Insights into Microbiota in Sjögren’s Syndrome
Abstract
:1. Introduction
2. The Interaction between Human Microbiota and Immune System
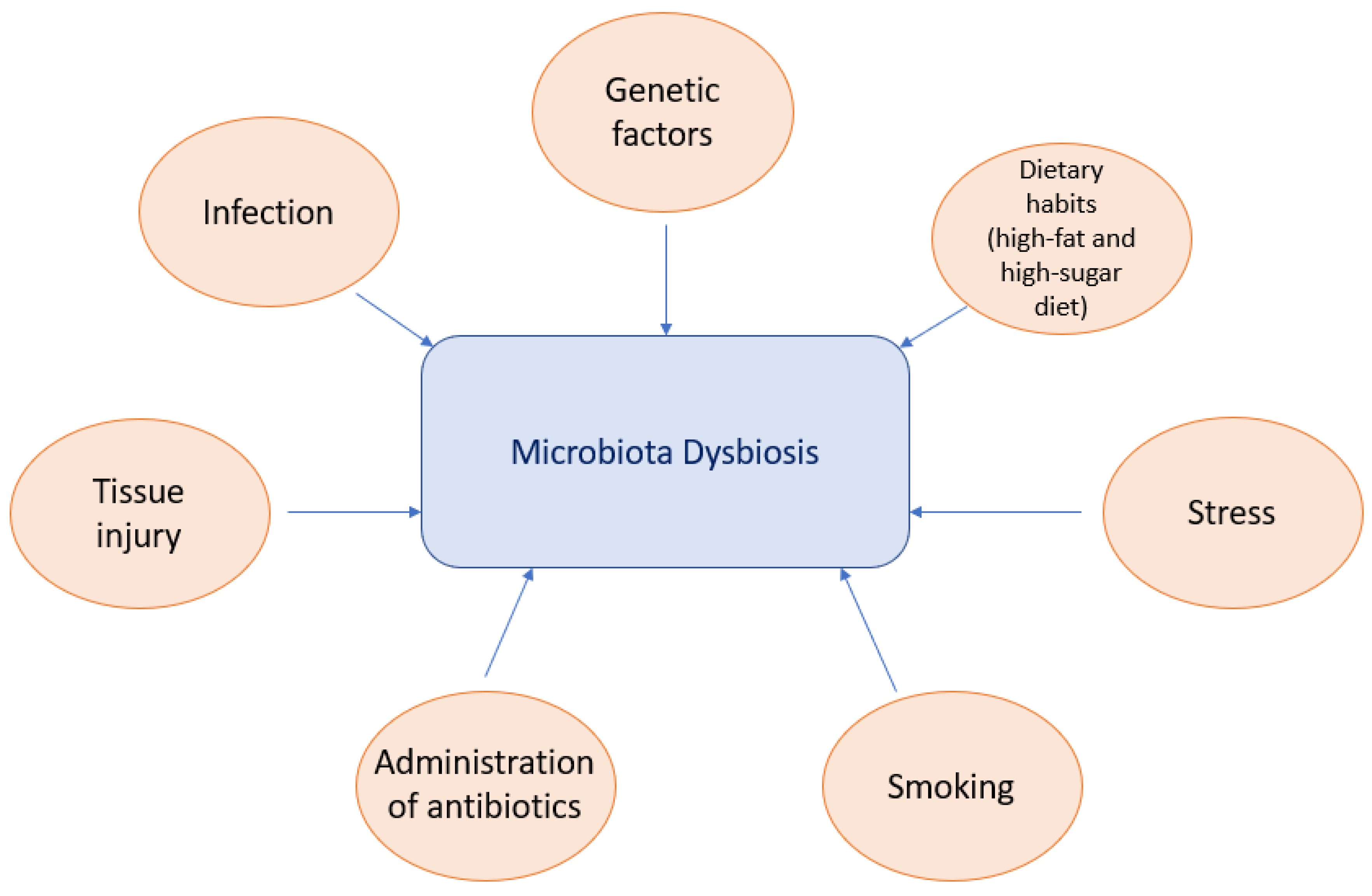
3. Factors Contributing to Microbiota Dysbiosis
4. Microbiota in Sjögren’s Syndrome
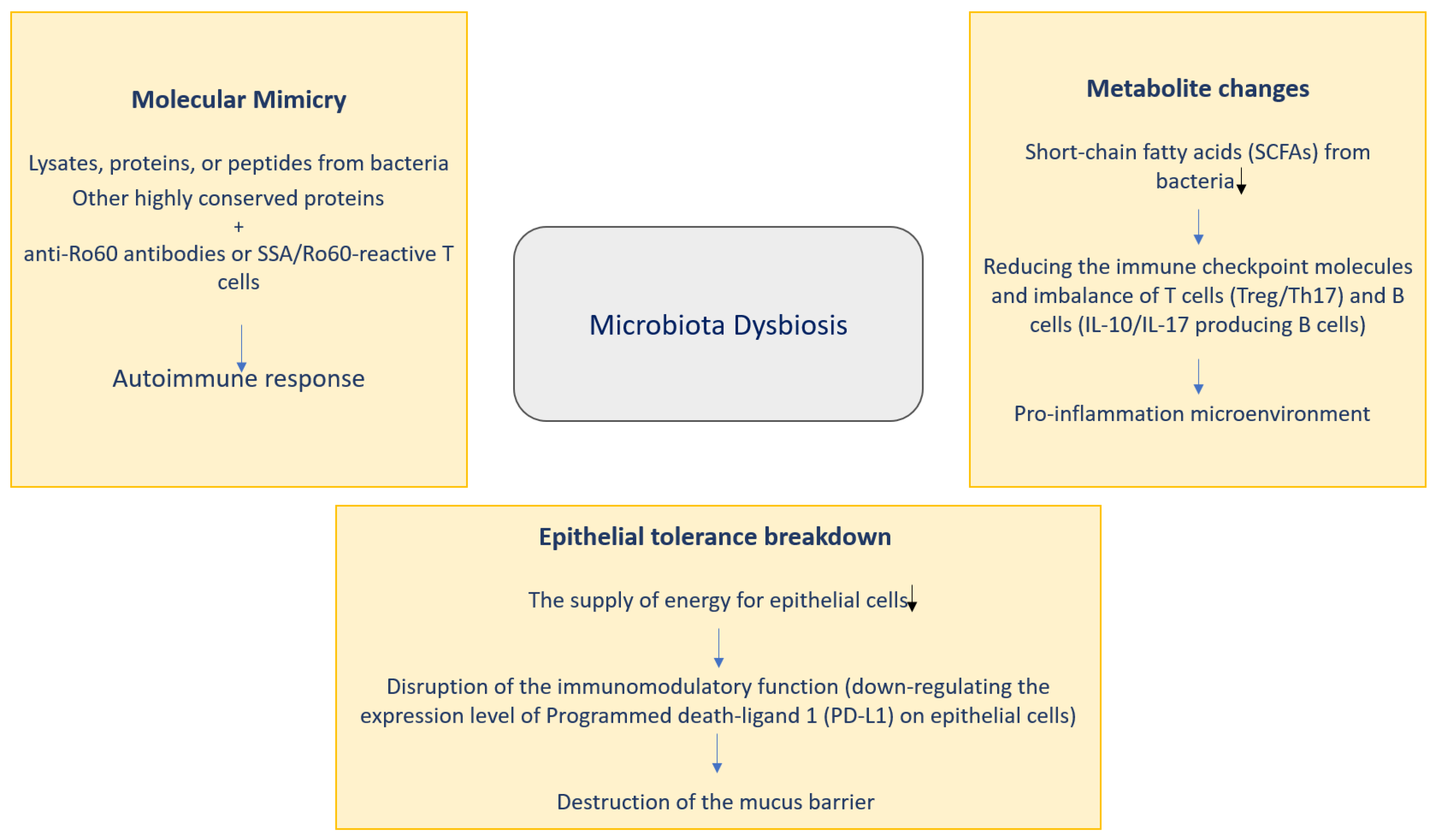
4.1. Effects of Microbiota on Sjögren’s Syndrome Pathogenesis
4.1.1. Molecular Mimicry
4.1.2. Metabolite Changes
4.1.3. Epithelial Tolerance Breakdown
4.2. Characteristic Changes in Microbiota in Patients with Sjögren’s Syndrome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manfrè:, V.; Chatzis, L.G.; Cafaro, G.; Fonzetti, S.S.; Calvacchi, S.; Fulvio, G.; Navarro Garcia, I.C.; La Rocca, G.; Ferro, F.; Perricone, C.; et al. Sjögren’s syndrome: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nakamura, H.; Kawakami, A. Role of the innate immunity signaling pathway in the pathogenesis of Sjogren’s syndrome. Int. J. Mol. Sci. 2021, 22, 3090. [Google Scholar] [CrossRef] [PubMed]
- Chivasso, C.; Sarrand, J.; Perret, J.; Delporte, C.; Soyfoo, M.S. The involvement of innate and adaptive immunity in the initiation and perpetuation of Sjogren’s syndrome. Int. J. Mol. Sci. 2021, 22, 658. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial–immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348. [Google Scholar] [CrossRef]
- Deng, C.; Xiao, Q.; Fei, Y. A Glimpse Into the Microbiome of Sjögren’s Syndrome. Front. Immunol. 2022, 13, 918619. [Google Scholar] [CrossRef]
- Hill, J.H.; Round, J.L. SnapShot: Microbiota effects on host physiology. Cell 2021, 184, 2796el. [Google Scholar] [CrossRef]
- Wolter, M.; Grant, E.T.; Boudaud, M.; Steimle, A.; Pereira, G.V.; Martens, E.C.; Desai, M.S. Leveraging diet to engineer the gut microbiome. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 885–902. [Google Scholar] [CrossRef]
- Svoboda, E. Gut feeling yields evidence of microbial involvement in autoimmunity. Nature 2021, 595, S54–S55. [Google Scholar] [CrossRef]
- van der Meulen, T.A.; Vissink, A.; Bootsma, H.; Spijkervet, F.K.L.; Kroese, F.G.M. Microbiome in Sjögren’s syndrome: Here we are. Ann. Rheum. Dis. 2022, 81, e114. [Google Scholar] [CrossRef]
- Hrestak, D.; Matijašič, M.; Cipčič Paljetak, H.; Ledič Drvar, D.; Ljubojevič Hadžavdič, S.; Perič, M. Skin Microbiota in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 3503. [Google Scholar] [CrossRef]
- Xue, X.; Yang, X.; Shi, X.; Deng, Z. Efficacy of probiotics in pediatric atopic dermatitis: A systematic review and meta-analysis. Clin. Transl. Allergy 2023, 13, e12283. [Google Scholar] [CrossRef] [PubMed]
- De Giani, A.; Zampolli, J.; Di Gennaro, P. Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: Diferent perspectives on their properties, challenges, and potential applications. Front. Microbiol. 2021, 12, 655150. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The host microbiome regulates and maintains human health: A primer and perspective for non-microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Bretin, A.; Lucas, C.; Larabi, A.; Dalmasso, G.; Billard, E.; Barnich, N.; Bonnet, R.; Nguyen, H.T.T. AIEC infection triggers modifcation of gut microbiota composition in genetically predisposed mice, contributing to intestinal infammation. Sci. Rep. 2018, 8, 12301. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Grossi, E.; Drago, L. Role of the human breast milk-associated microbiota on the newborns’ immune system: A mini review. Front. Microbiol. 2017, 8, 2100. [Google Scholar] [CrossRef]
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Freguia, C.F.; Pascual, D.W.; Fanger, G.R. Sjögren’s Syndrome Treatments in the Microbiome Era. Adv. Geriatr. Med. Res. 2023, 5, e230004. [Google Scholar]
- Requena, T.; Martinez-Cuesta, M.C.; Pelaez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Huang, Y.; Zheng, J.; Lu, Y.; Mai, Z.; Zhao, X.; Cui, L.; Huang, S. The oral microbiome in autoimmune diseases: Friend or foe? J. Transl. Med. 2023, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Fritz, B.M.; Munoz, B.; Yin, F.; Bauchle, C.; Atwood, B.K. A high-fat, high-sugar “western” diet alters dorsal striatal glutamate, opioid, and dopamine transmission in mice. Neuroscience 2018, 372, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Araki, Y.; Hanzawa, F.; Umeki, M.; Kojima, T.; Nishimura, N.; Ikedac, S.; Mochizuki, S.; Oda, H. High sucrose diet-induced dysbiosis of gut microbiota promotes fatty liver and hyperlipidemia in rats. J. Nutr. Biochem. 2021, 93, 108621. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yu, S.C.; Lo, Y.C.; Lin, I.H.; Tung, T.H.; Huang, S.Y. A high linoleic acid diet exacerbates metabolic responses and gut microbiota dysbiosis in obese rats with diabetes mellitus. Food Funct. 2019, 10, 786–798. [Google Scholar] [CrossRef]
- Chaves, I.M.; Zicker, M.C.; Laranjeira, A.O.; Silveira, A.L.M.; Aguiar, D.C.; Barrioni, B.R.; Ferreira, A.V.M.; Teixeira, M.M.; da Silva, T.A.; de Souza, D.G.; et al. Dysbiotic oral microbiota contributes to alveolar bone loss associated with obesity in mice. J. Appl. Oral. Sci. 2022, 30, e20220238. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Lv, X.-C.; Guo, W.-L.; Li, L.; Yu, X.-D.; Liu, B. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J. Funct. Foods 2019, 57, 48–58. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Nia, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Wu, H.; Ma, Y.; Peng, X.; Qiu, W.; Kong, L.; Ren, B.; Li, M.; Cheng, G.; Zhou, X.; Lei Cheng, L. Antibiotic-induced dysbiosis of the rat oral and gut microbiota and resistance to Salmonella. Arch. Oral. Biol. 2020, 114, 104730. [Google Scholar] [CrossRef]
- Vangoitsenhoven, R.; Cresci, G.A.M. Role of microbiome and antibiotics in autoimmune diseases. Nutr. Clin. Pract. 2020, 35, 406–416. [Google Scholar] [CrossRef]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J. Oral. Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef]
- Al-Attar, A.; Alimova, Y.; Kirakodu, S.; Kozal, A.; Novak, M.J.; Stromberg, A.J.; Orraca, M.J.L.; Gonzalez-Martinez, J.; Martinez, M.; Ebersole, J.L.; et al. Activation of notch-1 in oral epithelial cells by P. gingivalis triggers the expression of the antimicrobial protein PLA(2)-IIA. Mucosal Immunol. 2018, 11, 1047–1059. [Google Scholar] [CrossRef]
- Paudel, D.; Uehara, O.; Giri, S.; Yoshida, K.; Morikawa, T.; Kitagawa, T.; Matsuoka, H.; Miura, H.; Toyofuku, A.; Kuramitsu, Y.; et al. Effect of psychological stress on the oral-gut microbiota and the potential oral-gut-brain axis. Jpn. Dent. Sci. Rev. 2022, 58, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The dynamic interplay between the gut microbiota and autoimmune diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Watanabe, T.; Kamata, K.; Hara, A.; Minaga, K.; Kudo, M. Intestinal dysbiosis and autoimmune pancreatitis. Front Immunol. 2021, 12, 621532. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, Y.; Ma, L.; Ma, X.; Shen, L.; Chen, Z.; Chen, H.; Li, D.; Su, Z.; Chen, X. Constipation induced gut microbiota dysbiosis exacerbates experimental autoimmune encephalomyelitis in C57BL/6 mice. J. Transl. Med. 2021, 19, 317. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, H.; Xu, W.; Zhong, M. Gut microbiota and Sjögren’s syndrome: A two-sample Mendelian randomization study. Front. Immunol. 2023, 14, 1187906. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, C.; Zeng, X.; Wang, Q. The role of gut microbiota in the pathogenesis of rheumatic diseases. Clin. Rheumatol. 2018, 37, 25–34. [Google Scholar] [CrossRef]
- van der Meulen, T.A.; Harmsen, H.J.M.; Vila, A.V.; Kurilshikov, A.; Liefers, S.C.; Zhernakova, A.; Fud, J.; Wijmengad, C.; Weersma, R.K.; de Leeuwe, K.; et al. Shared gut, but distinct oral microbiota composition in primary sjögren’s syndrome and systemic lupus erythematosus. J. Autoimmun. 2019, 97, 77–87. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “Known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Xu, J.; Wei, X.; Yang, J.; Liu, Y.; Li, H.; Zhao, C.; Wang, Y.; Zhang, L.; et al. Helicobacter pylori infection aggravates dysbiosis of gut microbiome in children with gastritis. Front. Cell. Infect. Microbiol. 2019, 9, 375. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Jiang, H.; Xiang, S.; Zhao, Y.; Xiao, M.; Du, F.; Ji, H.; Kaboli, P.H.; Wu, X.; et al. Metagenome analysis of intestinal bacteria in healthy people, patients with inflammatory bowel disease and colorectal cancer. Front. Cell. Infect. Microbiol. 2021, 11, 599734. [Google Scholar] [CrossRef]
- Greiling, T.M.; Dehner, C.; Chen, X.; Hughes, K.; Iñiguez, A.J.; Boccitto, M.; Ruiz, D.Z.; Renfroe, S.C.; Vieira, S.M.; Ru, W.E.; et al. Commensal Orthologs of the Human Autoantigen Ro60 as Triggers of Autoimmunity in Lupus. Sci. Transl. Med. 2018, 10, eaan2306. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Ueshiba, H.; Abe, Y.; Kato, H.; Higuchi, T.; Yagi, J. Outer Membrane Protein of Gut Commensal Microorganism Induces Autoantibody Production and Extra-Intestinal Gland Inflammation in Mice. Int. J. Mol. Sci. 2018, 19, 3241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Minardi, L.M.; Kuenstner, J.T.; Zekan, S.M.; Kruzelock, R. Anti-Microbial Antibodies, Host Immunity, and Autoimmune Disease. Front. Med. 2018, 5, 153. [Google Scholar] [CrossRef]
- Bellocchi, C.; Fernández-Ochoa, Á.; Montanelli, G.; Vigone, B.; Santaniello, A.; Quirantes-Piné, R.; Borrás-Linares, I.; Gerosa, M.; Artusi, C.; Gualtierotti, R.; et al. Identification of a shared microbiomic and metabolomic profile in systemic autoimmune diseases. J. Clin. Med. 2019, 8, 1291. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Choi, S.H.; Yoon, C.H.; Kim, M.K. Gut dysbiosis is prevailing in sjögren’s syndrome and is related to dry eye severity. PLoS ONE 2020, 15, e0229029. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Laborda-Illanes, A.; Plaza-Andrades, I.; Membrillo Del Pozo, A.; Villarrubia Cuadrado, A.; Rodrıguez Calvo de Mora, M.; Leiva-Gea, I.; Sanchez-Alcoholado, L.; Queipo-Ortuño, M.I. Connection between the gut microbiome, systemic inflammation, gut permeability and FOXP3 expression in patients with primary Sjögren’s syndrome. Int. J. Mol. Sci. 2020, 21, 8733. [Google Scholar] [CrossRef]
- Lee, J.; Alam, J.; Choi, E.; Ko, Y.K.; Lee, A.; Choi, Y. Association of a dysbiotic oral microbiota with the development of focal lymphocytic sialadenitis in IkB-z-deficient mice. NPJ Biofilms Microbiomes 2020, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Woo, J.S.; Min, H.K.; Choi, J.W.; Moon, J.H.; Park, M.J.; Kwok, S.K.; Park, S.H.; Cho, M.L. Short-Chain Fatty Acid Butyrate Induces IL-10-Producing B Cells by Regulating Circadian-Clock-Related Genes to Ameliorate Sjögren’s Syndrome. J. Autoimmun. 2021, 119, 102611. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Lee, A.; Lee, J.; Kwon, D.I.; Park, H.K.; Park, J.H.; Jeon, S.; Baek, K.; Lee, J.; Park, S.H.; et al. Dysbiotic Oral Microbiota and Infected Salivary Glands in Sjögren’s Syndrome. PLoS ONE 2020, 15, e0230667. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Yang, H.Y.; Lin, W.T.; Chang, C.B.; Chien, H.C.; Wang, H.P.; Chen, C.M.; Wang, J.T.; Li, C.; Wu, S.F.; et al. Salivary Dysbiosis in Sjögren’s Syndrome and a Commensal-Mediated Immunomodulatory Effect of Salivary Gland Epithelial Cells. NPJ Biofilms Microbiomes 2021, 7, 21. [Google Scholar] [CrossRef]
- Wright, D.P.; Rosendale, D.I.; Robertson, A.M. Prevotella Enzymes Involved in Mucin Oligosaccharide Degradation and Evidence for a Small Operon of Genes Expressed During Growth on Mucin. FEMS Microbiol. Lett. 2000, 190, 73–79. [Google Scholar] [CrossRef]
- Terzulli, M.; Contreras-Ruiz, L.; Kugadas, A.; Masli, S.; Gadjeva, M. TSP-1 Deficiency Alters Ocular Microbiota: Implications for Sjögren’s Syndrome Pathogenesis. J. Ocul. Pharmacol. Ther. 2015, 31, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Mandl, T.; Marsal, J.; Olsson, P.; Ohlsson, B.; Andréasson, K. Severe Intestinal Dysbiosis is Prevalent in Primary Sjögren’s Syndrome and is Associated With Systemic Disease Activity. Arthritis Res. Ther. 2017, 19, 237. [Google Scholar] [CrossRef]
- de Paiva, C.S.; Jones, D.B.; Stern, M.E.; Bian, F.; Moore, Q.L.; Corbiere, S.; Streckfus, C.F.; Hutchinson, D.S.; Ajami, N.J.; Petrosino, J.F.; et al. Altered Mucosal Microbiome Diversity and Disease Severity in Sjögren Syndrome. Sci. Rep. 2016, 6, 23561. [Google Scholar] [CrossRef]
- Pal, S.; Vani, G.; Donthineni, P.R.; Basu, S.; Arunasri, K. Tear film microbiome in Sjogren’s and Non-Sjogren’s aqueous deficiency dry eye. Indian J. Ophthalmol. 2023, 71, 1566–1573. [Google Scholar] [CrossRef]
- Wang, X.; Pang, K.; Wang, J.; Zhang, B.; Liu, Z.; Lu, S.; Xu, X.; Zhu, L.; Zhou, Z.; Niu, M.; et al. Microbiota dysbiosis in primary Sjogren’s syndrome and the ameliorative effect of hydroxychloroquine. Cell Rep. 2022, 40, 111352. [Google Scholar] [CrossRef]
- Xing, H.; Liu, H.; Pan, J. High-Throughput Sequencing of Oral Microbiota in Candida Carriage Sjögren’s Syndrome Patients: A Pilot Cross-Sectional Study. J. Clin. Med. 2023, 12, 1559. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, M.; He, Y.; Liu, T.; Xu, M. Anxiety Disorders and Gut Dysbiosis in Primary Sjögren’s Syndrome-Mediated Dry Eye Patients. Int. J. Gen. Med. 2023, 16, 1735–1746. [Google Scholar] [CrossRef]
- Orliaguet, M.; Fong, S.B.; Le Pottier, L.; Meuric, V.; Boisramé, S.; Bonnaure-Mallet, M.; Pers, J.O. Tolerance to intraoral biofilms and their effectiveness in improving mouth dryness and modifying oral microbiota in patients with primary Sjögren’s syndrome: “Predelfi study”. Front. Microbiol. 2023, 14, 1071683. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.; Wang, C.; Bian, F.; Yu, Z.; Hernandez, H.; de Souza, R.G.; Simmons, K.T.; Schady, D.; Swennes, A.G.; Pflugfelder, S.C.; et al. Protective Role of Commensal Bacteria in Sjögren Syndrome. J. Autoimmun. 2018, 93, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhufeng, Y.; Chen, Z.; Xu, J.; Cheng, Y. The composition and function profile of the gut microbiota of patients with primary Sjögren’s syndrome. Clin. Rheumatol. 2023, 42, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Hwang, S.H.; Yang, S.C.; Lee, K.H.; Lee, Y.S.; Choi, J.W.; Park, J.S.; Jhun, J.Y.; Park, S.H.; Cho, M.L. Lactobacillus acidophilus and propionate attenuate Sjögren’s syndrome by modulating the STIM1-STING signaling pathway. Cell Commun. Signal. 2023, 21, 135. [Google Scholar] [CrossRef]
| Reference | Sample | Case and Control | Conclusion of the Study |
|---|---|---|---|
| [38] | Gut microbiome | - | Family Porphyromonadaceae, genus Subdoligranulum, genus Butyricicoccus and genus Lachnospiraceae are associated with a lower chance of SS developing. Genus Fusicatenibacter and genus Ruminiclostridium9 increase chance of SS developing. |
| [60] | Tear film |
| Changes in the phyla and genera in SS and non-SS are significant (compared with healthy controls). An expected relation of prevalent pro-inflammatory bacteria with SS and non-SS. |
| [61] | Fecal, oral and vaginal samples |
| Microbial shifts could appear prior to pSS. |
| [62] | Saliva |
| The primary genera for all: Treponema, Lactobacillus, Streptococcus, Selenomonas and Veillonella. The most abundant significantly mutative taxonomy (I001): Veillonella parvula. Microbial diversity significantly increased in SS. Different microbial compositional heterogeneity in SS. Microbial dysbiosis differs in SS independent of oral Candida carriage and DMFT. |
| [63] | Fecal samples |
| Correlation between anxiety disorders and intestinal dysbiosis. Prevotella is associated with seriousness of dry eye. |
| [64] | Single-center, prospective, comparative, randomized, double-blind, cross-over controlled study to assess the tolerance and effectiveness of adhesive biofilms |
| The sodium alginate biofilm increased the abundance of the Treponema genus in mouth. The prebiotic biofilm increased the abundance of the genera Veillonella and Prevotella. Pre-treatment with the prebiotic biofilm prevented the emergence of the Treponema genus induced by subsequent treatment with the sodium alginate biofilm. Pre-treatment with the prebiotic biofilm has a potential protective effect. |
| [65] | Fecal samples |
| The composition and function of the intestinal microbiota are common in pSS patients. Certain genera and species are associated with seriousness and therapy defiance of pSS disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieliauskaitė, D.; Kontenis, V. Insights into Microbiota in Sjögren’s Syndrome. Medicina 2023, 59, 1661. https://doi.org/10.3390/medicina59091661
Mieliauskaitė D, Kontenis V. Insights into Microbiota in Sjögren’s Syndrome. Medicina. 2023; 59(9):1661. https://doi.org/10.3390/medicina59091661
Chicago/Turabian StyleMieliauskaitė, Diana, and Vilius Kontenis. 2023. "Insights into Microbiota in Sjögren’s Syndrome" Medicina 59, no. 9: 1661. https://doi.org/10.3390/medicina59091661





