IgE-Mediated and Non-IgE-Mediated Fish Allergy in Pediatric Age: A Holistic Approach—A Consensus by Diagnostic Commission of the Italian Society of Pediatric Allergy and Immunology
Abstract
:1. Introduction
2. Allergenic Source
2.1. Taxonomic Classification
2.2. Fish Muscle
2.3. Fish Roe
2.4. Fish Gelatin
3. Epidemiology
3.1. Epidemiology of IgE-Mediated Fish Allergy: Self-Reported Data
3.2. Epidemiology of IgE-Mediated Fish Allergy: In Vivo or In Vitro Diagnostic Tests
3.3. Epidemiology of IgE-Mediated Fish Allergy: In Vivo or In Vitro Diagnostic Tests
3.4. FPIES Induced by Fish: Epidemiological Data
4. Pathogenesis and Clinical Features
4.1. IgE-Mediated Pediatric Fish Allergy
4.2. Non-IgE-Mediated Pediatric Fish Allergy
4.3. Clinical Clusters of IgE-Mediated Fish Allergy
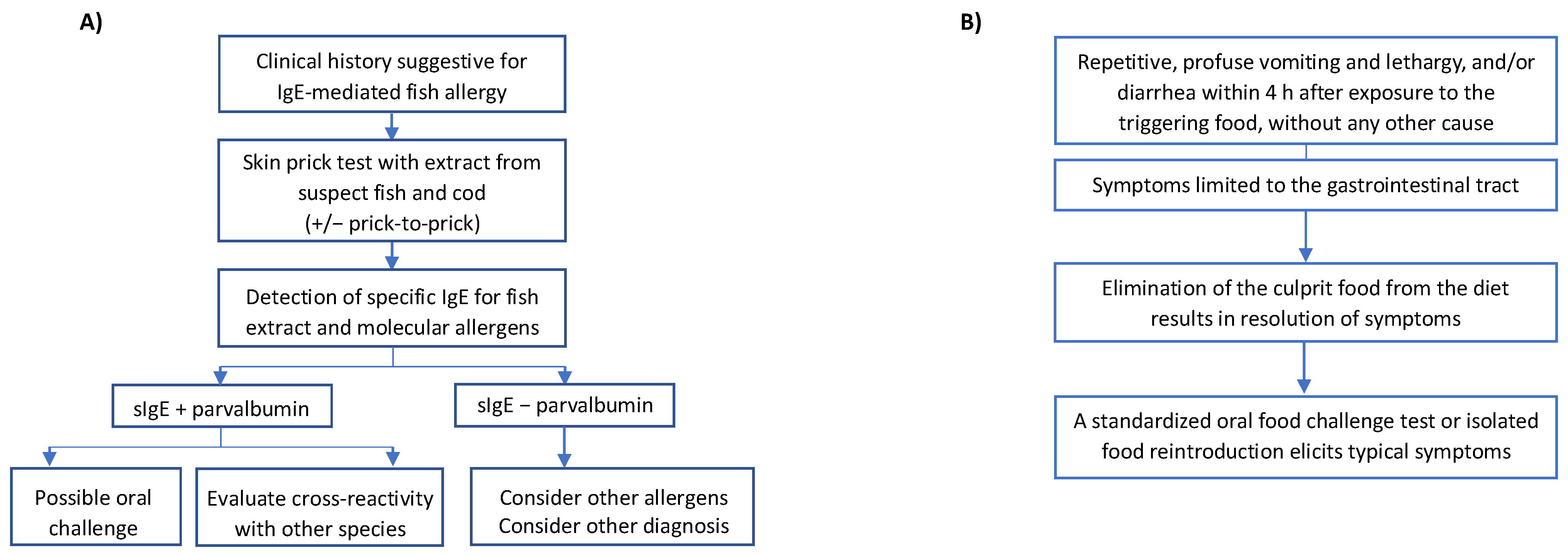
5. Diagnosis
6. Fish Allergens and Cross-Reactivity
7. Differential Diagnosis
- -
- Anisakiasis: Infestation by the parasite Anisakis (Nematode), with clinical manifestations mainly in the gastrointestinal tract. Requires ingestion of live parasites. Then, it is contracted only after consuming raw, undercooked, or pickled fish [78]. Within a few hours from ingestion, gastrointestinal symptoms occur (abdominal pain, vomiting, malnutrition). A severe eosinophilic granulomatous response can appear if larvae pass into the bowel. Diagnosis needs a gastroscopic examination by visualization of larvae which must be removed or biopsy with tissue histopathologic examination.
- -
- Allergic reaction to Anisakis: IgE-mediated reaction to Anisakis due to sensitization of nematode proteins, which infest various fish species. The clinical presentation is not distinguishable from a fish allergy, but IgE is not directed against the fish but against the parasite proteins. Anisakis-specific prick tests and serum-specific IgE have been identified [79,80].
- -
- Scombroid poisoning: The most common cause of toxicosis caused by seafood products worldwide. It is due to the ingestion of poorly preserved fish (most frequently red meat fish such as Scombroidae and Scomberesocidae, including mackerel, bonito, albacore, and tuna), in which the excessive bacterial growth allows the conversion of histidine into histamine. Clinical symptoms mimic allergic reactions in rapid onset (about 30 min after ingestion) and objectivity (e.g., urticaria, oral allergy syndrome, nausea and vomiting, and, in rare cases, anaphylaxis). Patients who habitually have no history of fish allergy may report oral tingling sensation, rash spreading to the face and trunk from top to bottom, and a metallic taste upon ingesting the culprit fish. Generally, the same symptoms and signs are reported by other diners who consume the same food [81,82]. Symptoms usually resolve within 24 h. Laboratory tests of dosing histamine levels from fish and patient’s plasma can help defining the diagnosis.
- -
- Toxic Algae Poisoning: Adverse reaction caused by fish contaminated by toxic algae consumed by themselves. Clinical reactions are various and depend on the toxin involved: e.g., Ciguatera poisoning, due to the ciguatoxin, which is more commonly found in tropical fish (grouper, eel, mackerel), can present with skin (urticaria), gastrointestinal (nausea, vomiting), neurological (blurred vision, paresthesia, ataxia, convulsions), and cardiovascular symptoms (bradycardia/tachycardia, hypotension/hypertension, conduction block) [83,84].
- -
- Bacterial/viral contamination: Poisoning caused by eating fish farmed or harvested from contaminated waters (e.g., by hepatitis A virus, Shigella spp., Salmonella spp.). It can mainly trigger gastrointestinal clinical manifestations several hours after ingestion, usually accompanied by fever [3].
- -
- Seafood intolerance: Adverse reaction due to vasoactive biogenic amines in fish (histamine and tyramine), remarkably, if it is canned or pickled fish, or due to fish autolysis [85,86,87]. It usually presents with severe headache. It involves histamine as scombroid poisoning; however, clinical characteristics differ.
8. Clinical Management
8.1. Clinical Management of IgE-Mediated Fish Allergy
8.2. Clinical Management of Non-IgE-Mediated Fish Allergy
9. Nutritional Considerations
10. Prognosis and Natural History
10.1. Prognosis of IgE-Mediated Fish Allergy
10.2. Prognosis of Non-IgE-Mediated Fish Allergy
10.3. Prognosis and Natural History of IgE- and Non-IgE-Mediated Fish Allergy: Key Points
11. Unmet Needs and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klueber, J.; Schrama, D.; Rodrigues, P.M.; Dickel, H.; Kuehn, A. Fish Allergy Management: From Component-Resolved Diagnosis to Unmet Diagnostic Needs. Curr. Treat. Options Allergy 2019, 6, 322–337. [Google Scholar] [CrossRef]
- Peixoto, S.; Monteiro, T.; Carvalho, M.; Santos, M.; Matos, C.; Bartolomé, B.; Labrador-Horrillo, M.; Quaresma, M. Vertebrate Tropomyosin as an Allergen. J. Investig. Allergol. Clin. Immunol. 2018, 28, 51–53. [Google Scholar] [CrossRef]
- Ruethers, T.; Taki, A.C.; Johnston, E.B.; Nugraha, R.; Le, T.T.K.; Kalic, T.; McLean, T.R.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, A.; Scheuermann, T.; Hilger, C.; Hentges, F. Important variations in parvalbumin content in common fish species: A factor possibly contributing to variable allergenicity. Int. Arch. Allergy Immunol. 2010, 153, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tsabouri, S.; Triga, M.; Makris, M.; Kalogeromitros, D.; Church, M.K.; Priftis, K.N. Fish and shellfish allergy in children: Review of a persistent food allergy. Pediatr. Allergy Immunol. 2012, 23, 608–615. [Google Scholar] [CrossRef]
- Maciag, M.C.; Bartnikas, L.M.; Sicherer, S.H.; Herbert, L.J.; Young, M.C.; Matney, F.; Westcott-Chavez, A.A.; Petty, C.R.; Phipatanakul, W.; Bingemann, T.A. A Slice of Food Protein–Induced Enterocolitis Syndrome (FPIES): Insights from 441 Children with FPIES as Provided by Caregivers in the International FPIES Association. J. Allergy Clin. Immunol. Pract. 2020, 8, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Mehr, S.; Frith, K.; Barnes, E.H.; Campbell, D.E.; Allen, K.; Gold, M.; Joshi, P.; Kakakios, A.; Loh, R.; Peake, J.; et al. Food protein–induced enterocolitis syndrome in Australia: A population-based study, 2012–2014. J. Allergy Clin. Immunol. 2017, 140, 1323–1330. [Google Scholar] [CrossRef]
- Ruiz-García, M.; Díez, C.E.; García, S.S.; del Rio, P.R.; Ibáñez, M.D. Diagnosis and natural history of food protein-induced enterocolitis syndrome in children from a tertiary hospital in central Spain. J. Investig. Allergol. Clin. Immunol. 2014, 24, 354–356. [Google Scholar]
- Douros, K.; Tsabouri, S.; Feketea, G.; Grammeniatis, V.; Koliofoti, E.G.; Papadopoulos, M.; Sardeli, O.; Triga, M.; Priftis, K.N. Retrospective study identified fish and milk as the main culprits in cases of food protein-induced enterocolitis syndrome. Acta Paediatr. 2019, 108, 1901–1904. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Kitsioulis, N.A.; Manousakis, E.; Manolaraki, I.; Douladiris, N.; Papadopoulos, N.G. Remission Patterns of Food Protein-Induced Enterocolitis Syndrome in a Greek Pediatric Population. Int. Arch. Allergy Immunol. 2019, 180, 113–119. [Google Scholar] [CrossRef]
- Miceli Sopo, S.; Giorgio, V.; Dello Iacono, I.; Novembre, E.; Mori, F.; Onesimo, R. A multicenter retrospective study of 66 Italian children with food protein-induced enterocolitis syndrome: Different management for different phenotypes. Clin. Exp. Allergy 2012, 42, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Monaco, S.; Badina, L.; Barni, S.; Longo, G.; Novembre, E.; Viola, S.; Monti, G. Food protein-induced enterocolitis syndrome caused by fish and/or shellfish in Italy. Pediatr. Allergy Immunol. 2015, 26, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; García, V.; Rial, M.J.; Novoa, E.; Cacharron, T. Fish is a major trigger of solid food protein–induced enterocolitis syndrome in Spanish children. J. Allergy Clin. Immunol. Pract. 2015, 3, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Lagousi, T.; Hatzopoulou, E.; Korovessi, P.; Kostaridou, S.; Mermiri, D.-Z. Atypical Food protein-induced enterocolitis syndrome in children: Is IgE sensitization an issue longitudinally? Allergol. Immunopathol. 2021, 49, 73–82. [Google Scholar] [CrossRef]
- Vazquez-Ortiz, M.; Machinena, A.; Dominguez, O.; Alvaro, M.; Calvo-Campoverde, K.; Giner, M.T.; Jiménez-Feijoo, R.; Lozano, J.; Piquer, M.; Dias, M.; et al. Food protein–induced enterocolitis syndrome to fish and egg usually resolves by age 5 years in Spanish children. J. Allergy Clin. Immunol. Pract. 2017, 5, 512–515.e1. [Google Scholar] [CrossRef]
- Alonso, S.B.; Ezquiaga, J.G.; Berzal, P.T.; Tardón, S.D.; José, M.M.S.; López, P.A.; Bermejo, T.B.; Teruel, S.Q.; Zudaire, L.Á.E. Food protein–induced enterocolitis syndrome: Increased prevalence of this great unknown—Results of the PREVALE study. J. Allergy Clin. Immunol. 2018, 143, 430–433. [Google Scholar] [CrossRef]
- Díaz, J.J.; Espín, B.; Segarra, O.; Domínguez-Ortega, G.; Blasco-Alonso, J.; Cano, B.; Rayo, A.; Moreno, A.; on behalf of the Gastrointestinal Allergy Working Group of the Spanish Society of Pediatric Gastroenterology, Hepatology and Nutrition (SEGHNP). Food Protein-induced Enterocolitis Syndrome: Data from a Multicenter Retrospective Study in Spain. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 232–236. [Google Scholar] [CrossRef]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de Las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2023, 34 (Suppl. S28), e13854. [Google Scholar] [CrossRef]
- Hanaoka, K.; Takahagi, S.; Ishii, K.; Nakano, M.; Chinuki, Y.; Tanaka, A.; Yanase, Y.; Hide, M. Type-I-hypersensitivity to 15 kDa, 28 kDa and 54 kDa proteins in vitellogenin specific to Gadus chalcogrammus roe. Allergol. Int. 2020, 69, 253–260. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Toda, M.; Ebihara, T.; Irie, S.; Hori, H.; Imai, A.; Yanagida, M.; Miyazawa, H.; Ohsuna, H.; Ikezawa, Z.; et al. IgE antibody to fish gelatin (type I collagen) in patients with fish allergy. J. Allergy Clin. Immunol. 2000, 106, 579–584. [Google Scholar] [CrossRef]
- Caglayan-Sozmen, S.; Santoro, A.; Cipriani, F.; Mastrorilli, C.; Ricci, G.; Caffarelli, C. Hazardous Medications in Children with Egg, Red Meat, Gelatin, Fish, and Cow’s Milk Allergy. Medicina 2019, 55, 501. [Google Scholar] [CrossRef] [PubMed]
- Kalic, T.; Kamath, S.D.; Ruethers, T.; Taki, A.C.; Nugraha, R.; Le, T.T.K.; Humeniuk, P.; Williamson, N.A.; Hira, D.; Rolland, J.M.; et al. Collagen-An Important Fish Allergen for Improved Diagnosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3084–3092.e10. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.J.; Carder, M.; Money, A.; Evans, G.; Seed, M.; Agius, R.; van Tongeren, M. Occupational Asthma and Its Causation in the UK Seafood Processing Industry. Ann. Work. Expo. Health 2020, 64, 817–825. [Google Scholar] [CrossRef]
- Moonesinghe, H.; Mackenzie, H.; Venter, C.; Kilburn, S.; Turner, P.; Weir, K.; Dean, T. Prevalence of fish and shellfish allergy: A systematic review. Ann. Allergy Asthma Immunol. 2016, 117, 264–272.e4. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Fernandez-Perez, C.; Jedrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitisation and Food Allergy in Children Across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef]
- Lyons, S.A.; Burney, P.G.J.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Clausen, M.; Dubakiene, R.; Fernandez-Perez, C.; Fritsche, P.; Jedrzejczak-Czechowicz, M.; et al. Food Allergy in Adults: Substantial Variation in Prevalence and Causative Foods Across Europe. J. Allergy Clin. Immunol. Pract. 2019, 7, 1920–1928.e11. [Google Scholar] [CrossRef] [PubMed]
- Buyuktiryaki, B.; Masini, M.; Mori, F.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Giovannini, M.; du Toit, G.; Lopata, A.L.; et al. IgE-Mediated Fish Allergy in Children. Medicina 2021, 57, 76. [Google Scholar] [CrossRef]
- Pyrhönen, K.; Näyhä, S.; Kaila, M.; Hiltunen, L.; Läärä, E. Occurrence of parent-reported food hypersensitivities and food allergies among children aged 1–4 yr. Pediatr. Allergy Immunol. 2009, 20, 328–338. [Google Scholar] [CrossRef]
- Kajosaari, M. Food allergy in Finnish children aged 1 to 6 years. Acta Paediatr. Scand. 1982, 71, 815–819. [Google Scholar] [CrossRef]
- Dalal, I.; Binson, I.; Reifen, R.; Amitai, Z.; Shohat, T.; Rahmani, S.; Levine, A.; Ballin, A.; Somekh, E. Food allergy is a matter of geography after all: Sesame as a major cause of severe IgE-mediated food allergic reactions among infants and young children in Israel. Allergy 2002, 57, 362–365. [Google Scholar] [CrossRef]
- Al-Hammadi, S.; Al-Maskari, F.; Bernsen, R. Prevalence of food allergy among children in Al-Ain city, United Arab Emirates. Int. Arch. Allergy Immunol. 2010, 151, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Eggesbø, M.; Halvorsen, R.; Tambs, K.; Botten, G. Prevalence of parentally perceived adverse reactions to food in young children. Pediatr. Allergy Immunol. 1999, 10, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Muñoz-Furlong, A.; Sampson, H.A. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 2004, 114, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011, 128, e9–e17. [Google Scholar] [CrossRef]
- Chiang, W.C.; Kidon, M.I.; Liew, W.K.; Goh, A.; Tang, J.P.; Chay, O.M. The changing face of food hypersensitivity in an Asian community. Clin. Exp. Allergy 2007, 37, 1055–1061. [Google Scholar] [CrossRef]
- Connett, G.J.; Gerez, I.; Cabrera-Morales, E.A.; Yuenyongviwat, A.; Ngamphaiboon, J.; Chatchatee, P.; Sangsupawanich, P.; Soh, S.E.; Yap, G.C.; Shek, L.P.; et al. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int. Arch. Allergy Immunol. 2012, 159, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Laoaraya, M.; Trakultivakorn, M. Prevalence of food allergy among preschool children in northern Thailand. Pediatr. Int. 2012, 54, 238–243. [Google Scholar] [CrossRef]
- Le, T.T.K.; Nguyen, D.H.; Vu, A.T.L.; Ruethers, T.; Taki, A.C.; Lopata, A.L. A cross-sectional, population-based study on the prevalence of food allergies among children in two different socio-economic regions of Vietnam. Pediatr. Allergy Immunol. 2019, 30, 348–355. [Google Scholar] [CrossRef]
- Obeng, B.B.; Amoah, A.S.; Larbi, I.A.; Yazdanbakhsh, M.; van Ree, R.; Boakye, D.A.; Hartgers, F.C. Food allergy in Ghanaian schoolchildren: Data on sensitization and reported food allergy. Int. Arch. Allergy Immunol. 2011, 155, 63–73. [Google Scholar] [CrossRef]
- von Hertzen, L.; Mäkelä, M.J.; Petäys, T.; Jousilahti, P.; Kosunen, T.U.; Laatikainen, T.; Vartiainen, E.; Haahtela, T. Growing disparities in atopy between the Finns and the Russians: A comparison of 2 generations. J. Allergy Clin. Immunol. 2006, 117, 151–157. [Google Scholar] [CrossRef]
- Pereira, B.; Venter, C.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J. Allergy Clin. Immunol. 2005, 116, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Pénard-Morand, C.; Raherison, C.; Kopferschmitt, C.; Caillaud, D.; Lavaud, F.; Charpin, D.; Bousquet, J.; Annesi-Maesano, I. Prevalence of food allergy and its relationship to asthma and allergic rhinitis in schoolchildren. Allergy 2005, 60, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Allen, K.J.; Ho, M.H.; Li, H. The prevalence of food allergy in infants in Chongqing, China. Pediatr. Allergy Immunol. 2011, 22, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.F.; Pascual, C.; Burks, A.W.; Helm, R.M.; Esteban, M.M. Frequency of food allergy in a pediatric population from Spain. Pediatr. Allergy Immunol. 1995, 6, 39–43. [Google Scholar] [CrossRef]
- Osterballe, M.; Hansen, T.K.; Mortz, C.G.; Høst, A.; Bindslev-Jensen, C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr. Allergy Immunol. 2005, 16, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Pereira, B.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: A population-based study. Pediatr. Allergy Immunol. 2006, 17, 356–363. [Google Scholar] [CrossRef]
- Orhan, F.; Karakas, T.; Cakir, M.; Aksoy, A.; Baki, A.; Gedik, Y. Prevalence of immunoglobulin E-mediated food allergy in 6-9-year-old urban schoolchildren in the eastern Black Sea region of Turkey. Clin. Exp. Allergy 2009, 39, 1027–1035. [Google Scholar] [CrossRef]
- Kristinsdóttir, H.; Clausen, M.; Ragnarsdóttir, H.S.; Halldórsdóttir, I.H.; McBride, D.; Beyer, K.; Sigurdardóttir, S.T. Prevalence of food allergy in Icelandic infants during first year of life. Laeknabladid 2011, 97, 11–18. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef]
- Pascual, C.Y.; Reche, M.; Fiandor, A.; Valbuena, T.; Cuevas, T.; Esteban, M.M. Fish allergy in childhood. Pediatr. Allergy Immunol. 2008, 19, 573–579. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Untersmayr, E.; Klems, M.; Jensen-Jarolim, E. The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients 2018, 10, 1129. [Google Scholar] [CrossRef]
- Untersmayr, E.; Vestergaard, H.; Malling, H.J.; Jensen, L.B.; Platzer, M.H.; Boltz-Nitulescu, G.; Scheiner, O.; Skov, P.S.; Jensen-Jarolim, E.; Poulsen, L.K. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J. Allergy Clin. Immunol. 2007, 119, 711–717. [Google Scholar] [CrossRef]
- Lopata, A.L.; Zinn, C.; Potter, P.C. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J. Allergy Clin. Immunol. 1997, 100, 642–648. [Google Scholar] [CrossRef]
- Mullins, R.J.; Wainstein, B.K.; Barnes, E.H.; Liew, W.K.; Campbell, D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2016, 46, 1099–1110. [Google Scholar] [CrossRef]
- Patton, T.; Chugh, A.; Padhye, L.; DeGeeter, C.; Guandalini, S. Pediatric Celiac Disease and Eosinophilic Esophagitis: Outcome of Dietary Therapy. J. Pediatr. Gastroenterol. Nutr. 2019, 69, e43–e48. [Google Scholar] [CrossRef]
- Vinit, C.; Dieme, A.; Courbage, S.; Dehaine, C.; Dufeu, C.M.; Jacquemot, S.; Lajus, M.; Montigny, L.; Payen, E.; Yang, D.D.; et al. Eosinophilic esophagitis: Pathophysiology, diagnosis, and management. Arch. Pediatr. 2019, 26, 182–190. [Google Scholar] [CrossRef]
- Sasaki, A.; Sugimoto, M.; Tokaji, N.; Irahara, M.; Okamoto, K.; Uehara, H.; Kagami, S. Efficacy of an elimination diet in a patient with eosinophilic gastroenteritis: A pediatric case with multiple food allergies. J. Med. Investig. 2019, 66, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Berin, M.C.; Mehr, S. Food Protein-Induced Enterocolitis Syndrome. J. Allergy Clin. Immunol. Pract. 2020, 8, 24–35. [Google Scholar] [CrossRef] [PubMed]
- González-Delgado, P.; Caparrós, E.; Moreno, M.V.; Clemente, F.; Flores, E.; Velásquez, L.; Rubio, G.; Fernández, J. Clinical and immunological characteristics of a pediatric population with food protein-induced enterocolitis syndrome (FPIES) to fish. Pediatr. Allergy Immunol. 2016, 27, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Xepapadaki, P.; Christopoulou, G.; Stavroulakis, G.; Freidl, R.; Linhart, B.; Zuidmeer, L.; Lakoumentas, J.; van Ree, R.; Valenta, R.; Papadopoulos, N.G. Natural History of IgE-Mediated Fish Allergy in Children. J. Allergy Clin. Immunol. Pract. 2021, 9, 3147–3156.e5. [Google Scholar] [CrossRef]
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847–852.e7. [Google Scholar] [CrossRef]
- Lee, L.A.; Burks, A.W. Food allergies: Prevalence, molecular characterization, and treatment/prevention strategies. Annu. Rev. Nutr. 2006, 26, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Ho, D.G. Relationship between food specific IgE concentrations and the risk of positive food challenges in children and adolescents. J. Allergy Clin. Immunol. 1997, 100, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.; Kuehn, A.; Mills, E.N.C.; Costello, C.A.; Ollert, M.; Småbrekke, L.; Primicerio, R.; Wickman, M.; Klingenberg, C. Cross-reactivity in fish allergy: A double-blind, placebo-controlled food-challenge trial. J. Allergy Clin. Immunol. 2017, 140, 1170–1172. [Google Scholar] [CrossRef]
- Ruethers, T.; Taki, A.C.; Nugraha, R.; Cao, T.T.; Koeberl, M.; Kamath, S.D.; Williamson, N.A.; O’Callaghan, S.; Nie, S.; Mehr, S.S.; et al. Variability of allergens in commercial fish extracts for skin prick testing. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1352–1363. [Google Scholar] [CrossRef]
- Aibinu, I.E.; Smooker, P.M.; Lopata, A.L. Anisakis Nematodes in Fish and Shellfish- from infection to allergies. Int. J. Parasitol. Parasites Wildl. 2019, 9, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Buyuktiryaki, B.; Santos, A.F. Food allergy severity predictions based on cellular in vitro tests. Expert. Rev. Mol. Diagn. 2020, 20, 679–692. [Google Scholar] [CrossRef]
- Hemmings, O.; Kwok, M.; McKendry, R.; Santos, A.F. Basophil Activation Test: Old and New Applications in Allergy. Curr. Allergy Asthma Rep. 2018, 18, 77. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Leung, N.; Wang, L.X.; Lisann, L.; Sicherer, S.H.; Scurlock, A.M.; Pesek, R.; Perry, T.T.; Jones, S.M.; et al. Correlations between basophil activation, allergen-specific IgE with outcome and severity of oral food challenges. Ann. Allergy Asthma Immunol. 2015, 114, 319–326. [Google Scholar] [CrossRef]
- Imakiire, R.; Fujisawa, T.; Nagao, M.; Tokuda, R.; Hattori, T.; Kainuma, K.; Kawano, Y. Basophil Activation Test Based on CD203c Expression in the Diagnosis of Fish Allergy. Allergy Asthma Immunol. Res. 2020, 12, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Dickel, H.; Kuehn, A.; Dickel, B.; Bauer, A.; Becker, D.; Fartasch, M.; Haeberle, M.; John, S.M.; Mahler, V.; Skudlik, C.; et al. Assessment of the effects of a work-related allergy to seafood on the reduction of earning capacity in the context of BK No. 5101. Allergol. Select 2021, 5, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Bonlokke, J.H.; Bang, B.; Aasmoe, L.; Rahman, A.M.A.; Syron, L.N.; Andersson, E.; Dahlman-Höglund, A.; Lopata, A.L.; Jeebhay, M. Exposures and Health Effects of Bioaerosols in Seafood Processing Workers—A Position Statement. J. Agromedicine 2019, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, A.; Hilger, C.; Lehners-Weber, C.; Codreanu-Morel, F.; Morisset, M.; Metz-Favre, C.; Pauli, G.; de Blay, F.; Revets, D.; Muller, C.P.; et al. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: Component resolved diagnosis using parvalbumin and the new allergens. Clin. Exp. Allergy 2013, 43, 811–822. [Google Scholar] [CrossRef]
- Ruethers, T.; Taki, A.C.; Karnaneedi, S.; Nie, S.; Kalic, T.; Dai, D.; Daduang, S.; Leeming, M.; Williamson, N.A.; Breiteneder, H.; et al. Expanding the allergen repertoire of salmon and catfish. Allergy 2021, 76, 1443–1453. [Google Scholar] [CrossRef]
- Kuehn, A.; Hilger, C.; Hentges, F. Anaphylaxis provoked by ingestion of marshmallows containing fish gelatin. J. Allergy Clin. Immunol. 2009, 123, 708–709. [Google Scholar] [CrossRef]
- Ueno, R.; Takaoka, Y.; Shimojo, N.; Ohno, F.; Yamaguchi, T.; Matsunaga, K.; Kameda, M. A case of pediatric anaphylaxis caused by gummy tablets containing fish collagen. Asia Pac. Allergy 2020, 10, e35. [Google Scholar] [CrossRef]
- Baird, F.J.; Gasser, R.B.; Jabbar, A.; Lopata, A.L. Foodborne anisakiasis and allergy. Mol. Cell Probes 2014, 28, 167–174. [Google Scholar] [CrossRef]
- Bernardini, R.; Lombardi, E.; Novembre, E.; Ingargiola, A.; Pucci, N.; Favilli, T.; Vierucci, A. Predictors of Anisakis simplex symptoms. Allergy 2000, 55, 979–980. [Google Scholar] [CrossRef]
- Tripodi, S.; Pingitore, G.; Calvani, M.; Scala, G.; Rodriguez-Perez, R.; Sfika, I.; Asero, R. Anisakis Sensitivity in Italian Children: A Prospective Study. J. Investig. Allergol. Clin. Immunol. 2017, 27, 142–143. [Google Scholar] [CrossRef]
- Guergué-Díaz de Cerio, O.; Barrutia-Borque, A.; Gardeazabal-García, J. Scombroid Poisoning: A Practical Approach. Actas Dermosifiliogr. 2016, 107, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Teuber, S.; Gershwin, M.E. Histamine (Scombroid) Fish Poisoning: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wjst, M. Ciguatera: A multifaceted and puzzling disorder. MMW Fortschr. Med. 2016, 158, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Gopal, T.K. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef]
- Sharp, M.F.; Lopata, A.L. Fish allergy: In review. Clin. Rev. Allergy Immunol. 2014, 46, 258–271. [Google Scholar] [CrossRef]
- Dello Iacono, I.; Limongelli, M.G.; Parente, C.; Varricchio, E.; Basilicata, A.M.; Vetrano, G. Allergia al pesce e cross-reazioni. RIAP 2010, 2, 14–20. [Google Scholar]
- Kuehn, A.; Hutt-Kempf, E.; Hilger, C.; Hentges, F. Clinical monosensitivity to salmonid fish linked to specific IgEepitopes on salmon and trout beta-parvalbumins. Allergy 2011, 66, 299–301. [Google Scholar] [CrossRef]
- Kalic, T.; Morel-Codreanu, F.; Radauer, C.; Ruethers, T.; Taki, A.C.; Swoboda, I.; Hilger, C.; Hoffmann-Sommergruber, K.; Ollert, M.; Hafner, C.; et al. Patients allergic to fish tolerate ray based on the low allergenicity of its parvalbumin. J. Allergy Clin. Immunol. Pract. 2019, 7, 500–508.e11. [Google Scholar] [CrossRef]
- Lim, D.L.; Neo, K.H.; Goh, D.L.; Shek, L.P.; Lee, B.W. Missing parvalbumin: Implications in diagnostic testing for tuna allergy. J. Allergy Clin. Immunol. 2005, 115, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, L.; Infante, S.; Fuentes-Aparicio, V.; Cabrera-Freitag, P.; Antonucci, N.; Alvarez-Perea, A. IgE-mediated fish allergy in pediatric age: Does canned tuna have a chance for tolerance? Pediatr. Allergy Immunol. 2021, 32, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Kourani, E.; Corazza, F.; Michel, O.; Doyen, V. What Do We Know About Fish Allergy at the End of the Decade? J. Investig. Allergol. Clin. Immunol. 2019, 29, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Infante, S.; Marco, G.; Sánchez-Domínguez, M.; Rodríguez-Fernández, A.; Fuentes-Aparicio, V.; Alvarez-Perea, A.; Cabrera-Freitag, P.; Morales-Cabeza, C.; Zubeldia, J.M.; Zapatero, L. Food protein-induced enterocolitis syndrome by fish: Not necessarily a restricted diet. Allergy 2017, 73, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; McKenzie, R. Nutritional Issues in Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 166–178. [Google Scholar] [CrossRef]
- Agostoni, C.; Nobile, M.; Ciappolino, V.; Delvecchio, G.; Tesei, A.; Turolo, S.; Crippa, A.; Mazzocchi, A.; Altamura, C.A.; Brambilla, P. The Role of Omega-3 Fatty Acids in Developmental Psychopathology: A Systematic Review on Early Psychosis, Autism, and ADHD. Int. J. Mol. Sci. 2017, 18, 2608. [Google Scholar] [CrossRef]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep. Technol. Assess. 2016, 224, 1–826. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Shrestha, S.K.; Itsiopoulos, C.; Erbas, B. The role of fish intake on asthma in children: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 350–360. [Google Scholar] [CrossRef]
- Mark, B.J.; Beaty, A.D.; Slavin, R.G. Are fish oil supplements safe in finned fish-allergic patients? Allergy Asthma Proc. 2008, 29, 528–529. [Google Scholar] [CrossRef]
- Savage, J.; Sicherer, S.; Wood, R. The Natural History of Food Allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203, quiz 204. [Google Scholar] [CrossRef]
- Carvalho, S.; Marcelino, J.; Cabral Duarte, M.F.; Costa, C.; Barbosa, M.A.; Pereira Dos Santos, M.C. Role of Recombinant Parvalbumin Gad c 1 in the Diagnosis and Prognosis of Fish Allergy. J. Investig. Allergol. Clin. Immunol. 2020, 30, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Ng, I.; Kemp, A.; Campbell, D. Seafood allergy in children: A descriptive study. Ann. Allergy Asthma Immunol. 2011, 106, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Feketea, G.; Vassilopoulou, E.; Geropanta, F.; Berghea, E.C.; Bocsan, I.C. Alternative Fish Species for Nutritional Management of Children with Fish-FPIES-A Clinical Approach. Nutrients 2021, 14, 19. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Feketea, G.; Konstantinou, G.N.; Zekakos Xypolias, D.; Valianatou, M.; Petrodimopoulou, M.; Vourga, V.; Tasios, I.; Papadopoulos, N.G. Food Protein-Induced Allergic Proctocolitis: The Effect of Maternal Diet During Pregnancy and Breastfeeding in a Mediterranean Population. Front. Nutr. 2022, 9, 843437. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Country | Fish as Offending Food (%) | N | Age, Range–Mean |
|---|---|---|---|---|
| Maciag, 2020 [6] | Australia, Austria, Brazil, Egypt, Germany, Italy, UK, USA, Qatar | 9.3 | 441 | 1.25–4 y (2 y) |
| Mehr, 2017 [7] | Australia | 5 | 230 | 7–14 m (10 m) |
| Ruiz-Garcìa, 2017 [8] | Spain | 31 | 16 | 11 m–12 y (50.6 m) |
| Douros, 2019 [9] | Greece | 54 | 78 | 10.1–12.2 m (11.1) |
| Xepapadaki, 2019 [10] | Greece | 34.7 | 72 | 12.1–19.5 m (15.8) |
| Miceli Sopo, 2012 [11] | Italy | 12 | 35 | 10.6 ± 6.7 m |
| Miceli Sopo, 2015 [12] | Italy | 81 | 70 | 6–46 m (14) |
| Vila, 2015 [13] | Spain | 80.9 | 21 | 9 m–14 y (12 m) |
| Papadopoulou, 2020 [14] | Greece | 56 | 100 | 13 ± 6.7 m |
| Vazquez-Ortiz, 2017 [15] | Spain | 54.3 | 81 | 9–12 m (10) |
| Alonso, 2019 [16] | Spain | 37.5 | 8 | 4–24 m |
| Dìaz, 2019 [17] | Spain | 32.5 | 120 | 0.5 m–12 y (11.2 m) |
| Diagnosis | Prevalence | Age (Years) | Country | Author, Year | Ref. |
|---|---|---|---|---|---|
| Self-reported | 5% | 1–4 | Finland | Pyrhönen, 2009 | [28] |
| 7% | 1–6 | Finland | Kajosaari, 1982 | [29] | |
| 0.0001% | 0–2 | Israel | Dalal, 2002 | [30] | |
| 2.8% | 6–9 | United Arab Emirates | Al-Hammadi, 2010 | [31] | |
| 3% | 0–2 | Norway | Eggesbø, 1999 | [32] | |
| 0.1% | 0–18 | USA | Sicherer, 2004 | [33] | |
| 0.3%–0.6% | 0–2/>11 | USA | Gupta, 2011 | [34] | |
| 13% | 0–18 | Singapore | Chiang, 2007 | [35] | |
| 2.29% | 14–16 | Philippines | Connett, 2012 | [36] | |
| 1.1% | 0–5 | Thailand | Laoaraya, 2012 | [37] | |
| 1.62% | 2–6 | Vietnam | Le, 2019 | [38] | |
| 0.3% | 5–16 | Ghana | Obeng, 2011 | [39] | |
| Clinical history + specific IgE + skin prick test | 0.3% | 0–18 | Finland | Von Hertzen, 2006 | [40] |
| 1.3% | 13–18 | UK | Pereira, 2005 | [41] | |
| 0.7% | 5–18 | France | Pénard--Morand, 2005 | [42] | |
| 0.21% | 0–18 | China | Chen, 2011 | [43] | |
| 17.8% | 1–7 | Spain | Crespo, 1995 | [44] | |
| Oral challenge | 0% | 0–80 | Denmark | Osterballe, 2005 | [45] |
| 0.0006% | 6 | UK | Venter, 2006 | [46] | |
| 0.0002% | 6–9 | Turkey | Orhan, 2009 | [47] | |
| 0.2% | 1 | Island | Kristinsdottir, H. 2011 | [48] |
| Order | Species | Molecular Allergen | Biochemical Name | Prevalence (%) | Molecular Weight (kDa) |
|---|---|---|---|---|---|
| Clupeiformes | Herring (Clupea harengus) | Clu h 1 | parvalbumin | 45 | 12 |
| Sardine (Sardinops sagax) | Sar sa 1 | parvalbumin | 80 | 12 | |
| Cyprinoformes | Carp (Cyprinus carpio) | Cyp c 1 | parvalbumin | 100 | 12 |
| Cyp c 2 | enolase | 17 | 47 | ||
| Grass carp (Ctenopharyngodon idella) | Cten i 1 | parvalbumin | 94 | 9 | |
| Gadiformes | Cod (Gadus callarias) | Gad c 1 | parvalbumin | 100 | 12 |
| Cod (Gadus morhua) | Gad m 1 | parvalbumin | 100 | 12 | |
| Gad m 2 | enolase | 56 | 50 | ||
| Gad m 3 | aldolase | 37 | 40 | ||
| Perciformes | Tuna (Thunnus albacares) | Thu a 1 | parvalbumin | 95 | 11 |
| Thu a 2 | enolase | 19 | 50 | ||
| Thu a 3 | aldolase | 13 | 40 | ||
| Barramundi (Lates calcarifer) | Lat c 1 | parvalbumin | 77–83 | 11.5 | |
| Lat c 6 | collagen | 22 | 130–140 | ||
| Tilapia (Oreochromis mossambicus) | Ore m 4 | tropomyosin | 100 | 33 | |
| Indo-pacific mackerel (Rastrelliger kanagurta) | Ras k 1 | parvalbumin | 83 | 11.3 | |
| Mackerel (Scomber scombrus) | Sco s 1 | parvalbumin | 95 | 12 | |
| Swordfish (Xiphias gladius) | Xip g 1 | parvalbumin | 71 | 11.5 | |
| Pleuronectiformes | Yellow diamond (Lepidorhombus whiffiagonis) | Lep w 1 | parvalbumin | 100 | 11.5 |
| Salmoniformes | Keta Salmon (Oncorhynchus keta) | Onc k 5 | vitegenin | nd | 18 |
| Rainbow trout (Oncorhynchus mykiss) | Onc m 1 | parvalbumin | 95 | 12 | |
| Salmon (Salmo salar) | Sal s 1 | parvalbumin | 49–64 | 12 | |
| Sal s 2 | enolase | 24–34 | 50 | ||
| Sal s 3 | aldolase | 16–26 | 40 | ||
| Sal s 4 | tropomyosin | 13 | 37 | ||
| Sal s 6 | collagen | 22 | 130, 140 | ||
| Sal s 7 | creatine kinase | 14 | 43 | ||
| Sal s 8 | triose-P isomerase | 34 | 25 | ||
| Sal s 9 | nd | nd | nd | ||
| Siluriformes | Pangasius (Pangasianodon hypophthalmus) | Pan h 1 | parvalbumin | 42 | 11 |
| Pan h 2 | enolase | 21 | 50 | ||
| Pan h 3 | aldolase | 21 | 40 | ||
| Pan h 4 | tropomyosin | 6–32 | 35 | ||
| Pan h 7 | creatine kinase | 10 | 43 | ||
| Pan h 8 | triose-P isomerase | 19 | 21 | ||
| Pan h 9 | pyruvate kinase | 6 | 65 | ||
| Pan h 10 | lactate DH | 13 | 34 | ||
| Pan h 11 | glucose-6-P DH | 8 | 60 | ||
| Pan h 13 | glyceraldehyde-3-P DH | 6 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrorilli, C.; Arasi, S.; Barni, S.; Caimmi, D.; Chiera, F.; Comberiati, P.; Dinardo, G.; Giannetti, A.; Gismondi, M.; Gracci, S.; et al. IgE-Mediated and Non-IgE-Mediated Fish Allergy in Pediatric Age: A Holistic Approach—A Consensus by Diagnostic Commission of the Italian Society of Pediatric Allergy and Immunology. Medicina 2023, 59, 1651. https://doi.org/10.3390/medicina59091651
Mastrorilli C, Arasi S, Barni S, Caimmi D, Chiera F, Comberiati P, Dinardo G, Giannetti A, Gismondi M, Gracci S, et al. IgE-Mediated and Non-IgE-Mediated Fish Allergy in Pediatric Age: A Holistic Approach—A Consensus by Diagnostic Commission of the Italian Society of Pediatric Allergy and Immunology. Medicina. 2023; 59(9):1651. https://doi.org/10.3390/medicina59091651
Chicago/Turabian StyleMastrorilli, Carla, Stefania Arasi, Simona Barni, Davide Caimmi, Fernanda Chiera, Pasquale Comberiati, Giulio Dinardo, Arianna Giannetti, Marco Gismondi, Serena Gracci, and et al. 2023. "IgE-Mediated and Non-IgE-Mediated Fish Allergy in Pediatric Age: A Holistic Approach—A Consensus by Diagnostic Commission of the Italian Society of Pediatric Allergy and Immunology" Medicina 59, no. 9: 1651. https://doi.org/10.3390/medicina59091651
APA StyleMastrorilli, C., Arasi, S., Barni, S., Caimmi, D., Chiera, F., Comberiati, P., Dinardo, G., Giannetti, A., Gismondi, M., Gracci, S., Paravati, F., Pelosi, U., Miraglia Del Giudice, M., Bernardini, R., & Pecoraro, L. (2023). IgE-Mediated and Non-IgE-Mediated Fish Allergy in Pediatric Age: A Holistic Approach—A Consensus by Diagnostic Commission of the Italian Society of Pediatric Allergy and Immunology. Medicina, 59(9), 1651. https://doi.org/10.3390/medicina59091651








