Effects of Aphrodite (an Herbal Compound) on SSRI-Induced Sexual Dysfunctions and Depression in Females with Major Depressive Disorder: Findings from a Randomized Clinical Trial
Abstract
:1. Introduction
The Current Study
2. Method
2.1. Procedure
2.2. Randomization and Sample Size Calculation
2.3. Participants
2.4. Compounds
2.4.1. Aphrodite
2.4.2. Placebo
2.5. Measures
2.5.1. Sociodemographic Information
2.5.2. Assessing SSRI-Induced Sexual Dysfunction
2.5.3. Sexual Functions
2.5.4. Symptoms of Depression
2.5.5. Symptoms of Anxiety
2.5.6. Sleep Quality
2.6. Statistical Analysis
Statistics Were Performed per Protocol
3. Results
3.1. Sample Characteristics
3.2. Sexual Function, Symptoms of Depression and Anxiety, and Sleep Quality in the Aphrodite and Placebo Conditions
3.3. Sexual Function
3.3.1. Main Effects
3.3.2. Post-Hoc Comparisons
3.4. Symptoms of Depression
3.4.1. Main Effects
3.4.2. Post-Hoc Comparisons
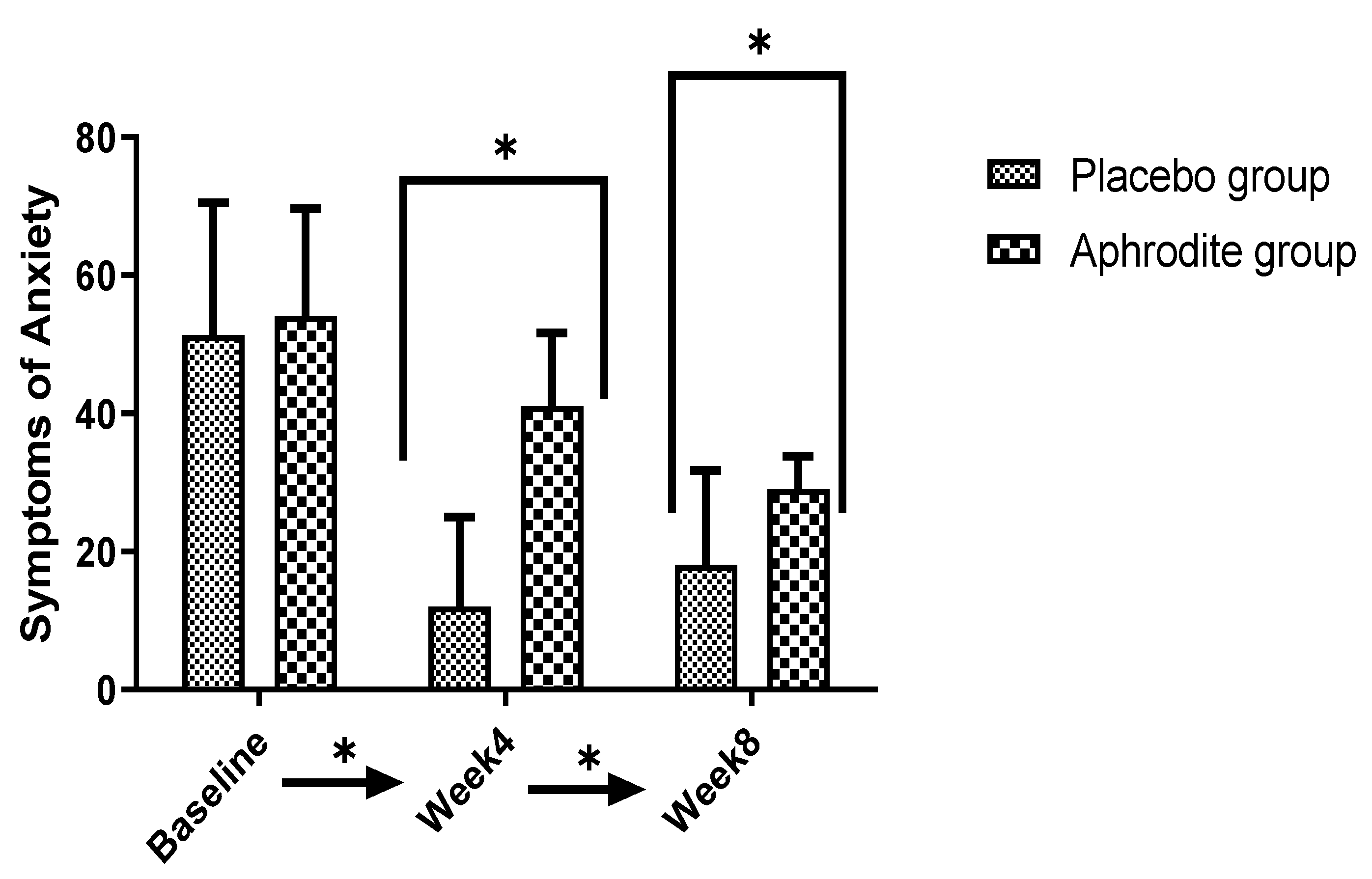
3.5. Symptoms of Anxiety
3.5.1. Main Effects
3.5.2. Post-Hoc Comparisons
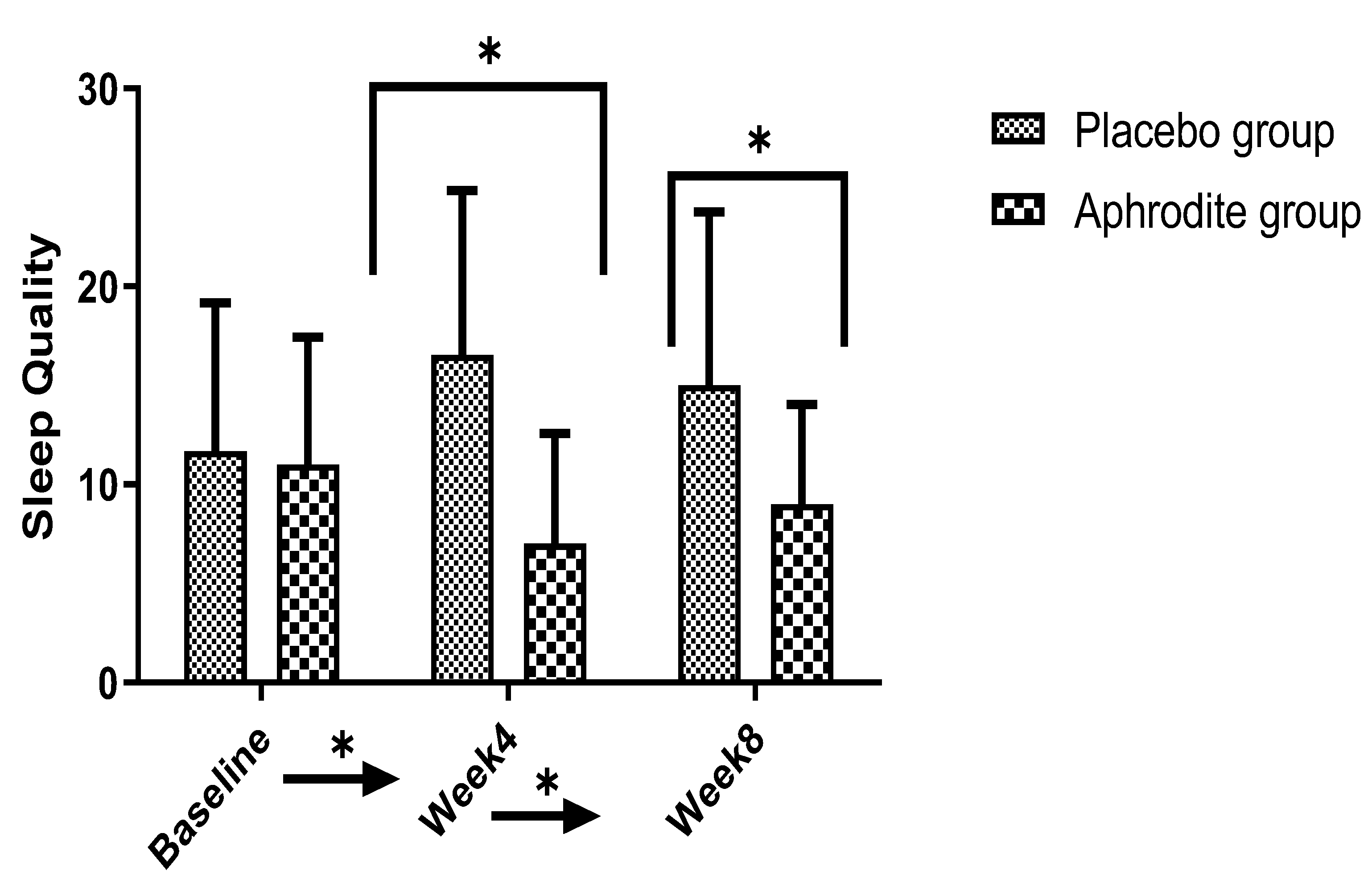
3.6. Sleep Quality
3.6.1. Main Effects
3.6.2. Post-Hoc Comparisons
4. Discussion
4.1. Sexual Dysfunctions
4.2. Symptoms of Depression
4.3. Symptoms of Anxiety
4.4. Sleep Disturbances
4.5. Psychiatric and Psychological Considerations
4.6. Strengths
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; DSM-IV-TR; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- World Health Organization. ICD-11: International Classification of Diseases, 11th revision ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Dubovsky, S.L.; Ghosh, B.M.; Serotte, J.C.; Cranwell, V. Psychotic Depression: Diagnosis, Differential Diagnosis, and Treatment. Psychother. Psychosom. 2021, 90, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Hadianfar, A.; Esmaily, H.; Ghayour-Mobarhan, M.; Aghajani, H.; Saki, A.; Tayefi, M.; Hosseini, F.; Sabouri, S. Spatial Modeling of Depression and Its Related Factors Using a Spatial Generalized Linear Mixed Model in Mashhad, Iran: A Cross-Sectional Study. Shiraz E-Med. J. 2019, 20, e87532. [Google Scholar] [CrossRef]
- Ahmadpanah, M.; Ramezanshams, F.; Ghaleiha, A.; Akhondzadeh, S.; Bahmani, D.S.; Brand, S. Crocus sativus L. (saffron) versus sertraline on symptoms of depression among older people with major depressive disorders–a double-blind, randomized intervention study. Psychiatry Res. 2019, 282, 112613. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, K.; Paludan-Müller, A.S.; Boesen, K. Considering the methodological limitations in the evidence base of antidepressants for depression: A reanalysis of a network meta-analysis. BMJ Open 2019, 9, e024886. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.P. Scientific debate instead of beef; challenging misleading arguments about the efficacy of antidepressants. Acta Neuropsychiatr. 2019, 31, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Maag, J.W.; Swearer, S.M. Cognitive-behavioral interventions for depression: Review and implications for school personell. Behav. Disord. 2005, 30, 259–276. [Google Scholar] [CrossRef]
- Cristea, I.A.; Huibers, M.J.; David, D.; Hollon, S.D.; Andersson, G.; Cuijpers, P. The effects of cognitive behavior therapy for adult depression on dysfunctional thinking: A meta-analysis. Clin. Psychol. Rev. 2015, 42, 62–71. [Google Scholar] [CrossRef]
- Cuijpers, P.; Andersson, G.; Donker, T.; van Straten, A. Psychological treatment of depression: Results of a series of meta-analyses. Nord. J. Psychiatry 2011, 65, 354–364. [Google Scholar] [CrossRef]
- Cuijpers, P.; Berking, M.; Andersson, G.; Quigley, L.; Kleiboer, A.; Dobson, K.S. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison, with other treatments. Can. J. Psychiatry 2013, 58, 376–385. [Google Scholar] [CrossRef]
- Cuijpers, P.; Noma, H.; Karyotaki, E.; Cipriani, A.; Furukawa, T.A. Effectiveness and Acceptability of Cognitive Behavior Therapy Delivery Formats in Adults With Depression: A Network Meta-analysis. JAMA Psychiatry 2019, 76, 700–707. [Google Scholar] [CrossRef]
- Khazaie, H.; Norouzi, E.; Rezaie, L.; Safari-Faramani, R. Effect of physical activity on sleep quality in patients with major depression disorder: A systematic review and meta-analysis of randomized controlled trials. Curr. Psychol. 2022, 1–11. [Google Scholar] [CrossRef]
- Yoldi-Negrete, M.; Gill, L.N.; Olivares, S.; Lauzière, A.; Désilets, M.; Tourjman, S.V. The effect of continuation and maintenance electroconvulsive therapy on cognition: A systematic review of the literature and meta-analysis. J. Affect. Disord. 2022, 316, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Hawken, E.R.; Sepehry, A.A.; Cabrera, C.A.; Vazquez, G. ECT beyond unipolar major depression: Systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr. Scand. 2019, 139, 214–226. [Google Scholar] [CrossRef]
- Haq, A.U.; Sitzmann, A.F.; Goldman, M.L.; Maixner, D.F.; Mickey, B.J. Response of depression to electroconvulsive therapy: A meta-analysis of clinical predictors. J. Clin. Psychiatry 2015, 76, 1374–1384. [Google Scholar] [CrossRef]
- Kellner, C.H.; Greenberg, R.M.; Murrough, J.W.; Bryson, E.O.; Briggs, M.C.; Pasculli, R.M. ECT in treatment-resistant depression. Am. J. Psychiatry 2012, 169, 1238–1244. [Google Scholar] [CrossRef]
- Rhee, T.G.; Shim, S.R.; Forester, B.P.; Nierenberg, A.A.; McIntyre, R.S.; Papakostas, G.I.; Krystal, J.H.; Sanacora, G.; Wilkinson, S.T. Efficacy and Safety of Ketamine vs. Electroconvulsive Therapy Among Patients With Major Depressive Episode: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 1162–1172. [Google Scholar] [CrossRef]
- Wilhelmy, S.; Brühl, A.B.; Himmighoffen, H.; Conca, A.; Grözinger, M. Electroconvulsive Therapy in Switzerland: A Survey on Contemporary Practice in Remembrance of a Historical Meeting. J. ECT 2023, 39, 197–201. [Google Scholar] [CrossRef]
- Jahangard, L.; Sadeghi, A.; Ahmadpanah, M.; Holsboer-Trachsler, E.; Bahmani, D.S.; Haghighi, M.; Brand, S. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders-Results from a double-blind, randomized and placebo-controlled clinical trial. J. Psychiatr. Res. 2018, 107, 48–56. [Google Scholar] [CrossRef]
- Apaydin, E.A.; Maher, A.R.; Shanman, R.; Booth, M.S.; Miles, J.N.; Sorbero, M.E.; Hempel, S. A systematic review of St. John’s wort for major depressive disorder. Syst. Rev. 2016, 5, 148. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Gómez, A.F. Mindfulness-Based Interventions for Anxiety and Depression. Psychiatr. Clin. N. Am. 2017, 40, 739–749. [Google Scholar] [CrossRef]
- Jakobsen, J.C.; Katakam, K.K.; Schou, A.; Hellmuth, S.G.; Stallknecht, S.E.; Leth-Møller, K.; Iversen, M.; Banke, M.B.; Petersen, I.J.; Klingenberg, S.L.; et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry 2017, 17, 58. [Google Scholar] [CrossRef]
- Gibbons, R.D.; Hur, K.; Brown, C.H.; Davis, J.M.; Mann, J.J. Benefits from antidepressants: Synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch. Gen. Psychiatry 2012, 69, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Henssler, J.; Alexander, D.; Schwarzer, G.; Bschor, T.; Baethge, C. Combining antidepressants vs. antidepressant monotherapy for treatment of patients with acute depression: A systematic review and meta-analysis. JAMA Psychiatry 2022, 79, 300–312. [Google Scholar] [CrossRef]
- Ekhart, G.C.; van Puijenbroek, E.P. Does sexual dysfunction persist upon discontinuation of selective serotonin reuptake inhibitors? Tijdschr. Psychiatr. 2014, 56, 336–340. [Google Scholar] [PubMed]
- Dubovicky, M.; Belovicova, K.; Csatlosova, K.; Bogi, E. Risks of using SSRI/SNRI antidepressants during pregnancy and lactation. Interdiscip. Toxicol. 2017, 10, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Zemishlany, Z.; Weizman, A. The impact of mental illness on sexual dysfunction. Sex. Dysfunct. 2008, 29, 89–106. [Google Scholar]
- Healy, D. Citizen petition: Sexual side effects of SSRIs and SNRIs. Int. J. Risk Saf. Med. 2018, 29, 135. [Google Scholar] [CrossRef]
- Rosenberg, K.P.; Bleiberg, K.L.; Koscis, J.; Gross, C. A survey of sexual side effects among severely mentally ill patients taking psychotropic medications: Impact on compliance. J. Sex Marital Ther. 2003, 29, 289–296. [Google Scholar] [CrossRef]
- Flynn, K.E.; Lin, L.; Bruner, D.W.; Cyranowski, J.M.; Hahn, E.A.; Jeffery, D.D.; Reese, J.B.; Reeve, B.B.; Shelby, R.A.; Weinfurt, K.P. Sexual Satisfaction and the Importance of Sexual Health to Quality of Life Throughout the Life Course of U.S. Adults. J. Sex. Med. 2016, 13, 1642–1650. [Google Scholar] [CrossRef]
- Dogan, T.; Tugut, N.; Golbasi, Z. The Relationship Between Sexual Quality of Life, Happiness, and Satisfaction with Life in Married Turkish Women. Sex. Disabil. 2013, 31, 239–247. [Google Scholar] [CrossRef]
- Sánchez-Fuentes, M.D.M.; Santos-Iglesias, P.; Sierra, J. A systematic review of sexual satisfaction. Int. J. Clin. Health Psychol. 2014, 14, 67–75. [Google Scholar] [CrossRef]
- Kessler, D.; Bennewith, O.; Lewis, G.; Sharp, D. Detection of depression and anxiety in primary care: Follow up study. BMJ 2002, 325, 1016–1017. [Google Scholar] [CrossRef] [PubMed]
- Unützer, J.; Klap, R.; Sturm, R.; Young, A.S.; Marmon, T.; Shatkin, J.; Wells, K.B. Mental disorders and the use of alternative medicine: Results from a national survey. Am. J. Psychiatry 2000, 157, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Makgahlela, M.; Mabidilala, M.; Lesolang, N.; Jidong, D.E.; Monera-Penduka, T.G. Using traditional medicine to help with bereavement loss and coping: An interpretative phenomenological analysis of traditional healers’ experiences. J. Ment. Health Train. Educ. Pract. 2022, 17, 145–158. [Google Scholar] [CrossRef]
- Hou, J.; Bhat, A.M.; Ahmad, S.; Raza, K.; Qazi, S. In silico analysis of ACE2 receptor to find potential herbal drugs in COVID-19 associated neurological dysfunctions. Nat. Prod. Commun. 2022, 17, 1934578X221118549. [Google Scholar] [CrossRef]
- Chan, K. Chinese medicinal materials and their interface with Western medical concepts. J. Ethnopharmacol. 2005, 96, 1–18. [Google Scholar] [CrossRef]
- Kessler, R.C.; Soukup, J.; Davis, R.B.; Foster, D.F.; Wilkey, S.A.; Van Rompay, M.I.; Eisenberg, D.M. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am. J. Psychiatry 2001, 158, 289–294. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Wang, Y.; Wang, P.; Li, Y.; Li, B. Herbal medicine for anxiety, depression and insomnia. Curr. Neuropharmacol. 2015, 13, 481–493. [Google Scholar] [CrossRef]
- Farnia, V.; Tatari, F.; Alikhani, M.; Yazdchi, K.; Taghizadeh, M.; Sadeghi, B.D.; Karbasizadeh, H.; Holsboer-Trachsler, E.; Brand, S. Rosa Damascena oil improved methadone-related sexual dysfunction in females with opioid use disorder under methadone maintenance therapy—results from a double-blind, randomized, and placebo-controlled trial. J. Psychiatr. Res. 2017, 95, 260–268. [Google Scholar] [CrossRef]
- Pearson, N.J.; Johnson, L.L.; Nahin, R.L. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch. Intern. Med. 2006, 166, 1775–1782. [Google Scholar] [CrossRef]
- Clayton, A.H.; El Haddad, S.; Iluonakhamhe, J.P.; Ponce Martinez, C.; Schuck, A.E. Sexual dysfunction associated with major depressive disorder and antidepressant treatment. Expert Opin. Drug Saf. 2014, 13, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y. Insight into the characteristics of research published in traditional, complementary, alternative, and integrative medicine journals: A bibliometric analysis. BMC Complement. Med. Ther. 2021, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Taavoni, S.; Ekbatani, N.N.; Gooshegir, A.; Haghani, H. Effect of Aphrodit capsule on somatic symptoms of postmenopausal women. J. Gorgan Univ. Med. Sci. 2016, 17, 10–15. [Google Scholar]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hariri, M.; Abbasi, B. Effects of Cinnamon, Cardamom, Saffron, and Ginger Consumption on Markers of Glycemic Control, Lipid Profile, Oxidative Stress, and Inflammation in Type 2 Diabetes Patients. Rev. Diabet. Stud. 2014, 11, 258–266. [Google Scholar] [CrossRef]
- Kavianipour, F.; Aryaeian, N.; Mokhtare, M.; Mirnasrollahiparsa, R.; Jannani, L.; Agah, S.; Fallah, S.; Moradi, N. The effect of saffron supplementation on some inflammatory and oxidative markers, leptin, adiponectin, and body composition in patients with nonalcoholic fatty liver disease: A double-blind randomized clinical trial. Phytother. Res. 2020, 34, 3367–3378. [Google Scholar] [CrossRef]
- Jalali, M.; Mahmoodi, M.; Moosavian, S.P.; Jalali, R.; Ferns, G.; Mosallanezhad, A.; Imanieh, M.H.; Mosallanezhad, Z. The effects of ginger supplementation on markers of inflammatory and oxidative stress: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2020, 34, 1723–1733. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A.; Gajjar, A. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, J.-W.; Zhao, J.-H.; Tang, L.-N.; Zhang, J.-J. Herbal insomnia medications that target GABAergic systems: A review of the psychopharmacological evidence. Curr. Neuropharmacol. 2014, 12, 289–302. [Google Scholar] [CrossRef]
- Srivastava, A.; Chaurasia, J.; Khan, R.; Dhand, C.; Verma, S. Role of medicinal plants of traditional use in recuperating devastating COVID-19 situation. Med. Aromat. Plants 2020, 9, 2167-0412. [Google Scholar]
- Sellandi, T.M.; Thakar, A.B.; Baghel, M.S. Clinical study of Tribulus terrestris Linn. in Oligozoospermia: A double blind study. Ayu 2012, 33, 356–364. [Google Scholar] [CrossRef]
- Neychev, V.K.; Mitev, V.I. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men. J. Ethnopharmacol. 2005, 101, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A. Mondia whitei, a medicinal plant from Africa with aphrodisiac and antidepressant properties: A review. J. Diet Suppl. 2012, 9, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Taavoni, S.; Nazem Ekbatani, N.; Haghani, H. The Effect of Aphrodite on Orgasm and Sexual Desire in Menopausal Women: A Randomized Clinical Trial. J. Hayat 2016, 22, 1–12. [Google Scholar]
- Luk, B.H.-K.; Loke, A.Y. The impact of infertility on the psychological well-being, marital relationships, sexual relationships, and quality of life of couples: A systematic review. J. Sex Marital Ther. 2015, 41, 610–625. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM 5; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- First, M. Structured Clinical Interview for the DSM (SCID); John Wiley & Sons, Inc: Hoboken, NJ, USA, 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Shokrollahi, P.; Mirmohamadi, M.; Mehrabi, F.; Babaei, G. Prevalence of sexual dysfunction in women seeking services at family planning centers in Tehran. J. Sex Marital Ther. 1999, 25, 211–215. [Google Scholar] [CrossRef]
- Syed, J.S.; Honig, S. Sexual Metrics in Transgender Women: Transitioning From International Index of Erectile Function to Female Sexual Function Index. Sex. Med. Rev. 2021, 9, 236–243. [Google Scholar] [CrossRef]
- Wiegel, M.; Meston, C.; Rosen, R. The female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. J. Sex Marital Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef]
- Ghassemzadeh, H.; Mojtabai, R.; Karamghadiri, N.; Ebrahimkhani, N. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second edition: BDI-II-PERSIAN. Depress. Anxiety 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Hojat, M.; Shapurian, R.; Mehryar, A.H. Psychometric properties of a Persian version of the short form of the Beck Depression Inventory for Iranian college students. Psychol. Rep. 1986, 59, 331–338. [Google Scholar] [CrossRef]
- Kaviani, H.; Mousavi, A.S. Psychometric properties of the Persian version of Beck Anxiety Inventory (BAI). Tehran Univ. Med. J. 2008, 66, 136–140. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Chehri, A.; Nourozi, M.; Eskandari, S.; Khazaie, H.; Hemati, N.; Jalali, A. Validation of the Persian version of the Pittsburgh Sleep Quality Index in elderly population. Sleep Sci. 2020, 13, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Farrahi, J.; Nakhaee, N.; Sheibani, V.; Garrusi, B.; Amirkafi, A. Psychometric properties of the Persian version of the Pittsburgh Sleep Quality Index addendum for PTSD (PSQI-A). Sleep Breath. 2009, 13, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Farrahi Moghaddam, J.; Nakhaee, N.; Sheibani, V.; Garrusi, B.; Amirkafi, A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. 2012, 16, 79–82. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Kashani, L.; Raisi, F.; Saroukhani, S.; Sohrabi, H.; Modabbernia, A.; Nasehi, A.A.; Jamshidi, A.; Ashrafi, M.; Mansouri, P.; Ghaeli, P.; et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women: Randomized double-blind placebo-controlled study. Hum. Psychopharmacol. 2013, 28, 54–60. [Google Scholar] [CrossRef]
- Gama, C.R.; Lasmar, R.; Gama, G.F.; Abreu, C.S.; Nunes, C.P.; Geller, M.; Oliveira, L.; Santos, A. Clinical Assessment of Tribulus terrestris Extract in the Treatment of Female Sexual Dysfunction. Clinical medicine insights. Women’s Health 2014, 7, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Vale, F.B.C.; Zanolla Dias de Souza, K.; Rezende, C.R.; Geber, S. Efficacy of Tribulus Terrestris for the treatment of premenopausal women with hypoactive sexual desire disorder: A randomized double-blinded, placebo-controlled trial. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2018, 34, 442–445. [Google Scholar] [CrossRef]
- Simon, J.A. Low sexual desire—Is it all in her head? Pathophysiology, diagnosis, and treatment of hypoactive sexual desire disorder. Postgrad. Med. 2010, 122, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lövheim, H. A new three-dimensional model for emotions and monoamine neurotransmitters. Med. Hypotheses 2012, 78, 341–348. [Google Scholar] [CrossRef]
- Kannur, D.; Kulkarni, A.; Paranjpe, M.; Navangul, M. Screening of antistress properties of herbal extracts and adaptogenic agents-a review. Pharmacogn. Rev. 2008, 2, 95. [Google Scholar]
- Kotta, S.; Ansari, S.H.; Ali, J. Exploring scientifically proven herbal aphrodisiacs. Pharmacogn. Rev. 2013, 7, 1–10. [Google Scholar]
- Monchaux De Oliveira, C.; Pourtau, L.; Vancassel, S.; Pouchieu, C.; Capuron, L.; Gaudout, D.; Castanon, N. Saffron extract-induced improvement of depressive-like behavior in mice is associated with modulation of monoaminergic neurotransmission. Nutrients 2021, 13, 904. [Google Scholar] [CrossRef]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 1768–1777. [Google Scholar] [CrossRef]
- Norouzi, E.; Mohammadi, R.; Fadaei, R.; Moradi, M.T.; Hosseini, H.; Rezaie, L.; Khazaiea, H. A systematic review and meta-analysis on the levels of brain-derived neurotrophic factor in insomnia patients with and without comorbid depression. Biol. Rhythm. Res. 2023, 54, 467–478. [Google Scholar] [CrossRef]
- Wei, X.H.; Cheng, X.M.; Shen, J.S.; Wang, Z.T. Antidepressant effect of Yueju-Wan ethanol extract and its fractions in mice models of despair. J. Ethnopharmacol. 2008, 117, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zhou, X.; Yi, N.; Jiang, L.; Tao, W.; Wu, R.; Wang, D.; Jiang, J.; Ge, X.; Wang, Y.; et al. Yueju pill rapidly induces antidepressant-like effects and acutely enhances BDNF expression in mouse brain. Evid. Based Complement. Altern. Med. Ecam 2013, 2013, 184367. [Google Scholar] [CrossRef]
- Lechtenberg, M.; Schepmann, D.; Niehues, M.; Hellenbrand, N.; Wünsch, B.; Hensel, A. Quality and functionality of saffron: Quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and sigma1 (sigma-1) receptors. Planta Med. 2008, 74, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, D.M.; El Makawy, A.I.; Ahmed, K.A.; Ramadan, M.F.; Ibrahim, F.M.J.E.S.; Research, P. Topiramate potential neurotoxicity and mitigating role of ginger oil in mice brain. Environ. Sci. Pollut. Res. 2022, 29, 87184–87199. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ali Redha, A.; Snoeck, E.R.; Singh, S.; Simal-Gandara, J.; Ibrahim, S.A.; Jafari, S.M. Anti-depressant properties of crocin molecules in saffron. Molecules 2022, 27, 2076. [Google Scholar] [CrossRef] [PubMed]
- Vahid, F.; Rahmani, D. Can an anti-inflammatory diet be effective in preventing or treating viral respiratory diseases? A systematic narrative review. Clin. Nutr. ESPEN 2021, 43, 9–15. [Google Scholar] [CrossRef]
- Seibel, R.; Schneider, R.H.; Gottlieb, M.G. Effects of spices (saffron, rosemary, cinnamon, turmeric and ginger) in Alzheimer’s disease. Curr. Alzheimer Res. 2021, 18, 347–357. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. PTR 2009, 23, 768–774. [Google Scholar] [CrossRef]
- Norton, P.J.; Barrera, T.L.; Mathew, A.R.; Chamberlain, L.D.; Szafranski, D.D.; Reddy, R.; Smith, M.A. Effect of transdiagnostic cbt for anxiety disorders on comorbid diagnoses. Depress. Anxiety 2013, 30, 168–173. [Google Scholar] [CrossRef]
- Norton, P.J.; Paulus, D.J. Toward a Unified Treatment for Emotional Disorders: Update on the Science and Practice. Behav Ther. 2016, 47, 854–868. [Google Scholar] [CrossRef]
- Norton, P.J.; Roberge, P. Transdiagnostic Therapy. Psychiatr Clin. North Am. 2017, 40, 675–687. [Google Scholar] [CrossRef]
- Pearl, S.B.; Norton, P.J. Transdiagnostic versus diagnosis specific cognitive behavioural therapies for anxiety: A meta-analysis. J. Anxiety Disord. 2017, 46, 11–24. [Google Scholar] [CrossRef]
- Guidi, J.; Offidani, E.; Rafanelli, C.; Roncuzzi, R.; Sonino, N.; Fava, G.A. The Assessment of Allostatic Overload in Patients with Congestive Heart Failure by Clinimetric Criteria. Stress Health 2016, 32, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Fava, G.A.; McEwen, B.S.; Guidi, J.; Gostoli, S.; Offidani, E.; Sonino, N. Clinical characterization of allostatic overload. Psychoneuroendocrinology 2019, 108, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Guidi, J.; Fava, G.A. The Clinical Science of Euthymia: A Conceptual Map. Psychother Psychosom. 2022, 91, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Schaub, A.C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: A randomized controlled trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Yamanbaeva, G.; Schaub, A.C.; Schneider, E.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Mählmann, L.; Brand, S.; Beglinger, C.; Borgwardt, S.; et al. Effects of a probiotic add-on treatment on fronto-limbic brain structure, function, and perfusion in depression: Secondary neuroimaging findings of a randomized controlled trial. J. Affect. Disord. 2023, 324, 529–538. [Google Scholar] [CrossRef]
- Giese, M.; Beck, J.; Brand, S.; Muheim, F.; Hemmeter, U.; Hatzinger, M.; Hatzinger, M.; Holsboer-Trachsler, E.; Eckert, A. Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. J. Psychiatr. Res. 2014, 59, 1–7. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar]
- Matinnia, N.; Faisal, I.; Hanafiah, J.M.; Herjar, A.R.; Moeini, B.; Osman, Z.J. Fears related to pregnancy and childbirth among primigravidae who requested caesarean versus vaginal delivery in Iran. Matern Child Health J. 2015, 19, 1121–1130. [Google Scholar] [CrossRef] [PubMed]



| Group | Statistics | ||
|---|---|---|---|
| Dimensions | Placebo | Aphrodite | |
| N | 19 | 21 | |
| Mean (SD) | Mean (SD) | ||
| Age (years) | 34.47 (8.79) | 36.64 (8.35) | t (39) = 1.89, p = 0.08 |
| Weight (kg) | 66.89 (10.39) | 69.36 (13.48) | t (39) = 0.97, p = 0.14 |
| Height (cm) | 160.63 (4.91) | 162.63 (6.26) | t (39) = 0.82, p = 0.16 |
| n (%) | n (%) | ||
| Occupational status | X2(N = 41; df = 1) =0.02, p = 0.91 | ||
| Unemployed | 13 | 11 | |
| Employed | 6 | 11 | |
| Educational level | X2(N = 41; df = 3) =0.5, p = 0.77 | ||
| Middle school | 5 | 8 | |
| High school | 5 | 3 | |
| Bachelor | 8 | 10 | |
| Master | 1 | 0 | |
| Aphrodite (n = 21) | Placebo (n = 19) | Factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Time | Time × Group Interaction | ||||||||
| Variable | M | SD | M | SD | F | ηp2 | F | ηp2 | F | ηp2 |
| Sexual function | 121.07 * | 0.71 | 60.18 ** | 0.60 | 150.90 ** | 0.79 | ||||
| Baseline | 18.86 | 6.60 | 17.94 | 7.69 | ||||||
| Week 4 | 43.95 | 7.37 | 12.94 | 4.39 | ||||||
| Week 8/study end | 49.63 | 2.42 | 10.63 | 4.49 | ||||||
| Depressive symptoms | 16.13 ** | 0.35 | 29.62 ** | 0.49 | 13.90 ** | 0.34 | ||||
| Baseline | 18.13 | 6.85 | 16.57 | 7.48 | ||||||
| Week 4 | 6.54 | 3.93 | 15.52 | 6.56 | ||||||
| Week 8/study end | 6.18 | 3.68 | 13.21 | 3.90 | ||||||
| Anxiety | 20.29 ** | 0.35 | 67.77 ** | 0.63 | 11.91 ** | 0.23 | ||||
| Baseline | 51.68 | 19.94 | 54.52 | 20.62 | ||||||
| Week 4 | 12.54 | 9.44 | 41.68 | 13.52 | ||||||
| Week 8/study end | 18.27 | 9.36 | 29.42 | 8.16 | ||||||
| Sleep quality | 19.75 ** | 0.33 | 0.85 | 0.004 | 11.60 ** | 0.22 | ||||
| Baseline | 11.81 | 5.51 | 11.31 | 4.89 | ||||||
| Week 4 | 7.09 | 4.25 | 16.47 | 6.38 | ||||||
| Week 8/study end | 9.00 | 5.35 | 15.31 | 5.36 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahmoradi, N.; Davarinejad, O.; Brühl, A.B.; Brand, S. Effects of Aphrodite (an Herbal Compound) on SSRI-Induced Sexual Dysfunctions and Depression in Females with Major Depressive Disorder: Findings from a Randomized Clinical Trial. Medicina 2023, 59, 1663. https://doi.org/10.3390/medicina59091663
Shahmoradi N, Davarinejad O, Brühl AB, Brand S. Effects of Aphrodite (an Herbal Compound) on SSRI-Induced Sexual Dysfunctions and Depression in Females with Major Depressive Disorder: Findings from a Randomized Clinical Trial. Medicina. 2023; 59(9):1663. https://doi.org/10.3390/medicina59091663
Chicago/Turabian StyleShahmoradi, Nasrin, Omran Davarinejad, Annette Beatrix Brühl, and Serge Brand. 2023. "Effects of Aphrodite (an Herbal Compound) on SSRI-Induced Sexual Dysfunctions and Depression in Females with Major Depressive Disorder: Findings from a Randomized Clinical Trial" Medicina 59, no. 9: 1663. https://doi.org/10.3390/medicina59091663
APA StyleShahmoradi, N., Davarinejad, O., Brühl, A. B., & Brand, S. (2023). Effects of Aphrodite (an Herbal Compound) on SSRI-Induced Sexual Dysfunctions and Depression in Females with Major Depressive Disorder: Findings from a Randomized Clinical Trial. Medicina, 59(9), 1663. https://doi.org/10.3390/medicina59091663







