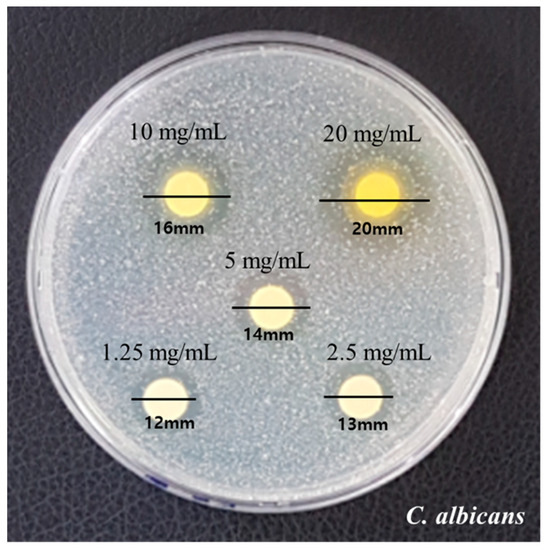
Figure 1.
Results of the clear zone of RVS extract against C. albicans.
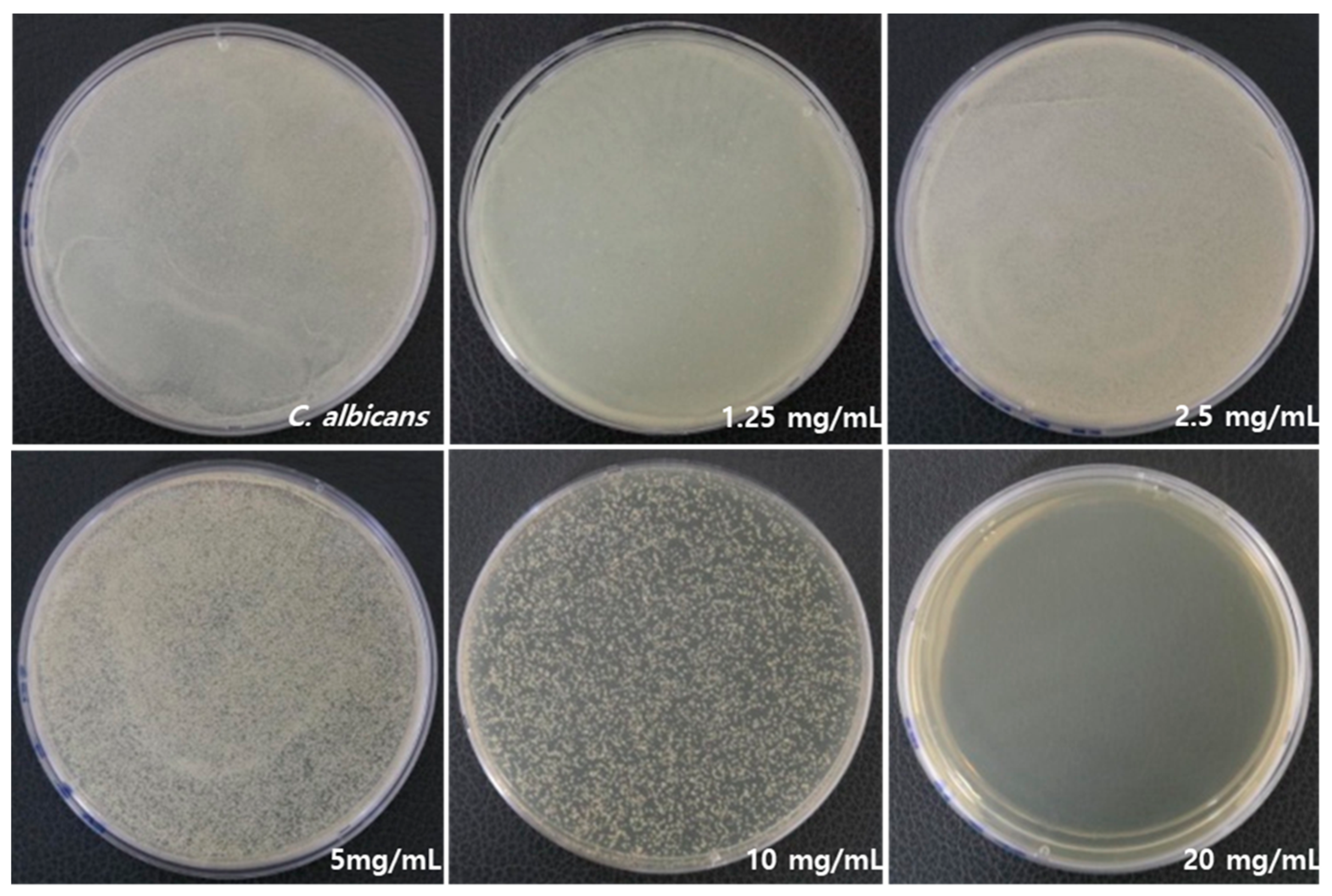
Figure 2 illustrates a significant difference in antifungal activity against C. albicans. RVS extract had an antifungal effect, and no fungal growth was observed at 20 mg/mL. Table 2 lists the mean and standard deviation (SD) values of C. albicans count, which were 2.5 ± 0.1 109. As the concentration of RVS extract increased, higher growth inhibition was observed. The minimal fungicidal concentration (MFC) was determined to be 1.25 mg/mL of RVS extract, and 99% of the fungi were killed compared to the control C. albicans as a result of CFU.
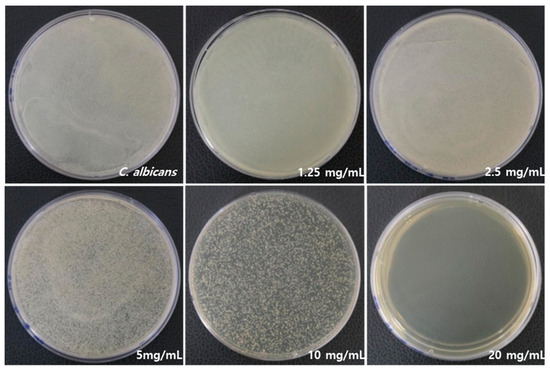
Figure 2.
Inhibitory effects on the growth of C. albicans by RVS extract at concentrations of 1.25, 2.5, 5, 10, and 20 mg/mL.

Table 2.
Mean ± S.D CFU values for the antifungal effect of C. albicans according to the RVS extract concentration (Unit: CFU/mL).
3.3. RVS Extract on the Growth of HaCaT Cells
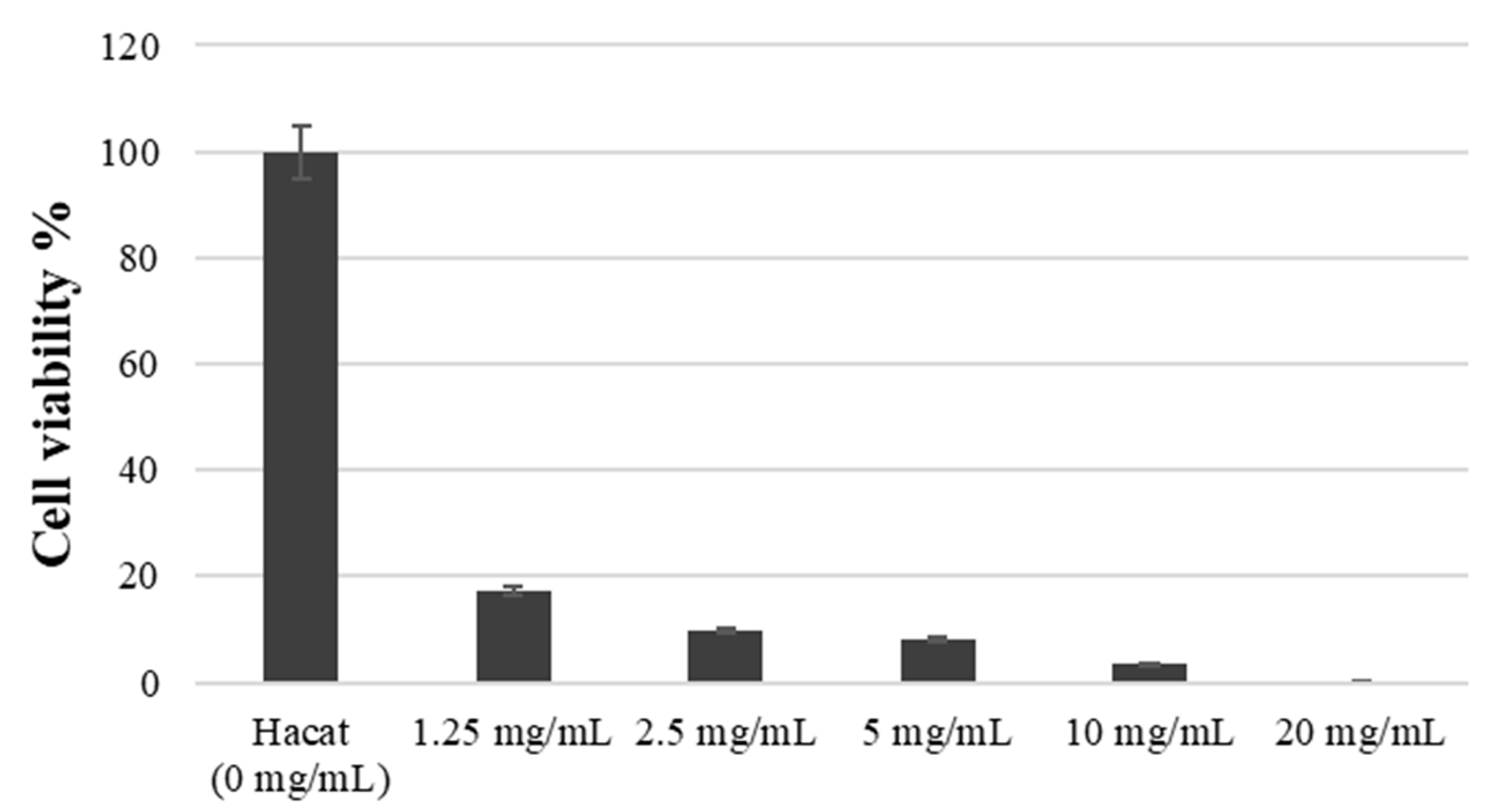
The number of viable cells by RVS extract was assessed using WST-1 assay. Figure 3 demonstrates the analysis results, with the absorbance value of the control group set at 100%. After exposure to concentrations of 1.25 mg/mL, 2.5 mg/mL, 5 mg/mL, 10 mg/mL, and 20 mg/mL for 3 h, cell viability was measured as 17.21 ± 2.02, 9.87 ± 3.01, 7.96 ± 2.10, 3.35 ± 1.73, 0.15 ± 0.05, respectively (p < 0.05). As depicted in Figure 3, the RVS extract induced HaCaT cell death at concentrations ranging from 1.25 to 20 mg/mL, indicating a significant reduction in cell viability.
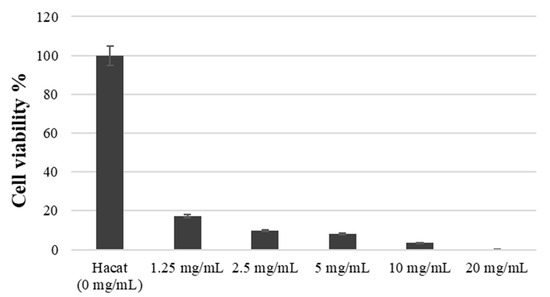
Figure 3.
Cell viability at various treatment concentrations of RVS extract by the WST-1 assay.
4. Discussion
Oral health is a multifaceted aspect of well-being that affects individuals physically, mentally, and socially. It needs to be approached as a part of general health and is an essential element in social and daily life [38]. The increase in life expectancy has led to a growing interest in quality of life, particularly oral health, which is closely related to dietary habits [39]. Therefore, effective oral health management is essential to prevent oral diseases and address their underlying causes [40].
C. albicans is an opportunistic fungus present in the human oral cavity and can be converted into disease-causing hyphae under nutrient-rich conditions [41]. C. albicans has an organic relationship with S. aureus, which strengthens bacterial colonization and biofilm formation, providing a scaffold for bacterial biofilm development. Thus, C. albicans survives even after antibiotic treatment and infects or destroys mucosal tissues [42]. It is eliminated with topical antifungal treatments. However, Nystatin oral solution and clotrimazole tablets have high concentrations of sugar, which may cause dental caries [43]. Fluconazole or itraconazole is known to be effective when patients are at risk of systemic fungal infections or do not respond to topical drug treatments. Nevertheless, these drugs have clinical limitations due to their discomfort, unpleasant taste, and the risk of developing azole-resistant strains when used repeatedly [44]. Therefore, research on alternative products continues, considering that natural substances isolated from plants used in traditional medicine are good alternatives to synthetic chemicals. Interest in adverse substances with environmentally friendly functions is increasing [45]. Recently, studies on disease treatment and prevention using natural products and the development of health-functional materials have been actively conducted [46]. Therefore, the viability of natural antioxidants is evaluated by measuring antioxidant compounds and their activity. Various physiological activities, such as antioxidant, antifungal, and anti-inflammatory properties, of phenolic compounds and flavonoids present in large amounts in plants are evaluated [47]. Phenolic compounds, which are secondary metabolites widely distributed in nature, typically exhibit physiological activities such as antioxidant effects as the total polyphenol content increases [48]. In addition, flavonoids, which are mainly present in plants, are highly active antioxidants and are known to have antiviral, anti-inflammatory, and anticancer effects similar to polyphenols [49]. DPPH is a substance with chemically stabilized free radicals and is reduced by substances such as ascorbic acid, tocopherol, polyhydroxy aromatic compounds, leading to the decolorization of its dark purple color. This is an extensively used method to measure antioxidant activity by determining the electron-donating ability of antioxidants derived from various natural materials [50]. The results of analyzing the antioxidant activity of RVS extract in this study reveal that the total polyphenol content was 501.405 mg GAE/g, the total flavonoid content was 1634.153 mg CE/g, and the DPPH radical scavenging activity was 72.228%. These values indicate a similar activity to the 78.508% activity of the control substance, gallic acid. According to Sim WS et al. [51], who studied the antioxidant activity of nine domestic forest plants, Geranium thunbergii showed the highest total polyphenol content at 303.94 ± 0.63 mg GAE/g, while Vitis ficifolia was found to have the highest flavonoid content at 279.00 ± 4.58 mg CE/g. These results indicate that RVS extract has a high polyphenol and flavonoid content. Although the polyphenol content and flavonoid content of natural products differ depending on their type, they generally exhibit anti-inflammatory properties. In particular, the RVS extract used in this study is a natural substance known for improving the oral health and has excellent anti-inflammatory properties. Therefore, these results are promising, suggesting the possibility of its application in oral health products. In addition, compared to a study [52] reporting that Osmunda japonica’s DPPH scavenging activity was 67.29%, the RVS extract demonstrated remarkably high radical scavenging activity. The RVS extract was also confirmed to have excellent antioxidant activity by effectively inhibiting the presence of reactive oxygen species. The RVS extract will be able to suppress body aging by activating the antioxidant defense system or scavenging active oxygen. This study confirmed the antifungal effect of RVS extract against C. albicans. The observed zone size in the disk diffusion test indicated that the higher the concentration of the RVS extract, the wider the clear zone. The antifungal activity of RVS extract exhibited more than 99% inhibition rate at a concentration of 1.25 mg/mL. Similar to the results of this study, Shin et al. [53] used Rubus coreanus Miquel and reported that it had a strong growth inhibitory effect of over 90% at 60 mg/mL. In addition, Choi et al. [54] reported that C. albicans was not detected at 50 mg/mL when applying Acanthopanax sessiliflorum extract. While the natural extract used in their study is excellent for inhibiting the growth of C. albicans, the RVS extract used in this study, even at lower concentrations, was effective in killing C. albicans.
Nevertheless, the peel of RVS extract mainly contains urushiol, an allergic compound, which limits its medical use [55]. HaCaT cells are derived from adult skin and are commonly used in scientific research as oral epithelial cells [56]. In this study, verifying the low cell viability using HaCaT cells was necessary to identify the effect of specific fungi using natural materials. Therefore, the application of RVS to HaCaT cells confirmed a significantly reduced survival rate. Consequently, while the RVS extract exhibited excellent antifungal properties against C. albicans, it also led to a decrease in cell viability.
Chelerythrine, extracted from Bocconia Houttuynia cordata Thunb, is an effective natural drug against C. albicans and possesses various properties, such as anti-cancer, anti-inflammatory, insecticide, and anti-fibrotic activities [57]. In addition, several other natural drugs, such as Perilla frutescence, Radix pulsatilla (Bai Tou Weng), Cortex phellodendri (Huang Bai), Rhizoma coptidis (Huang Lian), Cortex fraxini (Qin Pi), Coptis chinensis Franch, Phellodendron chinense C.K.Schneid, Paeonia suffruticosa, Paeonia Suffruticoas Andr, Magnolia officinalis, Dioscorea nipponica Makino, and Houttuynia cordata Thunb (Saururaceae family), are involved in innate immunity against C. albicans [58]. As such, studies on the antifungal efficacy of various natural drugs effective against C. albicans are being actively conducted, but studies on their cytotoxicity should also be conducted to ensure their safety. Therefore, this study is valuable because it confirmed the antioxidant activity of RVS extract, the growth inhibition effect against C. albicans, and the low survival rate against HaCaT cells.
The current study’s findings provide fundamental research data and suggest the possibility of developing antioxidant materials. However, this study has limitations. Firstly, anti-Candida properties were not confirmed using various methods. Therefore, further studies are needed to confirm the effect of RVS on biofilm formation and the morphological conversion of C. albicans. In addition, investigating reactive oxygen species (ROS) production, adenosine triphosphate (ATP) consumption, RNA extraction, and quantitative RT-PCR analysis is required to identify the mechanism underlying the potential antifungal effect. Secondly, the safety of the RVS extract was not secured using more diverse normal cells. Accordingly, it is essential to identify various cell types, such as human normal oral keratinocytes, human gingival fibroblasts, and B16 F10 mouse melanoma cells, in cytotoxicity experiments to ensure safety. Thirdly, comparing the results of this study with existing studies should be done cautiously because there is a difference in the experimental method. Therefore, analyzing the inhibitory effect of C. albicans by extending the period to 48, 72, and 24 h and exploring various RVS extraction methods are needed to determine the most effective extraction method. Fourthly, since this study is conducted in a laboratory setting using bacterial cultures, it is difficult to generalize the research results because the human oral environment and conditions are not the same. Consequently, before RVS extract is used to treat oral candidiasis, its efficacy as a natural antifungal agent, and its non-toxicity, it is crucial to conduct extensive animal studies. In addition, after the safety of RVS is secured, a clinical study on the risk of recurrence of fungal infection should be conducted with an extended observation period in the future. The potential usability of RVS extract in the pharmaceutical industry can be realized through further research on its functionality and safety.
5. Conclusions
The study’s results confirmed that RVS extract had a more than 99% antifungal effect against C. albicans at a concentration of 1.25 mg/mL. Moreover, HaCaT cells, which are oral epithelial cells, showed cytotoxicity, and only 17.21% of them survived at a concentration of 1.25 mg/mL. Therefore, while RVS extract inhibits the growth of C. albicans, the survival rate of HaCaT cells is low. Hence, further research is needed to identify its appropriate concentration for its safe and effective use.
Author Contributions
Conceptualization, S.-H.N. and G.-C.K.; data curation, Y.-R.K.; methodology, Y.-R.K. and S.-H.N.; resources, S.-H.N. and G.-C.K.; supervision, S.-H.N. and G.-C.K.; validation, Y.-R.K., S.-H.N. and G.-C.K.; writing the original draft, S.-H.N. and Y.-R.K.; writing review and editing, S.-H.N. and G.-C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, S.L.; Kim, J.G. Anti-microbial activity of soybean extract against oral microbes. Korean J. Environ. Health 2006, 32, 192–197. [Google Scholar]
- Park, J.H.; Jang, J.E.; Choi, Y.H. The impact of the COVID-19 pandemic on oral health behavior and oral symptoms in young adults. J. Korean Acad. Oral Health 2021, 45, 192–197. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensive Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Patton, L.L.; Dongari-Bagtzoglou, A. Oral candidiasis: Pathogenesis, clinical presentation, diagnosis and treatment strategies. J. Calif. Dent. Assoc. 2013, 41, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Radwan, S.S. Candida Adherence to Epithelial Cells; CRC Press: Boca Raton, FL, USA, 1990; pp. 105–169. [Google Scholar]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Barnett, J.A. A history of research on yeasts 12: Medical yeasts part 1, Candida albicans. Yeast 2008, 25, 385–417. [Google Scholar] [CrossRef]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3, 5771. [Google Scholar] [CrossRef]
- Jham, B.C.; Franca, E.C.; Oliveira, R.R.; Santos, V.R.; Kowalski, L.P.; da Silva Freire, A.R. Candida oral colonization and infection in Brazilian patients undergoing head and neck radiotherapy: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 355–358. [Google Scholar] [CrossRef]
- Belazi, M.; Velegraki, A.; Koussidou-Eremondi, T.; Andreadis, D.; Hini, S.; Arsenis, G.; Eliopoulou, C.; Destouni, E.; Antoniades, D. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: Prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol. Immunol. 2004, 19, 347–351. [Google Scholar] [CrossRef]
- Aldred, M.J.; Addy, M.; Bagg, J.; Finlay, I. Oral health in the terminally ill: A cross-sectional pilot survey. Spec. Care Dent. 1991, 11, 59–62. [Google Scholar] [CrossRef]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker Lee, D.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Cushing, A.M.; Sheiham, A.; Maizels, J. Developing socio-dental indicators-the social impact of dental disease. Community Dent. Health 1986, 3, 3–17. [Google Scholar] [PubMed]
- Zhou, Y.; Cheng, L.; Lei, Y.L.; Ren, B.; Zhou, X. The interactions between Candida albicans and mucosal immunity. Front. Microbiol. 2021, 12, 652725. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; Ifrim, D.C.; Quintin, J.; Netea, M.G.; van de Veerdonk, F.L. Antifungal innate immunity: Recognition and inflammatory networks. Semin. Immunopathol. 2015, 37, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ellepola, A.N.; Samaranayake, L.P. Oral candidal infections and antimycotics. Crit. Rev. Oral Biol. Med. 2000, 11, 172–198. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef]
- Byun, E.B.; Kim, M.J.; Kim, S.J.; Oh, N.S.; Park, S.H.; Kim, W.S.; Byun, E.H. Antioxidant activity and neuroprotective effects of ethanol extracts from the core of Diospyros kaki. Korean J. Food Sci. Technol. 2020, 52, 60–66. [Google Scholar]
- Singla, R.K.; Dubey, A.K. Molecules and Metabolites from Natural Products as Inhibitors of Biofilm in Candida spp. Pathogens. Curr. Top. Med. Chem. 2019, 19, 2567–2578. [Google Scholar] [CrossRef]
- Jang, I.S.; Park, J.W.; Jo, E.B.; Cho, C.K.; Lee, Y.W.; Yoo, H.S.; Park, J.S.; Kim, J.H.; Jang, B.C.; Choi, J.S. Growth inhibitory and apoptosis-inducing effects of allergen-free Rhus verniciflua stokes extract on A549 human lung cancer cells. Oncol. Rep. 2016, 36, 3037–3043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, K.W.; Um, E.S.; Jung, B.B.; Choi, E.S.; Kim, E.Y.; Lee, S.; Jang, E.Y.; Lee, J.H.; Kim, Y. Rhus verniciflua stokes extract induces inhibition of cell growth and apoptosis in human chronic myelogenous leukemia K562 cells. Oncol. Rep. 2018, 39, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.W.; Lee, C.; Jin, Q.; Lee, M.K.; Lee, C.K.; Lee, M.K.; Hwang, B.Y. Flavonol glycosides from the aerial parts of Gynostemma pentaphyllum and their antioxidant activity. Arch. Pharm. Res. 2016, 39, 1232–1236. [Google Scholar] [CrossRef]
- Choi, W.C.; Jung, H.S.; Kim, K.S.; Lee, S.K.; Yoon, S.W.; Park, J.H.; Kim, J.H.; Cheon, S.H.; Eo, W.K.; Lee, S.H. Rhus verniciflua stokes against advanced cancer: A perspective from the Korean Integrative Cancer Center. J. Biomed. Biotechnol. 2012, 2012, 874276. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Moon, J.W.; Lee, S.K.; Kim, S.M.; Kim, N.; Ko, S.G.; Kim, H.S.; Park, S.H. Rhus verniciflua extract modulates survival of MCF-7 breast cancer cells through the modulation of AMPK-pathway. Biol. Pharm. Bull. 2014, 37, 794–801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.H.; Kim, H.P.; Jung, C.H.; Hong, M.H.; Hong, M.C.; Bae, H.S.; Lee, S.D.; Park, S.Y.; Park, J.H.; Ko, S.G. Inhibition of cell cycle progression via p27Kip1 upregulation and apoptosis induction by an ethanol extract of Rhus verniciflua stokes in AGS gastric cancer cells. Int. J. Mol. Med. 2006, 18, 201–208. [Google Scholar] [CrossRef][Green Version]
- Son, Y.O.; Lee, K.Y.; Lee, J.C.; Jang, H.S.; Kim, J.G.; Jeon, Y.M.; Jang, Y.S. Selective antiproliferative and apoptotic effects of flavonoids purified from Rhus verniciflua stokes on normal versus transformed hepatic cell lines. Toxicol. Lett. 2005, 155, 115–125. [Google Scholar] [CrossRef]
- Choi, H.S.; Yeo, S.H.; Jeong, S.T.; Choi, J.H.; Park, H.S.; Kim, M.K. Preparation and characterization of urushiol free fermented Rhus verniciflua stem bark (FRVSB) extracts. Korean J. Food Sci. Technol. 2012, 44, 173–178. [Google Scholar] [CrossRef]
- Jordan, S.A.; Cunningham, D.G.; Marles, R.J. Assessment of herbal medicinal products: Challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol. Appl. Pharmacol. 2010, 243, 198–216. [Google Scholar] [CrossRef]
- Posadzki, P.; Watson, L.K.; Ernst, E. Adverse effects of herbal medicines: An overview of systematic reviews. Clin. Med. 2013, 13, 7–12. [Google Scholar] [CrossRef]
- Elinav, E.; Pinsker, G.; Safadi, R.; Pappo, O.; Bromberg, M.; Anis, E.; Keinan-Boker, L.; Broide, E.; Ackerman, Z.; Kaluski, D.N.; et al. Association between consumption of Herbalife® nutritional supplements and acute hepatotoxicity. J. Hepatol. 2007, 47, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wider, B.; Shang, H.; Li, X.; Ernst, E. Quality of herbal medicines: Challenges and solutions. Complement. Ther. Med. 2012, 20, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Denis, W. On Phospaotungastic-phosohomdybdic Compounds as Color Reagents. J. Biol. Chem. 1912, 12, 239–249. [Google Scholar] [CrossRef]
- Davis, W.B. Determination of flavanones in citrus fruits. Anal. Chem. 1947, 19, 476–478. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1201–1205. [Google Scholar] [CrossRef]
- Lee, S.N. The antioxidant effect of rutin in human dermal fibroblasts damaged by reactive oxygen species. Korean J. Aesthet. Cosmetol. 2014, 12, 831–836. [Google Scholar]
- Richmond, S.; Chestnutt, I.; Shennan, J.; Brown, R. The relationship of medical and dental factors to perceived general and dental health. Community Dent. Oral Epidemiol. 2007, 35, 89–97. [Google Scholar] [CrossRef]
- Lee, H.K.; Son, K.B.; Lee, S.K.; Park, J.H.; Choi, Y.H. Association between tooth loss and cardiovascular risk indicators in the Korean elderly. J. Korean Acad. Dent. Health 2008, 32, 495–503. [Google Scholar]
- Park, C.S.; Kim, Y.I.; Jang, S.H. A study on the status of recognition, understanding of the use and practical application of oral hygiene devices in dental clinics patients. J. Korean Soc. Dent. Hyg. 2009, 9, 685–698. [Google Scholar]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial interactions: Impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharikova, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 2016, 7, e01365-16. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Polsky, B. Oropharyngeal candidiasis: A review of its clinical spectrum and current therapies. Clin. Ther. 1998, 20, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Rautemaa, R.; Richardson, M.; Pfaller, M.; Perheentupa, J.; Saxen, H. Reduction of fluconazole susceptibility of Candida albicans in APECED patients due to long-term use of ketoconazole and miconazole. Scand. J. Infect. Dis. 2008, 40, 904–907. [Google Scholar] [CrossRef]
- Prabu, G.R.; Gnanamani, A.; Sadulla, S. Guaijaverin—A plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006, 101, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.R.; Kang, C.H.; Bu, H.J. Antioxidant and anti-inflammatory activity of extracts from red beet (Beta vulagaris) root. Korean J. Food Preserv. 2017, 24, 413–420. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, H.J. Study on the bioactive characteristics of Morinda citrifolia as a cosmetic raw material. J. Soc. Cosmet. Sci. Korean 2016, 42, 183–193. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K. The stimulation of phenolics and antioxidant activity in pea (Pisum sativum) elicited by genetically transformed anise root extract. J. Food Biochem. 2001, 25, 361–377. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Kang, M.H.; Choi, C.S.; Kim, Z.S.; Chung, H.K.; Min, K.S.; Park, C.G.; Park, H.W. Antioxidative Activities of Ethanol Extract Prepared from Leaves, Seed, Branch and Aerial Part of Crotalaria sessiflora L. Korean J. Food Sci. 2002, 34, 1098–1102. [Google Scholar]
- Sim, W.S.; Lee, J.S.; Lee, S.R.; Choi, S.I.; Cho, B.Y.; Choi, S.H.; Xionggao, H.; Jang, G.W.; Kwon, H.Y.; Choi, Y.E.; et al. Antioxidant effect of extracts from 9 Species of Forest Plants in Korea. J. Food Hyg. Saf. 2019, 34, 404–411. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.Y.; Yu, M.R.; Kim, M.S.; Lee, S.H.; Lee, B.H. Total Polyphenols, Total Flavonoid Contents, and Antioxidant Activity of Korean Natural and Medicinal Plant. Korean J. Food Sci. Technol. 2012, 44, 337–342. [Google Scholar] [CrossRef]
- Shin, A.R.; Ohk, S.H.; Choi, C.H.; Hong, S.J. Growth inhibition effect of Rubus coreanus miquel on Candida albicans. J. Korean Acad. Oral Health 2015, 39, 168–173. [Google Scholar] [CrossRef][Green Version]
- Choi, Y.S.; Kim, N.H.; Kim, H.J.; An, S.J.; Lee, B.N.; Jung, M.J.; Hwang, J.Y.; Nam, S.H. A study on the antibacterial effect of Acanthopanax sessiliflorum on inflammatory diseases in the oral cavity. Biomed. Res. 2017, 28, 8376–8380. [Google Scholar]
- Jang, J.Y.; Shin, H.; Lim, J.W.; Ahn, J.H.; Jo, Y.H.; Lee, K.Y.; Hwang, B.Y.; Jung, S.J.; Kang, S.Y.; Lee, M.K. Comparison of antibacterial activity and phenolic constituents of bark, lignum, leaves and fruit of Rhus verniciflua. PLoS ONE 2018, 13, e0200257. [Google Scholar] [CrossRef]
- Aidoukovitch, A.; Bodahl, S.; Tufvesson, E.; Nilsson, B.O. Desquamated epithelial cells of unstimulated human whole saliva express both EGF transcropt and protein. Int. J. Dent. 2022, 2022, 3194703. [Google Scholar] [CrossRef]
- Niu, X.F.; Zhou, P.; Li, W.F.; Xu, H.B. Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia 2011, 82, 620–625. [Google Scholar] [CrossRef]
- Bao, M.Y.; Li, M.; Bu, Q.R.; Yang, Y.; Song, H.; Wang, C.Z.; Wang, T.M.; Li, N. The effect of herbal medicine in innate immunity to Candida albicans. Front. Immunol. 2023, 14, 1096383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).