Abstract
Background: Androgenetic alopecia (AGA) and alopecia areata (AA) are the most common types of non-cicatricial alopecia. Both diseases have limited effective therapeutic options and affect patient quality of life. Pharmacogenetic tests can help predict the most appropriate treatment option by evaluating the single nucleotide polymorphisms (SNPs) corresponding to genes related to alopecia. The objective of the study was to evaluate and compare selected SNPs and genes in AA and AGA patients from Romania and Brazil. Materials and Methods: We performed a retrospective study regarding the associations between AA and AGA and 45 tag SNPs of 15 genes in 287 Romanian and 882 Brazilian patients. The DNA samples were collected from oral mucosa using a swab. The SNPs were determined by the qPCR technique. Each genetic test displays the subject’s genotype of the selected gene and the prediction of a successful treatment (e.g., genotype AA of the GR-alpha gene is related to a predisposition to normal sensibility to topical glucocorticoid, and, therefore, glucocorticoids should be effective). Results: The GR-alpha, GPR44-2, SULT1A1, and CRABP2 genes were statistically significantly different in Brazil compared to Romania. The SULT1A1 activity that predicts the response to minoxidil treatment showed in our analysis that minoxidil is recommended in half of the cases of AGA and AA. Patients with AGA and a high expression of SRD5A1 or PTGFR-2 may benefit from Dutasteride or Latanoprost treatment, respectively. Most of the studied genes showed no differences between the two populations. Conclusions: The DNA analysis of the patients with alopecia may contribute to a successful treatment.
1. Background
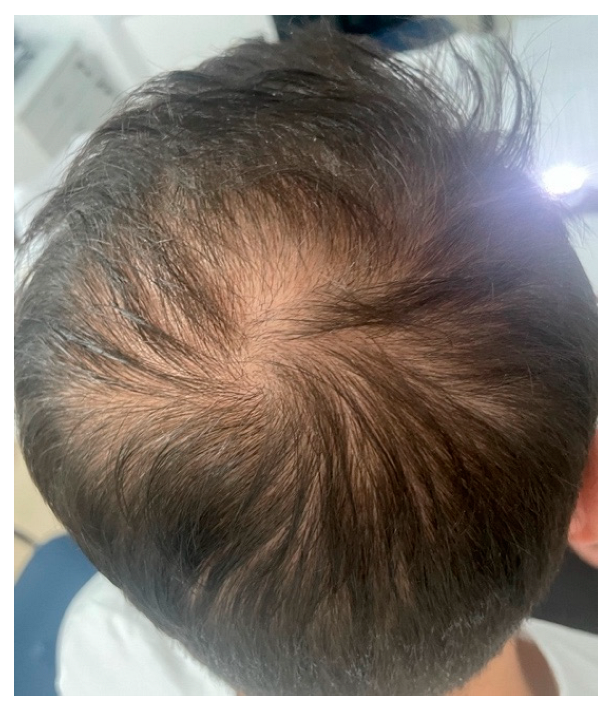
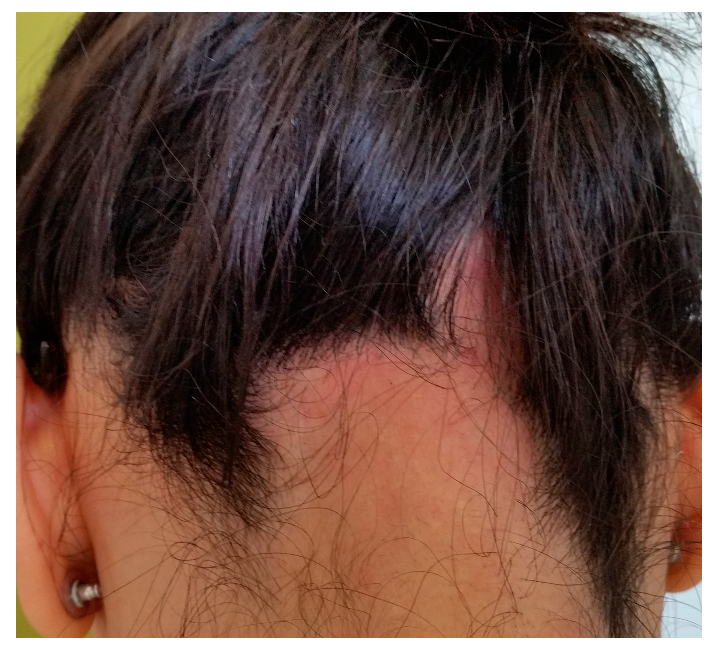
Androgenetic alopecia (AGA) and alopecia areata (AA) are among the most common types of non-cicatricial alopecia. AGA is characterized by a distinctively receding frontal hairline in men (Figure 1) and diffuse hair thinning with retention of the frontal hairline in women. Patients with AGA have a shorter anagen phase of the hair cycle, which will gradually diminish the hair follicle (HF) and cause the development of vellus hairs [1]. AGA’s etiology and pathophysiology are not entirely understood; however, genetics [2] and androgens are implicated [3]. AA is a complex autoimmune disease with a genetic substrate that targets the HF in the anagen phase [4]. It typically presents with round alopecic patches (Figure 2) and can affect children and adults. AA [5] and AGA [6] evaluation and diagnostic should include a medical history, clinical examination, dermoscopy, and hair pull test. Additional investigations for AA, such as scalp biopsy, are usually unnecessary [5]. The effectiveness of most treatments for AGA and AA is variable. Consequently, utilizing DNA variants may have an impact on treatment strategies.
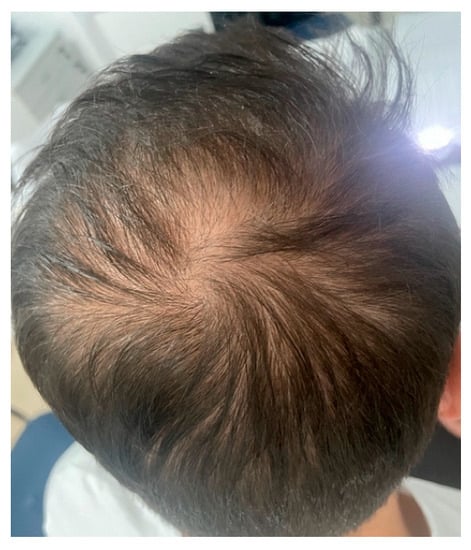
Figure 1.
Androgenetic alopecia vertex area. Photograph displayed with patient consent.
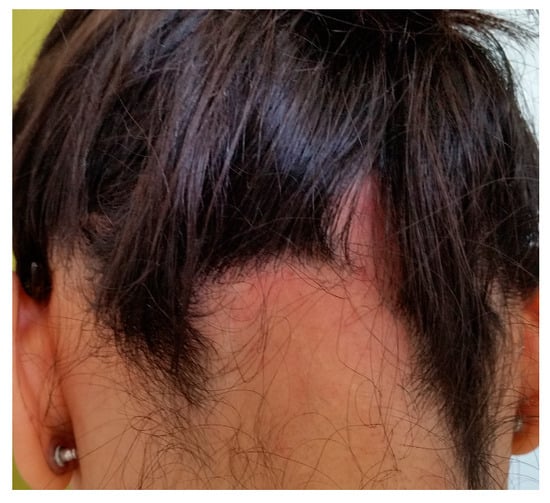
Figure 2.
Alopecia areata: round alopecic patches in the occipital area (ophiasis). Photograph displayed with patient consent.
The current analysis evaluates and compares the associations between non-cicatricial types of alopecia (AGA and AA) and 45 tag single nucleotide polymorphisms (SNPs) and 15 genes identified from previous AA and AGA association studies [7,8,9,10,11,12,13,14,15,16]. The evaluated population comprises 287 patients from Romania and 882 from Brazil, clinically diagnosed with AA or AGA.
Local treatments of AGA are limited and usually consist of minoxidil (2% BID or 5% QID), 5 alpha-reductase (finasteride and dutasteride), and antiandrogens [6]. Regarding AA, topical and intralesional corticosteroids remain the first-line treatment. Other topical treatments, such as minoxidil and prostaglandin F2α analogues, are also prescribed in AA [5].
2. The Objective of the Study
The study aims to evaluate and compare genetic data (selected SNPs and genes) in patients from Romania and Brazil diagnosed with AA and AGA.
3. Materials and Methods
We performed a retrospective study regarding associations between AA and AGA and 45 tag single nucleotide polymorphisms (SNPs) of 15 genes in a total of 1169 patients: 287 Romanian patients (group 1) and 882 Brazilian patients (group 2). All the patients of both groups were diagnosed with AA or AGA. The diagnosis of AGA or AA was confirmed by healthcare professionals in private clinics from different cities in Romania and Brazil, based on the evolution of the disease, the clinical aspect, and the trichoscopic examination. All patients signed the informed consent. The DNA samples were collected from oral mucosa using a swab. The sampling method was non-invasive and ensured adequate DNA quantity and quality. All the genotyping assays were performed by qPCR Taqman Assays (Thermo Fisher Scientific, USA) from the genomic DNA collected from the patient.
Fagron Genomics (Barcelona, Spain) provided all anonymized data from their database for the current retrospective study.
The evaluated SNPs and the related genes were rs9282861(SULT1A1 gene), rs523349 (SRD5A2 gene), rs39848 (SRD5A1 gene), rs10782665 (PTGFR-3 gene), rs1328441 (PTGFR-2 gene), rs6686438 (PTGFR-1 gene), rs13283456 (PTGES2 gene), rs2229765 (IGF1R gene), rs6198 (GR-alpha gene), rs533116 (GPR44-2 gene), rs545659 (GPR44-1 gene), rs2470152 (CYP19A1 gene), rs12724719 (CRABP2 gene), rs13078881 (BTD gene), and rs4343 (ACE gene). The study was approved by the Bioethics Committee of Colentina Clinical Hospital, Bucharest, Romania (approval no. 17/22.12.2022).
3.1. Statistical Analysis
Data were collected, analyzed, coded, and introduced in the Statistical Package for Social Science (IBM SPSS, New York, NY, USA) version 23. Quantitative data were presented as several patients from each country and percentages of the patients for each SNP in each alopecic disease (AGA and AA), as illustrated in Table 1.

Table 1.
Comparation between SNPs in patients with androgenetic alopecia and alopecia areata from Romania and Brazil.
The Chi-square test provided information about the relationship between the variables. The test was based on the observed frequencies fi, which represented the frequencies of occurrence of the component elements of each SNP and country. The SNP variable component elements were the country variable genotypes (Brazil and Romania). Based on the observed frequencies, the theoretical frequency for each observed frequency was determined according to a specific calculation algorithm. Each cell with observed frequencies (fi) corresponded to a cell with theoretical values (fti). Depending on the determined Chi-square value (χ2) and the degree of freedom (df), the Chi-square test provided the actual significance threshold, denoted by p, which was compared with the significance threshold considered in statistical research, in our case α = 0.05. The decision regarding the association (dependence) or non-association (independence) of the two variables was taken depending on the comparison between the thresholds p and α.
The tested research hypotheses were the null (H0) and alternative hypothesis (H1). The null hypothesis stood for no association between an SNP variable and its genotypes and the country variable (Brazil and Romania), with the variables being independent. The alternative hypothesis (H1) referred to an association between an SNP variable and its genotypes and the country variable (Brazil and Romania), with the variables being dependent. If p ≤ 0.05 (α), the null hypothesis was rejected, and there was a relationship between the two variables in the sense that the data (genotypes) of each SNP depended on the country. If p > 0.05 (α), the null hypothesis was accepted, and there was no connection between the two variables in the sense that the genotypes of each SNP did not depend on the country (they were similar).
3.2. Principal Component Analysis
Principal component analysis (PCA) is a statistical technique used by condensing the dimensions to the few principal components (PCs) that best describe the main patterns. It is used for analyzing data containing a large number of observations [17].
We performed a PCA for our 1169 patients with AA and AGA. The internal consistency coefficient (Cronbach’s Alpha) of the two specified dimensions and the percentage values for the variance on each dimension and for the total (18.142%) is displayed in Table 2.

Table 2.
Model Summary.
The Variance Accounted For table (Table 3) shows the coordinates of each element on each dimension relative to the centroid (0, 0). Furthermore, features with a very small mean coordinate indicate that these elements do not contribute substantially to the principal components. The SNPs rs39848 (SRD5A1 gene), rs523349 (SRD5A2 gene), rs12724719 (CRABP2 gene), and rs13078881 (BTD gene) have mean values less than or equal to 0.010, meaning they do not adequately contribute to the principal components. After transforming the variables through the optimal analysis approach, the Correlation-Transformed Variables table (Table 4) shows the correlations between the components. We observed that the elements contributing substantially to the main features have an eigenvalue > 1.

Table 3.
Variance Accounted For.

Table 4.
Component-Transformed Variables.
The Component-Transformed Variables table (Table 4) indicates the coordinates for each SNP on each dimension. Based on the data, the plotting of the analyzed SNPs according to the coordinates agree with to the two dimensions. We observed that the elements that contribute substantially to the main components are those with an eigenvalue > 1.
4. Results
In group 1 (287 Romanian patients), 90.2% were diagnosed with AGA and 9.8% with AA. In group 2 (882 Brazilian patients), 94.2% were diagnosed with AGA and 5.8% with AA.
Four SNPs (rs6198, rs533116, rs9282861, and rs 2724719) with the related genotypes (GPR44-2, GR-ALPHA, SULT1A1, and CRABP2) were statistically significantly associated in Brazil and Romania (Table 5). In these cases, the null hypothesis was rejected, with the data being significant from one population to the other. The non-statistically significant SNPs are shown in Table 6.

Table 5.
Genes and SNPs with significant differences in Brazil and Romania.

Table 6.
Genes and SNPs with non-significant differences in Brazil and Romania.
The most prevalent genotype (GA) was observed in the following SPNs: rs533116 (GPR44-2 gene), rs2470152 (CYP19A1 gene), rs1328441(PTGFR-2 gene), rs4343 (ACE gene), and rs2229765 (IGF1R gene). Prostaglandin D2 receptor 2 (GPR44 or CRTH2) variants were associated with an increased GPR44, resulting in higher responsiveness to prostaglandin D2 and HF regression. The aromatase gene (CYP19A1) showed low testosterone conversion in estrogens and high conversion into DHT. Prostaglandin F receptor 2 (PTGFR-2) was mainly related to the treatment efficacy with prostaglandin analogue and latanoprost. The angiotensin-converting enzyme (ACE) variants were associated with increased plasma levels of angiotensin in 49.0% of the patients with AA from Brazil and 54.8% from Romania, and insulin-like growth factor-I (IGF-I) variants were associated with lower plasma IGF-1 levels in half of the evaluated patients.
The genotype AA was more frequent for the tag SNP rs6198 (GR-ALPHA gene) and rs545659 (GPR44-1 gene) in both countries. The SNP rs6198 analysis indicated that the glucocorticoid receptor (GR or NR3C1) variants are associated with resistance or sensitivity to corticosteroids. Regarding rs545659 SPN, most subjects have an increased GPR44, leading to higher responsiveness to prostaglandin D2 and HF regression. Our analysis showed tha the AA genotype of the GPR44-1 gene was found in 68.6% of the cases from Brazil, 63.1% in AA, 75.0% of AGA cases from Romania, and 62.9% of AA cases.
For the tag SPNs rs9282861(SULT1A1), rs523349 (SRD5A2 gene), and rs13283456 (PTGES2 gene), the CC genotype was more prevalent. In our study, the treatment with minoxidil at a regular concentration showed good results in more than half of the patients with AGA (52.9% in Brazil and 50.0% in Romania) and AA (52.6% in Brazil and 53.3% in Romania). The SULT1A1 gene can predict a good response to minoxidil treatment.
The GG genotype was most frequently associated with the following SPNs: rs12724719 (CRABP2 gene), rs13078881 (BTD gene), and rs6686438 (PTGFR-1 gene). The SNP analysis did not indicate the necessity to supplement with retinoic acid or vitamin B as the levels were physiological. The genetic testing showed that latanoprost might not respond positively in 60.8% of AGA in Brazilian patients and 46.4% of Romanian patients.
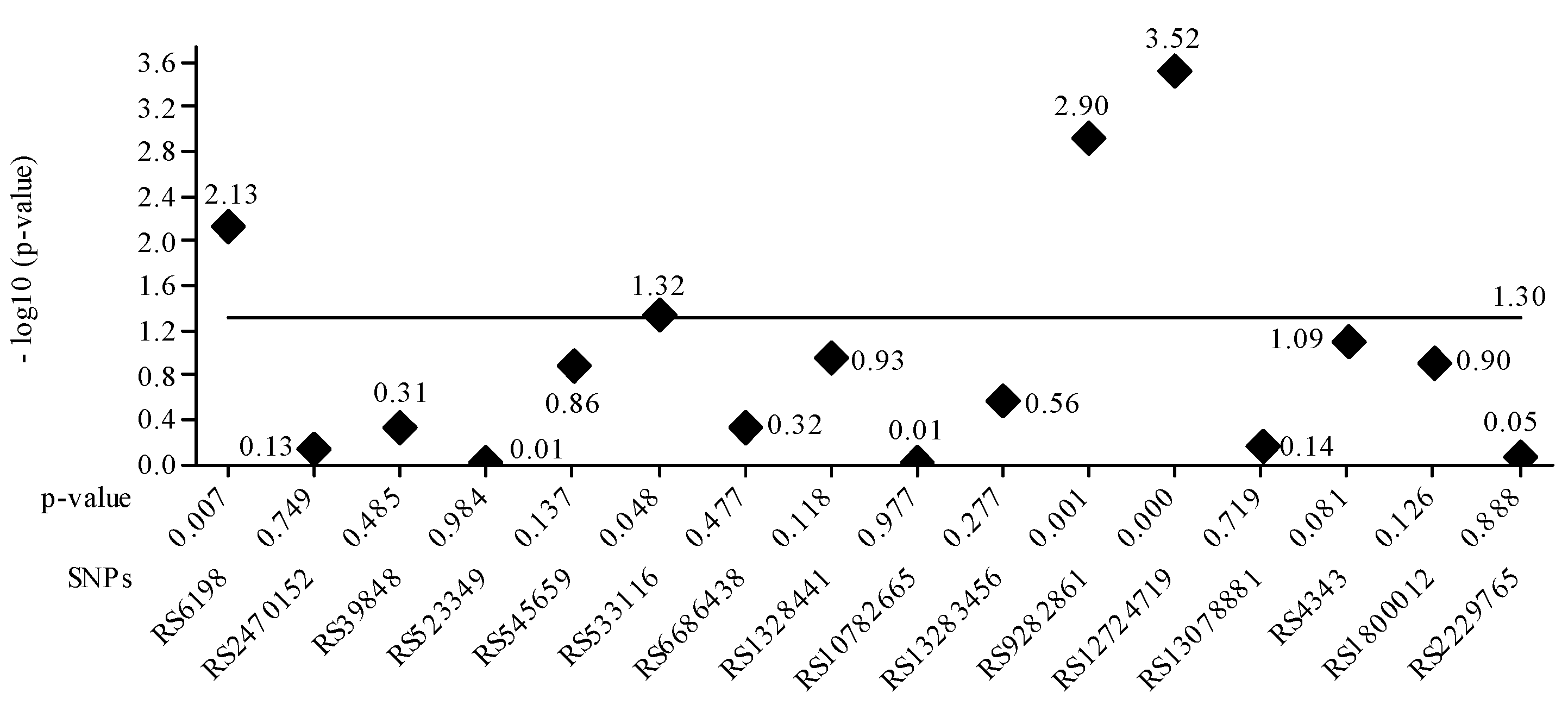
The gene PTGFR-3 SPN rs10782665 was commonly associated with genotype GT related to latanoprost treatment efficacy. Regarding the SPN rs39848 (SRD5A1 gene), the more common genotype was CT. The genetic analysis showed that increased SRD5A1 activity leads to increased DHT levels and hair growth inhibition, and treatment with Dutasteride is recommended in 43.1% of Brazilian and 53.6% of Romanian AGA patients. Figure 3 plots the p-value for each SPN. It is confirmed that the four values greater than 1.30 correspond to the SNPs seen in Table 5. The lowest nominal p-value was observed for the tag SNP RS9282861 gene SULTA1A (p = 0.001).
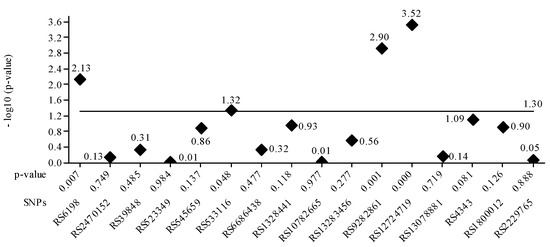
Figure 3.
The value of the base-10 logarithm of the p-value is always negative because the p-value takes values between 0 and 1. For this reason, for the representation of positive values on the Oy axis, the value of the logarithm minus (-log10 (p-value)) was used. The significance threshold considered was 0.05 -log10 0.05 = 1.30, the constant value, represented as a horizontal line. Statistically significant values had a p-value <= 0.05. For these, the -log10 values are above the line corresponding to p-value = 0.05, e.g., above 1.30. It is confirmed that the four values greater than 1.30 correspond to the SNPs in Table 5.
Regarding the type of alopecia (AA or AGA), the Chi-square test highlighted a significance threshold of p = 0.02 < 0.05. This result shows that AGA and AA are associated with the country variable, meaning that these types significantly differ from country to country, and the null hypothesis was rejected.
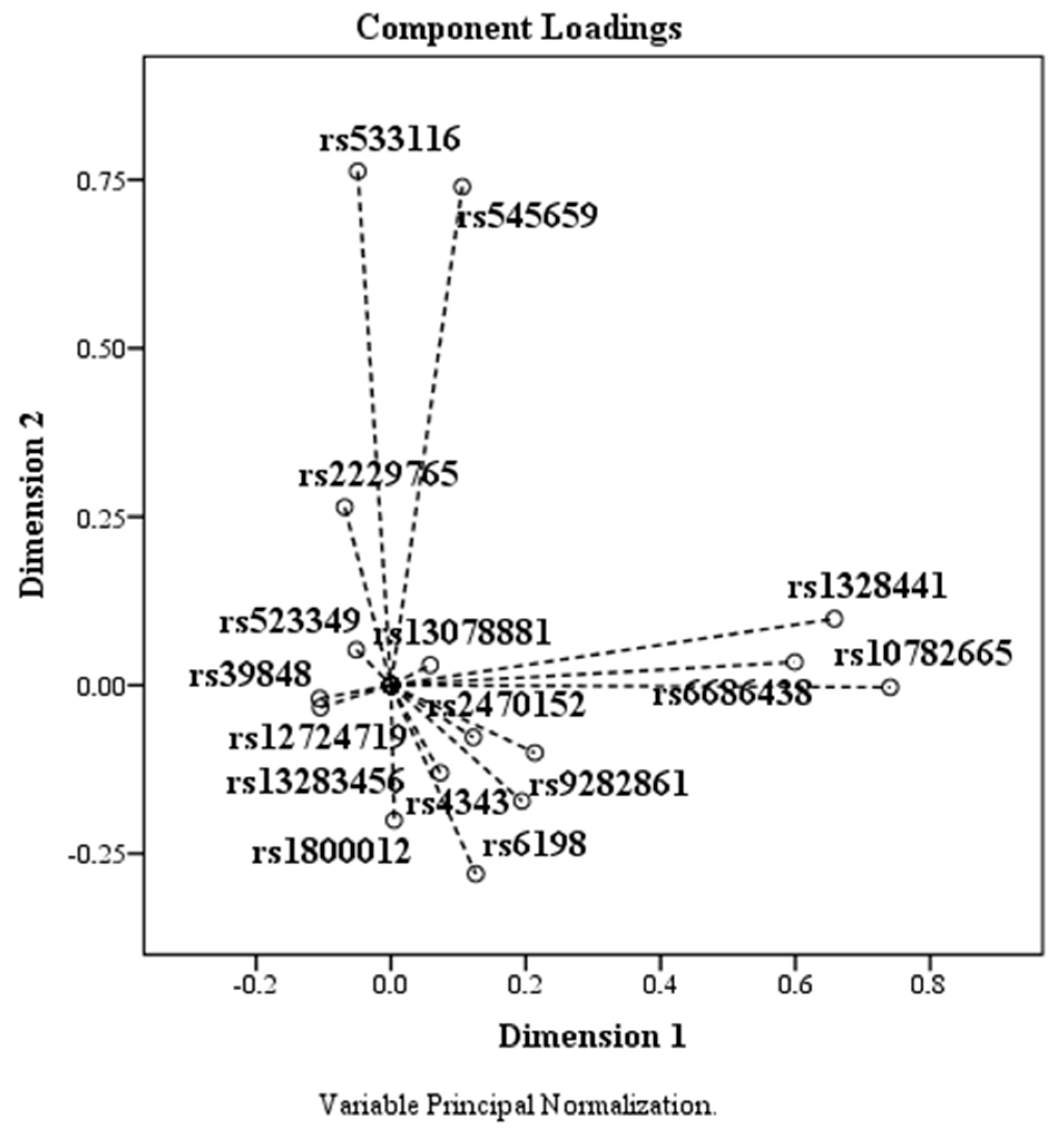
Based on the PCA data, the components plotted according to the coordinates corresponding to the two dimensions are shown in the Figure 4. SNPs rs523349, rs13078881, rs39848, rs12724719, and rs13283456 had means below 0.010, being localized around the coordinate (0, 0).
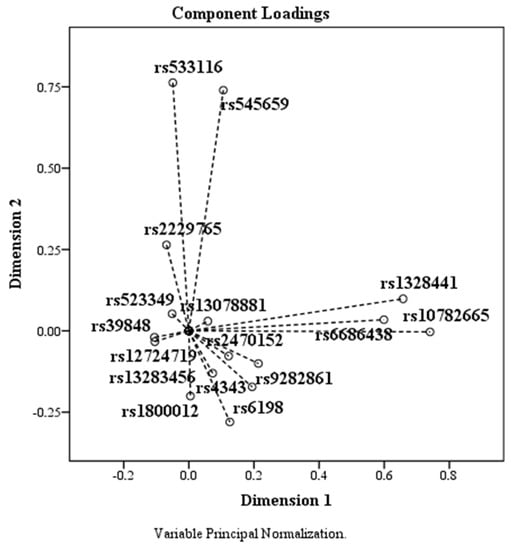
Figure 4.
The effect of PCA on SNPs. SNPs rs523349 (SRD5A2), rs13078881 (BTD), rs39848 (SRD5A1), rs12724719 (CRABP2), and rs13283456 (PTGES2) are located around the coordinate (0, 0).
5. Discussion
The possibility of linkages between SNPs in AA and AGA has only recently been investigated in a small number of Australian [7], European (Polish [5], UK, and German [8]), Chinese and European [9], and Korean [10] populations.
5.1. Aromatase Gene (CYP19A1 Gene)
Aromatase inhibitors (AIs), which inhibit the synthesis of estrogens, cause a relative increase in the activity of 5-reductase. Aromatase is known to reduce intrafollicular testosterone [5] and DHT levels [2,6] and to catalyze the conversion of testosterone to estradiol. This increase in the aromatase can determine “pseudo male pattern androgenetic alopecia,” which results in male pattern hair loss that mimics female androgenetic alopecia (FAGA) [11]. Aromatase expression varies between alopecic and non-alopecic scalps [18]. According to Yip et al., the aromatase gene (CYP19A1) may predispose women to types of non-scarring alopecia [7]. Estrogens can both prevent and modulate hair growth; hence, CYP19A1 plays a crucial protective role in frontal hair lines [7]. Compared to men, women have more significant levels of CYP19A1 in their frontal and occipital follicles [19].
Fifteen menopausal women with hormone receptor-positive breast cancer who received AIs were the subjects of a study by Rossi et al. [11]. After receiving AI therapy for a year, the patients began to develop frontotemporal follicle miniaturization that mimicked FAGA. Yip et al. [7] performed a gene-wide study of the association between female pattern hair loss (FPHL) and the aromatase gene-encoding CYP19A1. The investigators examined 61 CYP19A1 SNPs in the Australian population of 484 Caucasian women with FPHL and 471 controls. The CC genotype of rs4646 was substantially more common in FPHL (58%) than in the controls (48%), indicating a recessive genetic influence, even though no significant relationship was established at the allelic levels. Even though our study did not analyze the sex of the patients with AGA, the most prevalent genotype of the CYP19A1 gene (SPN rs2470152) was GA (47.4% in Brazil and 47.7% in Romania), showing a predisposition to reduced CYP19A1 activity.
5.2. Angiotensin-Converting Enzyme (ACE Gene)
The immune-dependent disease known as AA is defined by the interaction of T cells with follicular antigens. Studies have revealed the presence of a local renin–angiotensin system in the skin, where the angiotensin-converting enzyme (ACE) is involved in autoimmunity and causes alopecia due to HF’s chronic inflammation [20,21]. A few cases of non-cicatricial alopecia induced by ACE inhibitors have been identified. Lisinopril-induced alopecia in a patient of Kataria et al. [10] and enalapril-induced alopecia in a patient of Ahmad et al. [22] were reported. Hair re-growth was observed after the cessation of the treatment. A patient of Motel et al. received ACE treatment for one month, and after the medication was stopped, hair thinning was observed at a follow-up examination [12].
Namazi et al. assessed the serum activity of ACE in a study with 19 patients with AA and 16 healthy control participants. Although the findings were not statistically significant, AA patients had higher serum ACE levels. Moreover, in the patient group, there was no association between ACE activity and the severity or duration of the condition [13]. Fahim et al. found a significant correlation between serum ACE and disease severity despite the lower serum ACE levels in AA patients. Angiotensin I, which may also be involved in the inflammation of AA, is considered to be the cause of ACE consumption and reduced tissue levels of the enzyme [21]. In our study, 49.0% of the patients with AA from Brazil and 54.8% from Romania expressed the GA genotype, resulting in a predisposition to an increased angiotensin conversion activity.
5.3. 5-Alpha-Reductase (SRD5A1 and SRD5A2 Genes)
Genetics plays an essential role in AGA [2]. The role of the 5-alpha-reductase (5AR) enzyme in AGA is implicated in the metabolism of testosterone to dihydrotestosterone (DHT), hence the 5AR inhibitors effect in treating AGA [14]. Finasteride and dutasteride are 5AR inhibitors commonly used in AGA. Dutasteride is an inhibitor of both type I and II 5ARs and is considered an effective treatment for AGA [23,24]. The treatment response to dutasteride varies in each individual; however, it is unknown if the genetic factors contribute to these variations [23]. It was also observed that the response to dutasteride might be related to variations of genes involved in the metabolism of steroid hormones, such as SRD5A1. Finasteride inhibits type II 5ARs only, while dutasteride inhibits type I and type II 5ARs simultaneously. Accordingly, a difference in SRD5A1 could influence how well an individual responds to dutasteride when treated for AGA [24]. Moreover, in the Korean population, the analysis of the polymorphism of SRD5A1 and SRD5A2 genes showed that it might not be directly associated with the development of AGA [15]. Limited data are available regarding other populations. Our analysis shows that dutasteride at standard doses is recommended in AGA in 43.1% of Brazilian and 53.6% of Romanian patients and Finasteride is recommended in 51.0% of AGA in Brazilian patients and 42.9% of AGA in Romanian patients.
5.4. Glucocorticoid Receptor (GR-Alpha Gene)
Glucocorticoid receptor (GR) is known to be expressed in human hair follicles [25]. Early studies showed significant findings on glucocorticoid biochemical pathways. The GR is elevated in AA patients who are “glucocorticoid-resistant” and unable to bind glucocorticoids, suppressing cellular transcription [18]. Dexamethasone (DEX) is a synthetic glucocorticoid that inhibits the proliferation of human hair dermal papilla (DP) cells. DEX also decreases the expression of growth factors required for hair growth [26]. In addition, DEX promotes the catagen phase of the hair cycle [25]. Corticosteroids are known to be a treatment for AA. In our AA cases, tag SPN rs6198 GR-alpha showed that glucocorticoids should be effective in 73.6% of Brazilian and 67.6% of Romanian patients.
5.5. Insulin-like Growth Factor-1 (IGF-1 Gene)
The regulation of insulin-like growth factor-1 (IGF-1) by androgens is well established. IGF-1 increases HF development by controlling cellular proliferation, according to in vitro research. In organ cultures, in the absence of IGF-1 or insulin, anagen HF enters the catagen phase [27]. Panchaprateep et al. reported significantly lower levels of IGF-1 and its binding proteins (IGFBP-2 and IGFBP-4) in DP cells obtained from alopecic versus non-alopecic scalps [27]. We report similar results, as more than half of the patients are predisposed to lower IGF-1 levels.
5.6. Prostaglandin D, Prostaglandin E, and Prostaglandin F (PTGFR-1, PTGFR-2, PTGFR-3, PTGES2, GPR44-1, and GPR44-2 Genes)
One of the representative lipid mediator groups, which includes prostaglandins (PGs), is the prostanoid family. They have a variety of physiological impacts on controlling inflammatory reactions, cell proliferation, and apoptosis. Prostanoids are classified into five types: PGD2, PGE2, PGF2α, and PGI2. PGD2 binds to prostaglandin D2 receptors (DP1 and DP2) [28], while PGE2 binds to prostaglandin E2 receptors (EP1, EP2, EP3, and EP4) and PGF2α to the prostaglandin F2α receptor (FP) [29].
Colombe et al. investigated the expression patterns of the vital enzymes involved in the metabolism of PGs in human HF. They reported that PGD2, PGE2, and PGF2 metabolism are expressed in human hair follicles (HFs) and might be associated with hair growth and differentiation [30]. Androgens can regulate prostaglandin D2 synthase (PTGDS) to produce prostaglandin D2 (PGD2) [31]. It was reported that the expression levels of PGD2 in men with male pattern alopecia were higher than in the controls [31]. Studies in mice and humans [32,33] have revealed that PGD2 inhibits the re-growth of the HF and hair development, pointing to a role for the DP2 receptor in alopecia. Increased androgen levels may induce PGD2 because androgens have been found to stimulate PTGDS [30]. Kang et al. [30] hypothesized that the expression levels of the DP2 receptor affect hair cycle progression because mRNA is highly expressed in the early catagen phase, and strong staining in the outer root sheath, dermal papilla, and hair matrix was seen. They observed that in mice hair growth cycles, DP2 receptor expression is highest when the hair follicle regresses. Prostaglandin F2 (PTGF2) and Prostaglandin E2 (PTGE2) act synergistically in hair follicles to stimulate hair growth and prolong the anagen phase, which competes with PTGD2. According to a study, PTGES, the enzyme that produces PTGE2, is overexpressed in male patients with early-stage AGA [34].
Rafati et al. [33] observed significant hair re-growth in AA during the treatment with a latanoprost 0.005% solution. In our analysis, PTGFR-2 showed an increased likelihood of having a positive response to latanoprost in AA for 48.4% of patients in Brazil and 48.4% in Romania, similar to PTGFR-3 with 43.8% of patients in Brazil and 50.2% in Romania. In AGA, there was a 33.3–60.7% range of recommendation for latanoprost at regular doses.
The aromatase gene showed a low conversion of testosterone in estrogens, and a high conversion into DHT and prostaglandin D2 receptor 2 (GPR44-2 or CRTH2) variants were associated with an expressed GPR44 leading to higher responsiveness to prostaglandin D2 and HF regression. The prostaglandin F receptors (PTGFRs) were mainly related to latanoprost treatment efficacy, known as a prostaglandin analog.
More than 67.9% of the analyzed patients had a predisposition to normal PGE2 levels. The statistical analysis observed a predisposition to normal GPR44 levels in 43.4% (PTGFR-1) and 63.4% (PTGFR-2) of the Brazilian patients and 46.7% (PTGFR-1) and 64.1% of the Romanian patients. Our SNP analysis did not indicate any treatment with prostaglandin D2 inhibitors in patients from both countries. SPN rs545659 showed that most subjects have an increased GPR44, which leads to higher responsiveness to prostaglandin D2 and hair follicle regression.
5.7. Cellular Retinoic Acid-Binding Protein 2 (CRABP2 Gene)
Alopecia areata (AA) is an autoimmune disease affecting the hair follicles in the anagen phase. According to Duncan et al., vitamin A regulates the immune response and the hair cycle to slow the extension of AA [35]. Retinol, a form of vitamin A linked to retinol-binding protein 4 (RBP4) that is converted to retinoic acid by retinal dehydrogenases and transported to the nucleus by CRABP2. Retinol can be esterified or eliminated without or when RBP1/CRBP is saturated [35]. It was observed that RA induces AA, which may be attributed to an array of factors, such as dysregulated immunological function [35]. Genetic studies in mice with AA revealed increased synthesis and retinoic acid (RA) levels in the involved genes. In contrast, RA degradation genes were reduced in AA patients compared to the controls. The facts were confirmed by immunohistochemistry in biopsies from patients with AA, and both control mice and AA models and RA levels were also elevated in C3H/HeJ mice with AA.
In our analysis, 66.1% of AA patients from Brazil and 76.4% from Romania did not require vitamin A supplementation. In comparison, 30.8% of patients with AA from Brazil and 22.8% of patients from Romania were predisposed to reduced retinoic acid transport, and vitamin A would be recommended.
5.8. Sulfotransferase (SULT1A1 Gene)
Recent and older studies reported a correlation between SULT1A1 expression in the scalp and the response to minoxidil [16,35]. Topical minoxidil is the only FDA-approved treatment for AGA and the most commonly prescribed topical treatment for female androgenetic alopecia (FAGA) [7]. Minoxidil is a pro-drug that needs to be bio-activated into minoxidil sulfate to stimulate hair growth by vasodilation. Minoxidil sulfotransferase (SULT1A1) catalyzes the reaction in the hair follicle [36]. Individual differences in SULT1A1 expression in the scalp explain the varieties of clinical responses to topical minoxidil [37]. Therefore, dermatologists could achieve long-lasting persistent results from AGA treatment by using the SULT1A1 activity assay [35]. The SULT1A1 gene analysis showed that minoxidil at regular concentrations would be highly recommended in 52.9% of AGA cases and 52.6% of AA cases in Brazil and 50% of AGA cases and 53.3% of AA cases from Romania.
5.9. Biotinidase (BTD Gene)
Biotinidase deficiency (BTD) can be categorized as primary and secondary. Primary BTD is an autosomal recessive inherited neuro-cutaneous disorder [38], while a common cause of acquired biotin deficiency is associated with raw egg consumption or several treatments (anticonvulsants, isotretinoin, and antibiotics) [39].
According to Georgala et al. [40], biotinidase deficiency can induce alopecia. The study showed that 12 out of 19 patients with significantly decreased biotinidase activity compared to the controls experienced clinical remission of AA after biotin supplementation. Moreover, Patel et al. [39] performed a literature analysis of 10 cases of alopecia—most patients with an inherited biotinidase enzyme deficiency or being treated with valproic acid. Eight patients experienced hair re-growth after biotin (vitamin B7) supplementation.
Our SNP analysis shows no indication of vitamin B supplementation in more than 90% of the cases of AA and AGA from both countries. Most of the evaluated subjects had normal biotinidase activity.
Our study had several limitations. First, more data are needed regarding the severity, timing, personal and family history, and therapeutic options for the disease. Second, the study’s population was entirely Brazilian or Romanian, which may have limited the results’ applicability to a broader community. Also, the number of the studied groups of patients was unequal, and the sample size can influence the research results.
6. Conclusions
The SULT1A1 gene was expressed in more than half of the patients with AA and AGA from both groups but was higher in the Romanian population than in the Brazilian one. Higher responsiveness to prostaglandin D2 and hair follicle regression due to an expressed GPR44 was found in half of the Romanian subjects with AGA and three quarters of the Brazilian subjects. Most of the studied genes showed no differences between the two populations, suggesting a common genetic background. Furthermore, the ACE gene variants were associated with increased plasma levels of angiotensin, and IGF-I variants were associated with lower plasma IGF-1 levels in half of the studied patients. Additionally, our analysis does not indicate the necessity to supplement with vitamin B in most AA and AGA cases from both groups.
Additionally, the PCA showed that the SRD5A1, SRD5A2, PTGES2, CRABP2, and BTD genes do not contribute substantially to the principal components.
Individual genes frequently provide only polygenic conditions, but identifying essential genes may reveal new and significant treatment targets. Our research indicates that AGA and AA are likely to become predicted by DNA with sufficient accuracy to support common practical uses. Further and extensive studies are necessary.
Author Contributions
Conceptualization, M.P. and G.T.; methodology, M.P. and G.T.; validation, G.S.Ț., M.P. and V.M.C.; writing—original draft preparation, M.P. and G.S.Ț.; writing—review and editing, M.P., G.S.Ț. and V.M.C.; visualization, G.S.Ț. and V.M.C.; supervision, G.S.Ț. and V.M.C.; English translation and supervision, G.S.Ț. and M.P.; pictures and graphics, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The University of Medicine and Pharmacy Carol Davila of Bucharest, Romania, supports the article processing charges of this publication.
Institutional Review Board Statement
Approval for the study was obtained from the Bioethics Committee of Colentina Clinical Hospital, Bucharest, Romania, no. 17/22 December 2022.
Informed Consent Statement
Informed consent was obtained for all participants.
Data Availability Statement
Available on request.
Acknowledgments
We gratefully acknowledge the Fagron Lab for supporting and providing the data. We also thank all the subjects who participated in the study. This article is part of the Ph.D. thesis of Monica Paun. We thank the University of Medicine and Pharmacy Carol Davila of Bucharest, Romania, for offering support to the PhD students.
Conflicts of Interest
Monica Păun received consulting fees from the Lóreal Group with no influence in the representation or interpretation of the reported research results. George-Sorin Țiplica received consulting fees from Genesis Biopharm Romania, Leo Pharma Romania, and Antibiotice Romania with no influence in the representation or interpretation of the reported research results. The other authors have no conflict of interest.
References
- Bergfeld, W.F. Androgenetic alopecia: An autosomal dominant disorder. Am. J. Med. 1995, 98, 95S–98S. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, D.; Sinclair, R. Male androgenetic alopecia. Expert Opin. Pharmacother. 2010, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Reygagne, P.; Benech, P.; Jean-Louis, F.; Scalvino, S.; Ly Ka So, S.; Hamidou, Z.; Bianovici, S.; Pouch, J.; Ducos, B. Study of gene expression alteration in male androgenetic alopecia: Evidence of predominant molecular signalling pathways. Br. J. Dermatol. 2017, 177, 5. [Google Scholar] [CrossRef]
- Biran, R.; Zlotogorski, A.; Ramot, Y. The genetics of alopecia areata: New approaches, new findings, new treatments. J. Dermatol. Sci. 2015, 78, 1. [Google Scholar] [CrossRef] [PubMed]
- Lintzeri, D.A.; Constantinou, A.; Hillmann, K.; Ghoreschi, K.; Vogt, A.; Blume-Peytavi, U. Alopecia areata—Current understanding and management. J. Dtsch. Dermatol. Ges. 2022, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Sood, T.; Zito, P.M. Androgenetic Alopecia; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Br. J. Dermatol. 2009, 161, 2. [Google Scholar] [CrossRef] [PubMed]
- Redler, S.; Birch, M.P.; Drichel, D.; Dobson, K.; Brockschmidt, F.F.; Tazi-Ahnini, R.; Giehl, K.A.; Kluck, N.; Kruse, R.; Lutz, G. Investigation of variants of thearomatase gene (CYP19A1) in female pattern hair loss. Br. J. Dermatol. 2011, 165, 703–705. [Google Scholar] [CrossRef]
- Liu, F.; Hamer, M.; Heilmann, S.; Herold, C.; Moebus, S.; Hofman, A.; Uitterlinden, A.G.; Nöthen, M.M.; M van Duijn, C.; Ec Nijsten, T. Prediction of male-pattern baldness from genotypes. Eur. J. Hum. Genet. 2016, 24, 895–902. [Google Scholar] [CrossRef]
- Seok, H.; Jeon, H.S.; Park, H.J.; Kim, S.K.; Choi, J.H.; Lew, B.L.; Chung, J.H.; Sim, W.Y. Association of HSPA1B SNP rs6457452 with Alopecia Areata in the Korean population. Immunol. Investig. 2014, 43, 212–223. [Google Scholar] [CrossRef]
- Rossi, A.; Iorio, A.; Scali, E.; Fortuna, M.C.; Mari, E.; Maxia, C.; Gerardi, M.; Framarino, M.; Carlesimo, M. Aromatase inhibitors induce ‘male pattern hair loss’ in women? Ann. Oncol. 2013, 24, 6. [Google Scholar] [CrossRef]
- Motel, P. Captopril and alopecia: A case report and review of known cutaneous reactions in captopril use. J. Am. Acad. Dermatol. 1990, 23, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.R.; Ashraf, A.; Handjani, F.; Eftekhar, E.; Kalafi, A. Angiotensin Converting Enzyme Activity in Alopecia Areata. Enzym. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Stebbing, M.; Harrap, S.B. Genetic analysis of male pattern baldness and the 5 alpha reductase genes. J. Investig. Dermatol. 1998, 110, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Seog-Jun, H.; Jung-Soo, K.; Jae-Wook, M.; Hyun-Jeong, L.; Jin-Wou, K. Analysis of genetic polymorphisms of steroid 5α-reductase type 1 and 2 genes in Korean men with androgenetic alopecia. J. Dermatol. Sci. 2003, 31, 2. [Google Scholar] [CrossRef]
- Pietrauszka, K.; Bergler-Czop, B. Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: A review. Adv. Dermatol. Allergol. 2022, 39, 3. [Google Scholar] [CrossRef]
- Reich, D.; Price, A.; Patterson, N. Principal component analysis of genetic data. Nat. Genet. 2008, 40, 491–492. [Google Scholar] [CrossRef]
- Sawaya, M.E.; Hordinsky, M.K. Glucocorticoid Regulation of Hair Growth in Alopecia Areata. J. Investig. Dermatol. 1995, 104, 5. [Google Scholar] [CrossRef]
- Price, V.H. Androgenetic Alopecia in Women. J. Investig. Dermatol. Symp. Proc. 2003, 8, 24–27. [Google Scholar] [CrossRef]
- Kataria, V.; Wang, H.; Wald, J.W.; Phan, Y.L. Lisinopril-Induced Alopecia: A Case Report. J. Pharm. Pract. 2017, 30, 5. [Google Scholar] [CrossRef]
- Fahim, S.; Montazer, F.; Tohidinik, H.R.; Naraghi, Z.S.; Abedini, R.; Nasimi, M.; Ghandi, N. Serum and tissue angiotensin-converting enzyme in patients with alopecia areata. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 295. [Google Scholar] [CrossRef]
- Ahmad, S. Enalapril and reversible alopecia. Arch. Intern. Med. 1991, 151, 404. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S. The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Rhie, A.; Son, H.-Y.; Kwak, S.J.; Lee, S.; Kim, D.Y.; Lew, B.-K.; Sim, W.-Y.; Seo, J.-S.; Kwon, O.; Kim, J.-I.; et al. Genetic variations associated with response to dutasteride in the treatment of male subjects with androgenetic alopecia. PLoS ONE 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Lee, J.H.; Seo, C.H.; Kim, J.C.; Kim, M.K.; Sung, Y.K. Dickkopf-1 is involved in dexamethasone-mediated hair follicle regression. Exp. Dermatol. 2017, 26, 952–954. [Google Scholar] [CrossRef]
- Choi, S.J.; Cho, A.R.; Jo, S.J.; Hwang, S.T.; Kim, K.H.; Kwon, O.S. Effects of glucocorticoid on human dermal papilla cells in vitro. J. Steroid Biochem. Mol. Biol. 2013, 135, 24–29. [Google Scholar] [CrossRef]
- Panchaprateep, R.; Asawanonda, P. Insulin-like growth factor-1: Roles in androgenetic alopecia. Exp. Dermatol. 2014, 23, 3. [Google Scholar] [CrossRef]
- Kang, Y.R.; Bak, S.-S.; Kim, M.-K.; Joo, H.W.; Mali, N.M.; Shin, M.J.; Kim, M.K.; Kim, J.C.; Oh, J.W.; Sung, Y.K. Expression Level of Prostaglandin D2 Receptor 2 Regulates Hair Regression. J. Investig. Dermatol. 2019, 139, 8. [Google Scholar] [CrossRef]
- Shin, D.W. The physiological and pharmacological roles of prostaglandins in hair growth. Korean J. Physiol. Pharmacol. 2022, 26, 6. [Google Scholar] [CrossRef]
- Colombe, L.; Michelet, J.F.; Bernard, B.A. Prostanoid receptors in anagen human hair follicles. Exp. Dermatol. 2008, 17, 63–72. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C. Prostaglandin D2 Inhibits Hair Growth and Is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Sci. Transl. Med. 2012, 4, 126. [Google Scholar] [CrossRef]
- Jeong, K.; Jung, J.; Kim, J.; Kang, H. Prostaglandin D2-Mediated DP2 and AKT Signal Regulate the Activation of Androgen Receptors in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2018, 19, 556. [Google Scholar] [CrossRef] [PubMed]
- Rafati, M.; Mahmoudian, R.; Golpour, M.; Kazeminejad, A.; Saeedi, M.; Nekouka, Z. The effect of latanoprost 0.005% solution in the management of scalp alopecia areata, a randomized double-blind placebo-controlled trial. Dermatol. Ther. 2022, 35. [Google Scholar] [CrossRef]
- Villarreal-Villarreal, C.D.; Sinclair, R.D.; Martínez-Jacobo, L.; Garza-Rodríguez, V.; Rodríguez-León, S.A.; Lamadrid-Zertuche, A.C.; Rodríguez-Gutierrez, R.; Ortiz-Lopez, R.; Rojas-Martinez, A.; Ocampo-Candiani, J. Prostaglandins in androgenetic alopecia in 12 men and four female. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 5. [Google Scholar] [CrossRef]
- Duncan, F.J.; Silva, K.A.; Johnson, C.J.; King, B.L.; Szatkiewicz, J.P.; Kamdar, S.P.; Ong, D.E.; Napoli, J.L.; Wang, J.; King, L.E., Jr. Endogenous Retinoids in the Pathogenesis of Alopecia Areata. J. Investig. Dermatol. 2013, 133, 2. [Google Scholar] [CrossRef]
- Buhl, A.E.; Baker, C.A.; Dietz, A.J. Minoxidil sulfotransferase activity influences the efficiency of Rogaine® topical solutions (ts)—Enzyme studies using scalp and platelets. J. Investig. Dermatol. 1994, 102, 534. [Google Scholar]
- Goren, A.; Mccoy, J.; Kovacevic, M.; Situm, M.; Chitalia, J.; Dhurat, R.; Naccarato, T.; Lotti, T. The effect of topical minoxidil treatment on follicular sulfotransferase enzymatic activity. J. Biol. Regul. Homeost. Agents. 2018, 32, 937–940. [Google Scholar]
- Wolf, B. Biotinidase Deficiency. In GeneReviews; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 2023. [Google Scholar]
- Patel, D.P.; Swink, S.M.; Castelo-Soccio, L. A Review of the Use of Biotin for Hair Loss. Ski. Appendage Disord. 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Georgala, S.; Schulpis, K.; Papakonstantinou, E.D.; Kalogirou, S.; Michas, T. Possible involvement of partial biotinidase deficiency in alopecia areata. J. Eur. Acad. Dermatol. Venereol. 1996, 135–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).