Age Differences in Cardiopulmonary Exercise Testing Parameters in Heart Failure with Reduced Ejection Fraction
Abstract
:1. Introduction
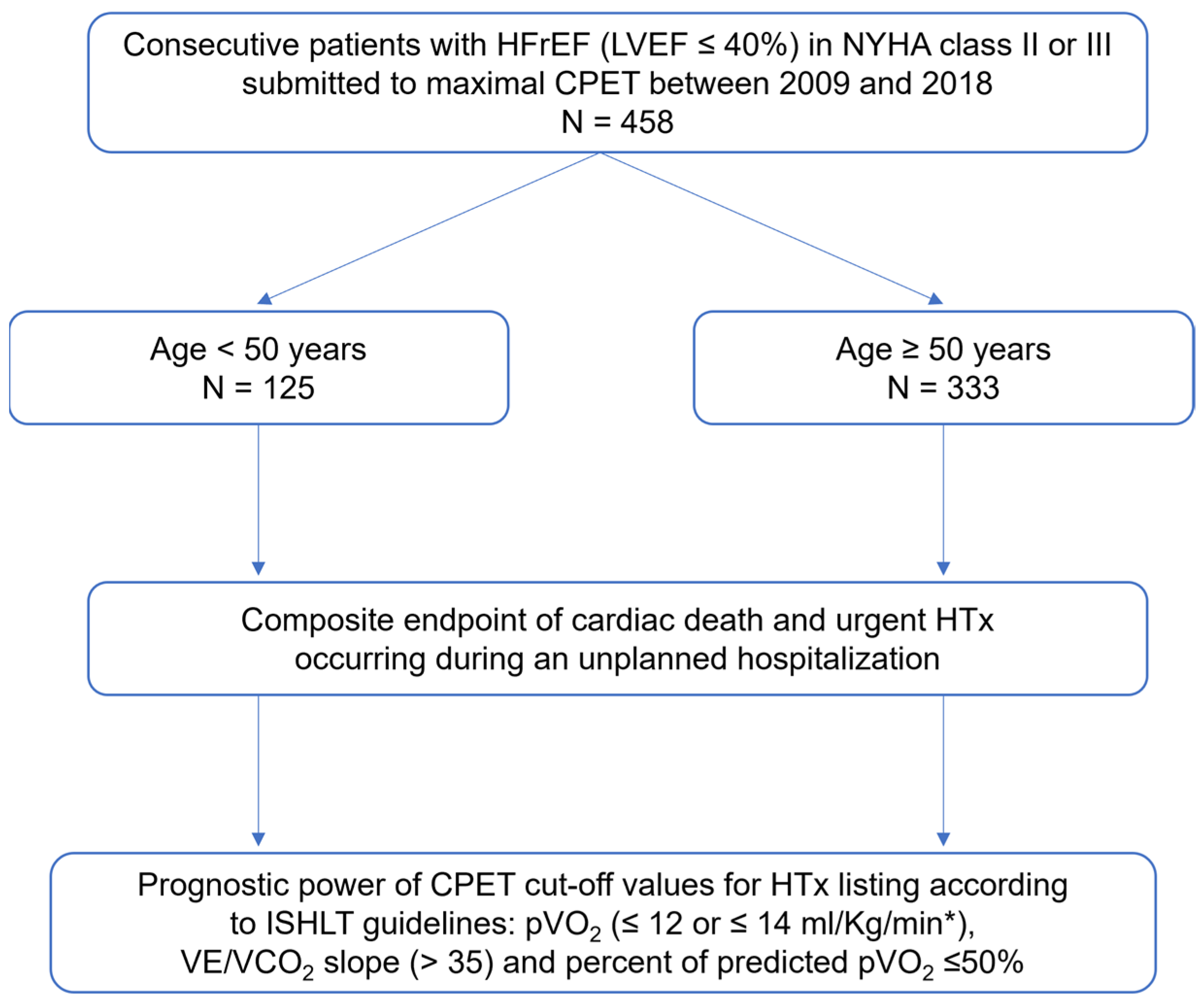
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Exclusion Criteria
- Younger than 18 years;
- Planned or recent coronary revascularization or cardiac surgery;
- Exercise-limiting comorbidities (cerebrovascular disease, severe peripheral vascular disease, or orthopedic disorders);
- Previous HTx;
- Elective HTx during the follow-up period;
- Submaximal CPET (defined as one with a peak RER of ≤1.05 [7]);
- Lost to follow-up.
2.4. Cardiorespiratory Exercise Testing
2.5. Primary Endpoint
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Composite Endpoint
3.3. Relationship between Cardiopulmonary Exercise Test Prognostic Parameters and the Primary Endpoint
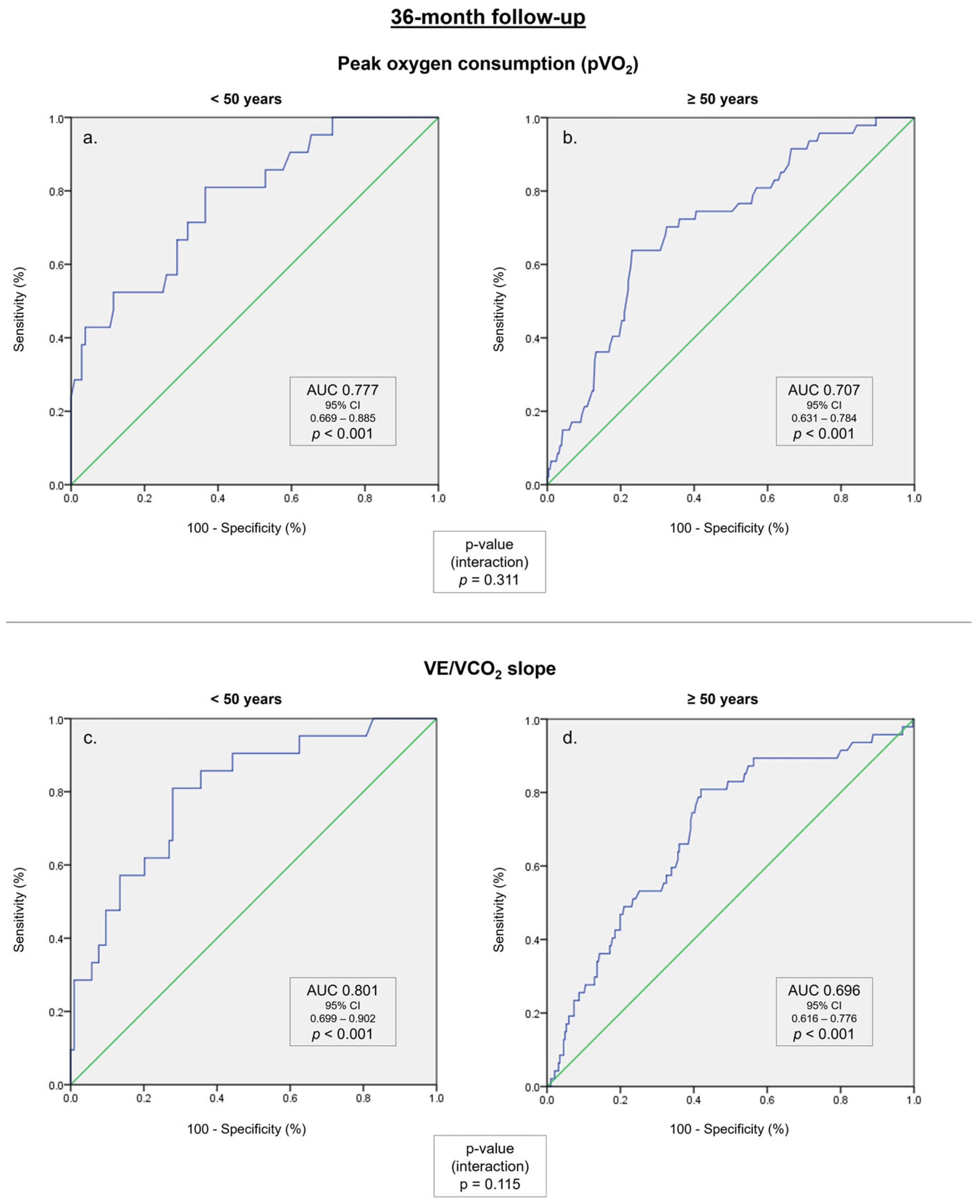
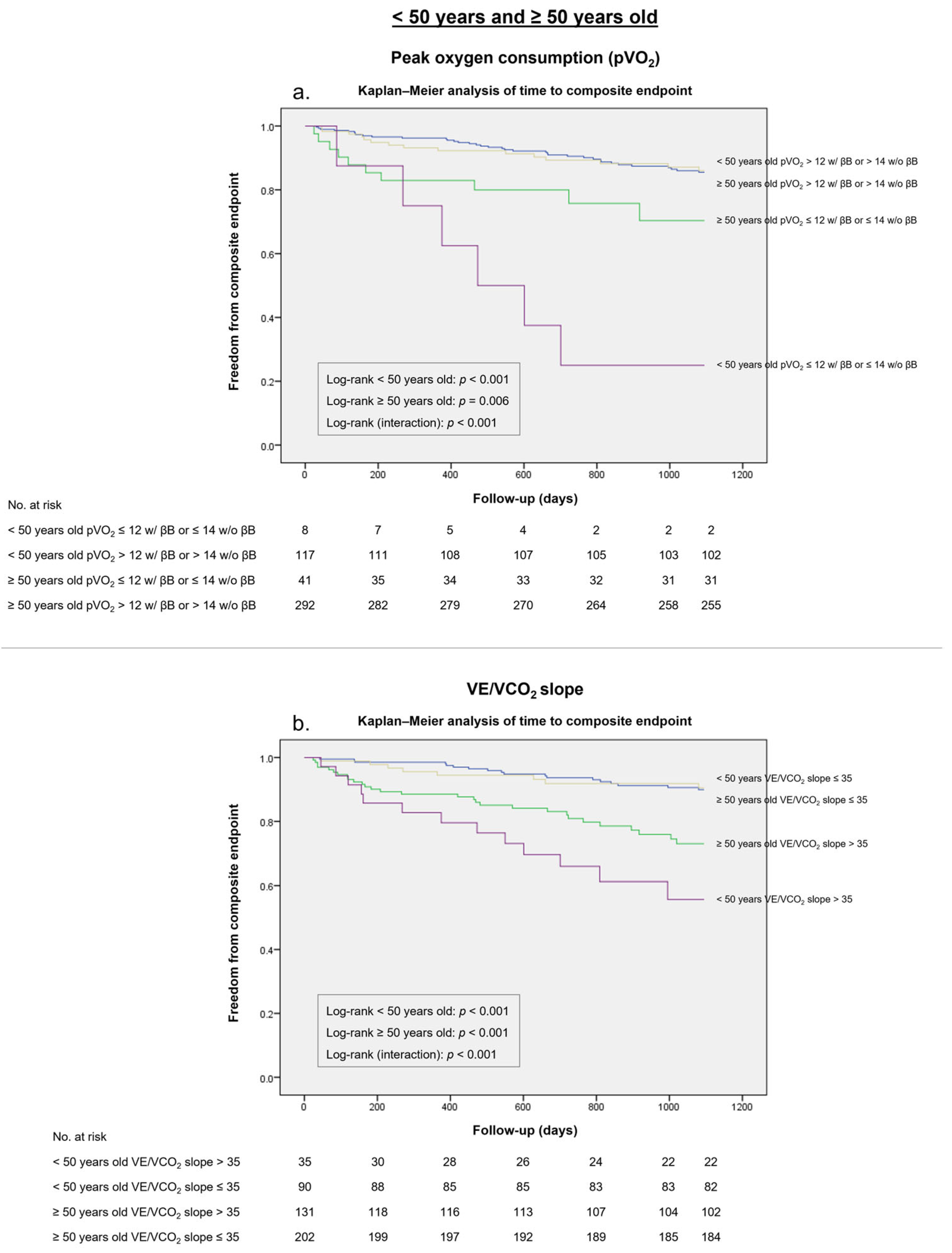
3.4. ISHL Thresholds for HTx Listing
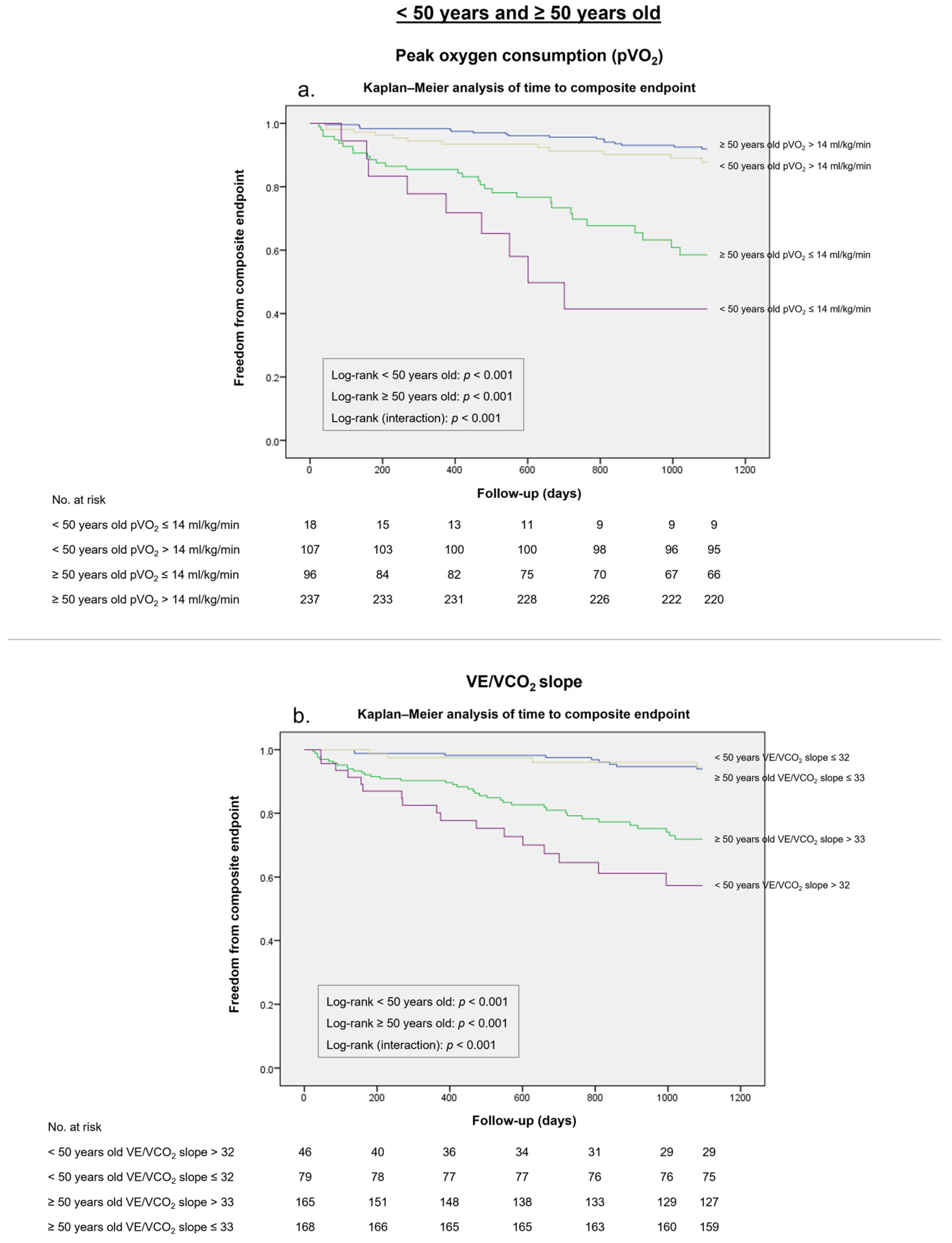
3.5. Alternative pVO2 and VE/VCO2 Slope Cut-Offs
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corra, U.; Agostoni, P.G.; Anker, S.D.; Coats, A.J.; Crespo Leiro, M.G.; de Boer, R.A.; Harjola, V.P.; Hill, L.; Lainscak, M.; Lund, L.H.; et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.P.; Ponikowski, P.; Harrington, D.; Anker, S.D.; Webb-Peploe, K.; Clark, A.L.; Poole-Wilson, P.A.; Coats, A.J. Clinical Correlates and Prognostic Significance of the Ventilatory Response to Exercise in Chronic Heart Failure. J. Am. Coll. Cardiol. 1997, 29, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.P.; Shamim, W.; Davies, L.C.; Piepoli, M.F.; Ponikowski, P.; Anker, S.D.; Coats, A.J.S. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO2 slope and peak VO2. Eur. Heart J. 2000, 21, 154–161. [Google Scholar] [CrossRef]
- Arena, R.; Humphrey, R. Comparison of ventilatory expired gas parameters used to predict hospitalization in patients with heart failure. Am. Heart J. 2002, 143, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Aslam, S.S.; Varughese, E.B.; Peberdy, M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004, 147, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Kleber, F.X.; Vietzke, G.; Wernecke, K.D.; Bauer, U.; Opitz, C.; Wensel, R.; Sperfeld, A.; Glaser, S. Impairment of Ventilatory Efficiency in Heart Failure. Circulation 2000, 101, 2803–2809. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Peterson, L.R.; Schechtman, K.B.; Ewald, G.A.; Geltman, E.M.; De Las Fuentes, L.; Meyer, T.; Krekeler, P.; Moore, M.L.; Rogers, J.G. Timing of cardiac transplantation in patients with heart failure receiving β-adrenergic blockers. J. Heart Lung Transplant. 2003, 22, 1141–1148. [Google Scholar] [CrossRef]
- Corrà, U.; Agostoni, P.; Giordano, A.; Cattadori, G.; Battaia, E.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; et al. Sex Profile and Risk Assessment with Cardiopulmonary Exercise Testing in Heart Failure: Propensity Score Matching for Sex Selection Bias. Can. J. Cardiol. 2016, 32, 754–759. [Google Scholar] [CrossRef]
- Lund, L.H.; Mancini, D.M. Peak VO2 in elderly patients with heart failure. Int. J. Cardiol. 2008, 125, 166–171. [Google Scholar] [CrossRef]
- Forman, D.E.; Clare, R.; Kitzman, D.W.; Ellis, S.J.; Fleg, J.L.; Chiara, T.; Fletcher, G.; Kraus, W.E.; HF-ACTION Investigators. Relationship of age and exercise performance in patients with heart failure: The HF-ACTION study. Am. Heart J. 2009, 158, S6–S15. [Google Scholar] [CrossRef] [PubMed]
- Palau, P.; Domínguez, E.; Núñez, J. Sex differences on peak oxygen uptake in heart failure. ESC Heart Fail. 2019, 6, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Corrà, U.; Piepoli, M.F.; Adamopoulos, S.; Agostoni, P.; Coats, A.J.; Conraads, V.; Lambrinou, E.; Pieske, B.; Piotrowicz, E.; Schmid, J.P.; et al. Cardiopulmonary exercise testing in systolic heart failure in 2014: The evolving prognostic role. Eur. J. Heart Fail. 2014, 16, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Scardovi, A.B.; De Maria, R.; Celestini, A.; Perna, S.; Coletta, C.; Feola, M.; Aspromonte, N.; Rosso, G.L.; Carunchio, A.; Ferraironi, A.; et al. Additive prognostic value of cardiopulmonary exercise testing in elderly patients with heart failure. Clin. Sci. 2009, 116, 415–422. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Abella, J.; Pinkstaff, S.; Brubaker, P.; Kitzman, D.W.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Cardiopulmonary exercise testing is equally prognostic in young, middle-aged and older individuals diagnosed with heart failure. Int. J. Cardiol. 2011, 151, 278–283. [Google Scholar] [CrossRef]
- Carubelli, V.; Metra, M.; Corrà, U.; Magrì, D.; Passino, C.; Lombardi, C.; Scrutinio, D.; Correale, M.; Cattadori, G.; Piepoli, M.F.; et al. Exercise Performance Is a Prognostic Indicator in Elderly Patients with Chronic Heart Failure—Application of Metabolic Exercise Cardiac Kidney Indexes Score. Circ. J. 2015, 79, 2608–2615. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, Y.; Oh, J.; Jang, J.; Kang, S.M. Characteristics and Safety of Cardiopulmonary Exercise Testing in Elderly Patients with Cardiovascular Diseases in Korea. Yonsei Med. J. 2019, 60, 547. [Google Scholar] [CrossRef]
- Sciomer, S.; Moscucci, F.; Salvioni, E.; Marchese, G.; Bussotti, M.; Corrà, U.; Piepoli, M.F. Role of gender, age and BMI in prognosis of heart failure. Eur. J. Prev. Cardiol. 2020, 27, 46–51. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Bello, R.; Shin, J.J.; Stevens, G.; Zolty, R.; Maybaum, S.; D’Alessandro, D. Outcomes of cardiac transplantation in septuagenarians. J. Heart Lung Transplant. 2012, 31, 679–685. [Google Scholar] [CrossRef]
- Moore, B.; Brubaker, P.H.; Stewart, K.P.; Kitzman, D.W. VE/VCO2 Slope in Older Heart Failure Patients with Normal Versus Reduced Ejection Fraction Compared with Age-Matched Healthy Controls. J. Card. Fail. 2007, 13, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Mejhert, M.; Linder-Klingsell, E.; Edner, M.; Kahan, T.; Persson, H. Ventilatory variables are strong prognostic markers in elderly patients with heart failure. Heart 2002, 88, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cicoira, M.; Davos, C.H.; Florea, V.; Shamim, W.; Doehner, W.; Coats, A.J.; Anker, S.D. Chronic heart failure in the very elderly: Clinical status, survival, and prognostic factors in 188 patients more than 70 years old. Am. Heart J. 2001, 142, 174–180. [Google Scholar] [CrossRef]
- Marburger, C.; Brubaker, P.; Pollock, W.; Morgan, T.; Kitzman, D. Reproducibility of cardiopulmonary exercise testing in elderly patients with congestive heart failure. Am. J. Cardiol. 1998, 82, 905–909. [Google Scholar] [CrossRef]
- Koch, B.; Schäper, C.; Ittermann, T.; Spielhagen, T.; Dörr, M.; Völzke, H.; Opitz, C.F.; Ewert, R.; Gläser, S. Reference values for cardiopulmonary exercise testing in healthy volunteers: The SHIP study. Eur. Respir. J. 2008, 33, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Hansen, J.E.; Garatachea, N.; Storer, T.W.; Wasserman, K. Ventilatory Efficiency during Exercise in Healthy Subjects. Am. J. Respir. Crit. Care Med. 2002, 166, 1443–1448. [Google Scholar] [CrossRef]
- Salvioni, E.; Corrà, U.; Piepoli, M.; Rovai, S.; Correale, M.; Paolillo, S.; Pasquali, M.; Magrì, D.; Vitale, G.; Fusini, L.; et al. Gender and age normalization and ventilation efficiency during exercise in heart failure with reduced ejection fraction. ESC Heart Fail. 2020, 7, 368–377. [Google Scholar] [CrossRef]
- Nicholls, D.; O’Dochartaigh, C.; Riley, M. Circulatory power—A new perspective on an old friend. Eur. Heart J. 2002, 23, 1242–1245. [Google Scholar] [CrossRef]
- Lala, A.; Shah, K.B.; Lanfear, D.E.; Thibodeau, J.T.; Palardy, M.; Ambardekar, A.V.; McNamara, D.M.; Taddei-Peters, W.C.; Baldwin, J.T.; Jeffries, N.; et al. Predictive Value of Cardiopulmonary Exercise Testing Parameters in Ambulatory Advanced Heart Failure. JACC Heart Fail. 2021, 9, 226–236. [Google Scholar] [CrossRef]
- Lewis, G.D.; Zlotoff, D.A. Cardiopulmonary Exercise Testing-Based Risk Stratification in the Modern Era of Advanced Heart Failure Management. JACC Heart Fail. 2021, 9, 237–240. [Google Scholar] [CrossRef]
- Agdamag, A.C.; Van Iterson, E.H.; Tang, W.H.W.; Finet, J.E. Prognostic Role of Metabolic Exercise Testing in Heart Failure. J Clin. Med. 2023, 12, 4438. [Google Scholar] [CrossRef] [PubMed]
- Brischetto, M.J.; Millman, R.P.; Peterson, D.D.; Silage, D.A.; Pack, A.I. Effect of aging on ventilatory response to exercise and CO2. J. Appl. Physiol. 1984, 56, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.; Gyanwali, B.; Soh, J.; Koh, A.S.; Goh, J. The potential benefits of assessing post-cardiopulmonary exercise testing (CPET) in aging: A narrative review. BMC Sports Sci. Med. Rehabil. 2023, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Turgeon, P.Y.; Nadreau, É.; Grégoire, P.; Maltais, L.G.; Sénéchal, M.; Provencher, S.; Maltais, F. Prognostic Value of Oxygen Kinetics During Recovery from Cardiopulmonary Exercise Testing in Patients with Chronic Heart Failure. Can. J. Cardiol. 2015, 31, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verma, S.; Bhatt, D.L.; Connelly, K.A.; Swiggum, E.; Vaduganathan, M.; Zieroth, S.; Butler, J. Optimizing Foundational Therapies in Patients with HFrEF. JACC Basic Transl. Sci. 2022, 7, 504–517. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Paolillo, S.; Veglia, F.; Salvioni, E.; Corrà, U.; Piepoli, M.; Lagioia, R.; Limongelli, G.; Sinagra, G.; Cattadori, G.; Scardovi, A.B.; et al. Heart failure prognosis over time: How the prognostic role of oxygen consumption and ventilatory efficiency during exercise has changed in the last 20 years. Eur. J. Heart Fail. 2019, 21, 208–217. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 37, 993–1004. [Google Scholar] [CrossRef]
- Gorodeski, E.Z.; Chu, E.C.; Chow, C.H.; Levy, W.C.; Hsich, E.; Starling, R.C. Application of the Seattle Heart Failure Model in Ambulatory Patients Presented to an Advanced Heart Failure Therapeutics Committee. Circ. Heart Fail. 2010, 3, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Giamouzis, G.; Smith, A.L.; Agha, S.A.; Waheed, S.; Laskar, S.; Puskas, J.; Dunbar, S.; Vega, D.; et al. Utility of the Seattle Heart Failure Model in Patients with Advanced Heart Failure. J. Am. Coll. Cardiol. 2009, 53, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, K.D.; Schwartz, J.S.; Chen, T.M.; Wong, K.L.; Goin, J.E.; Mancini, D.M. Development and Prospective Validation of a Clinical Index to Predict Survival in Ambulatory Patients Referred for Cardiac Transplant Evaluation. Circulation 1997, 95, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Arena, R.; Wagoner, L.E.; Dardas, T.; Abraham, W.T. Prognostic Impact of the Addition of Ventilatory Efficiency to the Seattle Heart Failure Model in Patients with Heart Failure. J. Card. Fail. 2012, 18, 614–619. [Google Scholar] [CrossRef]
- Agostoni, P.; Corrà, U.; Cattadori, G.; Veglia, F.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; Limongelli, G.; et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int. J. Cardiol. 2013, 167, 2710–2718. [Google Scholar] [CrossRef]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2018, 39, 1144–1161. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary Exercise Testing. What Is its Value? J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Development of a Ventilatory Classification System in Patients with Heart Failure. Circulation 2007, 115, 2410–2417. [Google Scholar] [CrossRef]
| Overall (n = 458) | <50 Years (n = 125) | ≥50 Years (n = 333) | p-Value | |
|---|---|---|---|---|
| Clinical and demographic data | ||||
| Age (years) | 56 ± 12 | 39 ± 8 | 61 ± 8 | <0.001 |
| Male sex (n, %) | 363 (79) | 94 (75) | 269 (81) | 0.189 |
| Body mass index (kg/m2) | 27.1 ± 4.3 | 26.8 ± 5.1 | 27.2 ± 3.9 | 0.348 |
| Ischemic etiology (n, %) | 261 (57) | 62 (50) | 199 (60) | 0.073 |
| ACEi/ARB (n, %) | 361 (79) | 93 (74) | 268 (80) | 0.183 |
| ARNI (n, %) | 80 (17) | 28 (22) | 52 (16) | 0.149 |
| β-blocker (n, %) | 392 (86) | 104 (83) | 288 (86) | 0.422 |
| MRA (n, %) | 336 (73) | 93 (74) | 243 (73) | 0.624 |
| iSGLT2 (n, %) | 47 (10) | 9 (7) | 38 (11) | 0.124 |
| Digoxin (n, %) | 129 (28) | 38 (30) | 91 (27) | 0.560 |
| Diabetes | 104 (23) | 11 (9) | 93 (28) | <0.001 |
| CKD (n, %) | 145 (32) | 16 (13) | 129 (39) | <0.001 |
| AF (n, %) | 109 (24) | 18 (14) | 91 (27) | 0.004 |
| ICD * (n, %) | 293 (64) | 84 (67) | 210 (63) | 0.225 |
| CRT (n, %) | 102 (22) | 31 (25) | 71 (21) | 0.246 |
| NYHA class II | 347 (76) | 95 (76) | 252 (76) | 0.358 |
| NYHA class III | 111 (24) | 30 (24) | 81 (24) | 0.358 |
| HFSS | 8.6 ± 1.1 | 8.9 ± 1.2 | 8.6 ± 1.1 | 0.088 |
| Laboratory data | ||||
| eGFR, mL/min/1.73 m2 | 75.3 ± 29.2 | 92.4 ± 31.2 | 69.3 ± 25.9 | <0.001 |
| Sodium, mEq/L | 138.0 ± 3.0 | 137.5 ± 2.7 | 138.2 ± 3.1 | 0.036 |
| NT-proBNP, pg/mL | 2196 ± 2101 | 2116 ± 2013 | 2228 ± 2214 | 0.764 |
| Echocardiographic data | ||||
| LVEDD, mm/m2 | 67.4 ± 10.3 | 69.2 ± 10.7 | 66.8 ± 10.2 | 0.171 |
| LVEF, % | 29.7 ± 8.0 | 30.0 ± 8.1 | 29.5 ± 8.1 | 0.564 |
| MR III–IV, % | 67 (14) | 23 (18) | 44 (13) | 0.435 |
| RV dysfunction (n, %) | 69 (15) | 15 (12) | 54 (16) | 0.486 |
| CPET parameters | ||||
| Peak RER | 1.14 ± 0.07 | 1.14 ± 0.06 | 1.14 ± 0.07 | 0.916 |
| Delta HRt during exercise | 51 (37–68) | 64 (41–85) | 47 (35–62) | <0.001 |
| HHR1 | 17 (11–27) | 22 (14–34) | 16 (10–24) | <0.001 |
| pVO2, mL/kg/min | 18.5 ± 5.8 | 21.5 ± 7.0 | 17.4 ± 4.8 | <0.001 |
| Percentage of predicted pVO2 (%) | 63.8 ± 18.7 | 60.3 ± 18.7 | 65.1 ± 18.5 | 0.014 |
| VE/VCO2 slope | 33.9 ± 9.6 | 32.3 ± 10.5 | 34.6 ± 9.2 | 0.030 |
| pVO2, mL/kg/min at AT | 13.6 ± 4.6 | 14.4 ± 5.4 | 13.4 ± 4.2 | 0.857 |
| O2 pulse, mL/kg/beat | 0.14 ± 0.06 | 0.15 ± 0.03 | 0.14 ± 0.07 | 0.380 |
| Circulatory power, mmHg.mL/kg/min | 2883 ± 1543 | 3288 ± 1467 | 2730 ± 1545 | 0.001 |
| Ventilatory power, mmHg | 4.8 ± 1.7 | 5.0 ± 1.7 | 4.7 ± 1.6 | 0.073 |
| COP | 28.9 ± 7.2 | 27.9 ± 8.7 | 29.2 ± 6.6 | 0.393 |
| PetCO2 at rest, mmHg | 33.6 ± 4.8 | 34.3 ± 5.1 | 33.3 ± 4.7 | 0.080 |
| PetCO2 at AT, mmHg | 36.8 ± 6.0 | 38.2 ± 6.4 | 36.3 ± 5.8 | 0.006 |
| Overall (n = 458) | <50 Years (n = 125) | ≥50 Years (n = 333) | p-Value | |
|---|---|---|---|---|
| Combined primary endpoint (n, %) | 68 (14.8%) | 21 (16.8%) | 47 (14.1%) | 0.464 |
| Total mortality (n, %) | 67 (14.6%) | 15 (12%) | 52 (15.6%) | 0.121 |
| Cardiac mortality (n, %) | 54 (11.8%) | 14 (11.2%) | 40 (12.0%) | 0.683 |
| Sudden cardiac death (n, %) | 19 (4.1%) | 6 (4.8%) | 13 (3.9%) | 0.385 |
| Death from worsening HF (n, %) | 35 (7.6%) | 8 (6.4%) | 27 (8.1%) | 0.138 |
| Urgent HTx (n, %) | 14 (3.1%) | 7 (5.6%) | 7 (2.1%) | 0.002 |
| Total Cohort | ||||||
|---|---|---|---|---|---|---|
| Model | Univariable HR | 95% CI | p-Value | Multivariable HR | 95% CI | p-Value |
| Male sex | 1.547 | 0.791 to 3.026 | 0.203 | |||
| Age | 1.002 | 0.983 to 1.021 | 0.829 | |||
| BMI | 0.953 | 0.897 to 1.013 | 0.121 | 0.954 | 0.887 to 1.027 | 0.210 |
| LVEF | 0.927 | 0.900 to 0.955 | <0.001 | 0.935 | 0.905 to 0.966 | <0.001 |
| eGFR | 0.979 | 0.969 to 0.989 | <0.001 | 0.986 | 0.976 to 0.996 | 0.009 |
| Diabetes | 1.196 | 0.254 to 5.632 | 0.821 | |||
| Smoker | 1.716 | 1.405 to 2.820 | 0.033 | 1.395 | 0.835 to 2.328 | 0.203 |
| Peak VO2 | 0.835 | 0.789 to 0.883 | <0.001 | 0.856 | 0.804 to 0.912 | <0.001 |
| Percentage of predicted pVO2 | 0.948 | 0.934 to 0.963 | <0.001 | 0.955 | 0.939 to 0.971 | <0.001 |
| VE/VCO2 slope | 1.058 | 1.041 to 1.075 | <0.001 | 1.064 | 1.039 to 1.090 | <0.001 |
| Peak VO2 at AT, mL/kg/min | 0.854 | 0.737 to 0.989 | 0.035 | 0.879 | 0.687 to 1.124 | 0.305 |
| O2 pulse, mL/kg/beat | 0.858 | 0.791 to 0.932 | <0.001 | 0.865 | 0.780 to 0.961 | 0.007 |
| Circulatory power, mmHg.mL/kg/min | 0.999 | 0.999 to 0.999 | <0.001 | 0.999 | 0.998 to 1.000 | <0.001 |
| Ventilatory power, mmHg | 0.575 | 0.483 to 0.684 | <0.001 | 0.632 | 0.521 to 0.768 | <0.001 |
| COP | 1.118 | 1.054 to 1.186 | <0.001 | 1.060 | 0.956 to 1.174 | 0.268 |
| PetCO2 at rest, mmHg | 0.887 | 0.839 to 0.937 | <0.001 | 0.948 | 0.889 to 1.011 | 0.102 |
| PetCO2 at AT, mmHg | 0.862 | 0.826 to 0.900 | <0.001 | 0.890 | 0.845 to 0.993 | <0.001 |
| <50 years of age | ||||||
| Model | Univariable HR | 95% CI | p-value | Multivariable HR | 95% CI | p-value |
| Male sex | 2.172 | 0.639 to 7.378 | 0.214 | |||
| BMI | 0.926 | 0.835 to 1.027 | 0.146 | 0.947 | 0.810 to 1.107 | 0.493 |
| LVEF | 0.899 | 0.838 to 0.943 | <0.001 | 0.903 | 0.835 to 0.976 | 0.011 |
| eGFR | 0.974 | 0.958 to 0.991 | 0.002 | 0.971 | 0.952 to 0.991 | 0.005 |
| Diabetes | 1.752 | 0.513 to 5.979 | 0.371 | |||
| Smoker | 4.922 | 1.784 to 13.579 | 0.002 | 3.171 | 1.060 to 9.486 | 0.039 |
| Peak VO2 | 0.835 | 0.768 to 0.908 | <0.001 | 0.876 | 0.793 to 0.968 | 0.009 |
| Percentage of predicted pVO2 | 0.933 | 0.907 to 0.961 | <0.001 | 0.951 | 0.917 to 0.986 | 0.006 |
| VE/VCO2 slope | 1.061 | 1.037 to 1.084 | <0.001 | 1.058 | 1.016 to 1.101 | 0.006 |
| Peak VO2 at AT, mL/kg/min | 0.737 | 0.559 to 0.971 | 0.030 | 0.891 | 0.691 to 1.091 | 0.593 |
| O2 pulse, mL/kg/beat | 0.835 | 0.724 to 0.962 | 0.013 | 0.873 | 0.711 to 1.071 | 0.193 |
| Circulatory power, mmHg.mL/kg/min | 0.999 | 0.999 to 0.999 | <0.001 | 0.999 | 0.999 to 1.000 | 0.035 |
| Ventilatory power, mmHg | 0.514 | 0.375 to 0.703 | <0.001 | 0.662 | 0.462 to 0.948 | 0.024 |
| COP | 1.196 | 1.082 to 1.323 | <0.001 | 1.058 | 0.954 to 1.162 | 0.681 |
| PetCO2 at rest, mmHg | 0.861 | 0.785 to 0.944 | 0.001 | 0.949 | 0.868 to 1.037 | 0.949 |
| PetCO2 at AT, mmHg | 0.871 | 0.821 to 0.925 | <0.001 | 0.936 | 0.878 to 0.999 | 0.047 |
| ≥50 years of age | ||||||
| Model | Univariable HR | 95% CI | p-value | Multivariable HR | 95% CI | p-value |
| Male sex | 1.338 | 0.599 to 2.987 | 0.477 | |||
| BMI | 0.972 | 0.901 to 1.048 | 0.462 | |||
| LVEF | 0.941 | 0.908 to 0.974 | 0.001 | 0.948 | 0.913 to 0.984 | 0.005 |
| eGFR | 0.977 | 0.964 to 0.990 | 0.001 | 0.983 | 0.969 to 0.996 | 0.013 |
| Diabetes | 1.282 | 0.167 to 2.542 | 0.474 | |||
| Smoker | 1.135 | 0.628 to 2.050 | 0.675 | |||
| Peak VO2 | 0.824 | 0.765 to 0.887 | <0.001 | 0.823 | 0.761 to 0.891 | <0.001 |
| Percentage of predicted pVO2 | 0.954 | 0.937 to 0.972 | <0.001 | 0.958 | 0.939 to 0.977 | <0.001 |
| VE/VCO2 slope | 1.055 | 1.031 to 1.080 | <0.001 | 1.069 | 1.038 to 1.101 | <0.001 |
| Peak VO2 at AT, mL/kg/min | 0.961 | 0.809 to 1.142 | 0.654 | |||
| O2 pulse, mL/kg/beat | 0.868 | 0.784 to 0.960 | 0.006 | 0.837 | 0.738 to 0.950 | 0.006 |
| Circulatory power, mmHg.mL/kg/min | 0.999 | 0.999 to 0.999 | <0.001 | 0.999 | 0.999 to 0.999 | <0.001 |
| Ventilatory power, mmHg | 0.601 | 0.488 to 0.741 | <0.001 | 0.617 | 0.488 to 0.780 | <0.001 |
| COP | 1.037 | 0.941 to 1.142 | 0.465 | |||
| PetCO2 at rest, mmHg | 0.900 | 0.839 to 0.964 | 0.003 | 0.942 | 0.869 to 1.022 | 0.150 |
| PetCO2 at AT, mmHg | 0.857 | 0.808 to 0.910 | <0.001 | 0.868 | 0.810 to 0.931 | <0.001 |
| <50 Years (n = 125) | ≥50 Years (n = 333) | ||||||
|---|---|---|---|---|---|---|---|
| CPET Parameters | AUC | 95% CI | p-Value | AUC | 95% CI | p-Value | p-Value (Interaction) |
| pVO2, mL/kg/min | 0.777 | 0.669–0.885 | <0.001 | 0.707 | 0.631–0.784 | <0.001 | 0.311 |
| Predicted pVO2 (%) | 0.818 | 0.731–0.904 | <0.001 | 0.703 | 0.623–0.784 | <0.001 | 0.060 |
| VE/VCO2 slope | 0.801 | 0.699–0.902 | <0.001 | 0.696 | 0.616–0.776 | <0.001 | 0.115 |
| pVO2, mL/kg/min at AT | 0.723 | 0.511–0.935 | 0.018 | 0.524 | 0.312–0.735 | 0.824 | 0.088 |
| O2 pulse, mL/kg/beat | 0.656 | 0.519–0.793 | 0.024 | 0.631 | 0.547–0.716 | 0.004 | 0.803 |
| Circulatory power, mmHg.mL/kg/min | 0.778 | 0.663–0.894 | <0.001 | 0.709 | 0.639–0.780 | <0.001 | 0.399 |
| Ventilatory power, mmHg | 0.773 | 0.646–0.900 | <0.001 | 0.705 | 0.630–0.780 | <0.001 | 0.373 |
| COP | 0.661 | 0.572–0.750 | 0.001 | 0.568 | 0.479–0.654 | 0.520 | 0.168 |
| PetCO2 at rest, mmHg | 0.711 | 0.591–0.831 | 0.003 | 0.626 | 0.539–0.712 | 0.007 | 0.277 |
| PetCO2 at AT, mmHg | 0.804 | 0.706–0.903 | <0.001 | 0.706 | 0.620–0.792 | <0.001 | 0.145 |
| <50 Years (n = 125) | ≥50 Years (n = 333) | |||||
|---|---|---|---|---|---|---|
| CPET Parameters | Specificity | Sensitivity | Youden Index (J) | Specificity | Sensitivity | Youden Index (J) |
| pVO2 ≤ 12 mL/kg/min * | 98% | 29% | 0.27 | 89% | 21% | 0.10 |
| pVO2 ≤ 14 mL/kg/miN | 89% | 52% | 0.41 | 77% | 64% | 0.41 |
| VE/VCO2 slope >35 | 80% | 62% | 0.42 | 64% | 62% | 0.26 |
| VE/VCO2 slope >32 | 72% | 81% | 0.53 | 54% | 81% | 0.35 |
| VE/VCO2 slope >33 | 73% | 67% | 0.40 | 58% | 81% | 0.39 |
| Percentage of predicted pVO2 ≤ 50% | 71% | 62% | 0.33 | 83% | 47% | 0.30 |
| Percentage of predicted pVO2 ≤ 56% | 65% | 81% | 0.46 | 71% | 66% | 0.37 |
| Percentage of predicted pVO2 ≤ 58% | 64% | 86% | 0.50 | 67% | 68% | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia Brás, P.; Gonçalves, A.V.; Reis, J.F.; Moreira, R.I.; Pereira-da-Silva, T.; Rio, P.; Timóteo, A.T.; Silva, S.; Soares, R.M.; Ferreira, R.C. Age Differences in Cardiopulmonary Exercise Testing Parameters in Heart Failure with Reduced Ejection Fraction. Medicina 2023, 59, 1685. https://doi.org/10.3390/medicina59091685
Garcia Brás P, Gonçalves AV, Reis JF, Moreira RI, Pereira-da-Silva T, Rio P, Timóteo AT, Silva S, Soares RM, Ferreira RC. Age Differences in Cardiopulmonary Exercise Testing Parameters in Heart Failure with Reduced Ejection Fraction. Medicina. 2023; 59(9):1685. https://doi.org/10.3390/medicina59091685
Chicago/Turabian StyleGarcia Brás, Pedro, António Valentim Gonçalves, João Ferreira Reis, Rita Ilhão Moreira, Tiago Pereira-da-Silva, Pedro Rio, Ana Teresa Timóteo, Sofia Silva, Rui M. Soares, and Rui Cruz Ferreira. 2023. "Age Differences in Cardiopulmonary Exercise Testing Parameters in Heart Failure with Reduced Ejection Fraction" Medicina 59, no. 9: 1685. https://doi.org/10.3390/medicina59091685






