Idiopathic Slow Transit Constipation: Pathophysiology, Diagnosis, and Management
Abstract
1. Introduction
2. Definitions and Classification
3. Pathophysiology
3.1. Normal Physiology of the Colon
3.1.1. Control of Colonic Function
3.1.2. Fluid and Electrolyte Homeostasis
3.1.3. Motor Function
3.2. Pathophysiology of Constipation
4. Diagnosis
4.1. Differential Diagnoses
4.1.1. Other Phenotypes of Primary Chronic Constipation
4.1.2. Secondary Causes of Chronic Constipation
4.2. Clinical Assessment
4.3. Investigations and Diagnostic Workup
4.3.1. Investigations to Rule out Secondary Causes
4.3.2. Investigations for Primary Constipation
Assessment of Colonic Motility
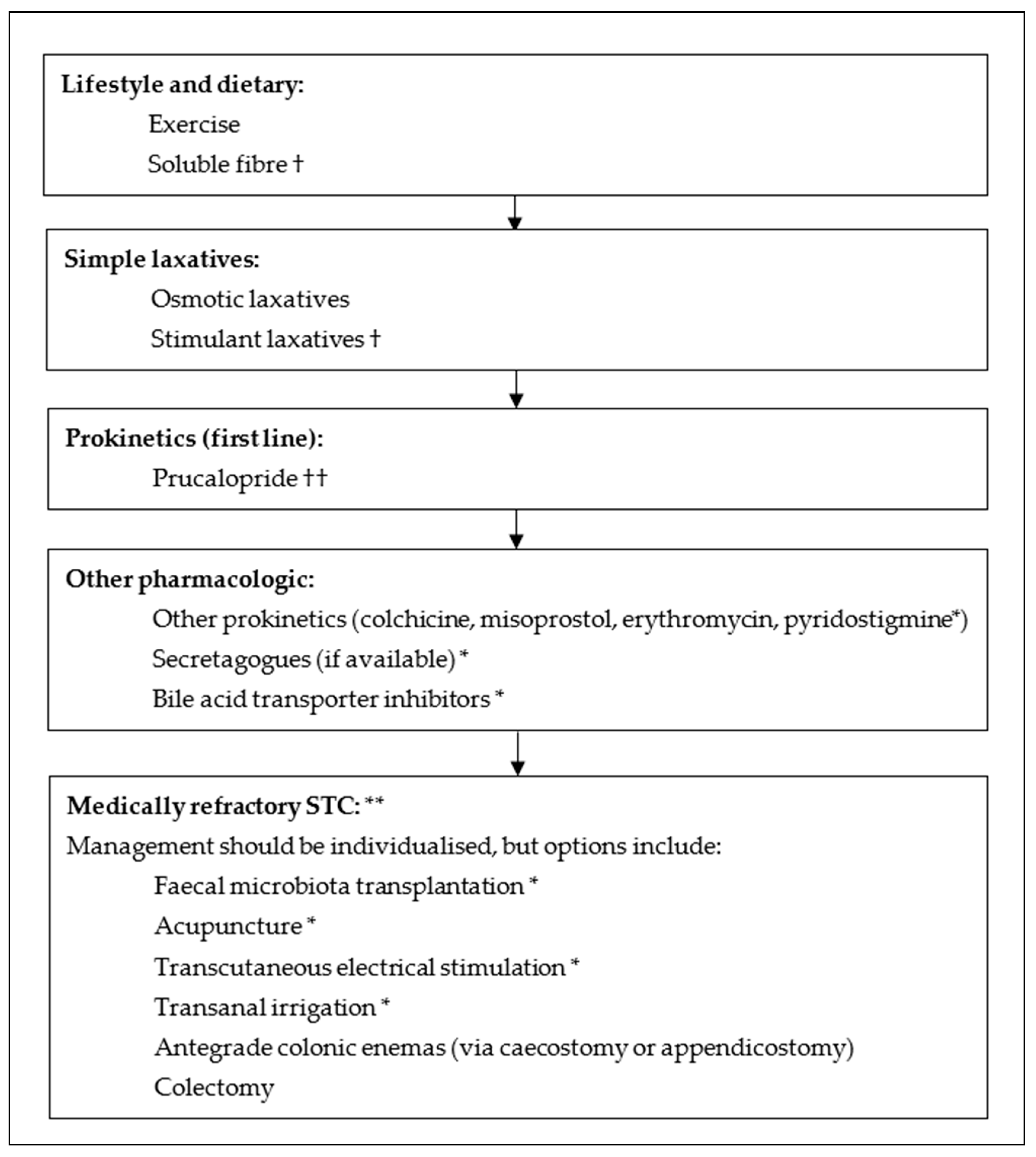
5. Management
5.1. Lifestyle, Dietary and Fibre Supplementation
5.2. Pharmacologic
5.2.1. Osmotic Laxatives
5.2.2. Stimulant Laxatives
5.2.3. Stool Softeners
5.2.4. Secretagogues
5.2.5. Bile Acid Transporter Inhibitors
5.2.6. Prokinetics
5.3. Interventional and Surgical
5.3.1. Faecal Microbiota Transplant
5.3.2. Electrical Stimulation
5.3.3. Acupuncture
5.3.4. Transanal Irrigation
5.3.5. Antegrade Colonic Enemas
5.3.6. Surgery
5.4. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Suares, N.C.; Ford, A.C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 1582–1591; quiz 1581, 1592. [Google Scholar] [CrossRef] [PubMed]
- Andromanakos, N.P.; Pinis, S.I.; Kostakis, A.I. Chronic severe constipation: Current pathophysiological aspects, new diagnostic approaches, and therapeutic options. Eur. J. Gastroenterol. Hepatol. 2015, 27, 204–214. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Pemberton, J.H.; Locke, G.R., 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology 2013, 144, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Ford, A.C. Chronic idiopathic constipation in adults: Epidemiology, pathophysiology, diagnosis and clinical management. Med. J. Aust. 2018, 209, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef]
- El-Salhy, M. Chronic idiopathic slow transit constipation: Pathophysiology and management. Colorectal Dis. 2003, 5, 288–296. [Google Scholar] [CrossRef]
- Andrews, C.N.; Storr, M. The pathophysiology of chronic constipation. Can. J. Gastroenterol. 2011, 25 (Suppl. B), 16B–21B. [Google Scholar] [CrossRef]
- Shahid, S.; Ramzan, Z.; Maurer, A.H.; Parkman, H.P.; Fisher, R.S. Chronic idiopathic constipation: More than a simple colonic transit disorder. J. Clin. Gastroenterol. 2012, 46, 150–154. [Google Scholar] [CrossRef]
- Bassotti, G.; Roberto, G.D.; Sediari, L.; Morelli, A. Toward a definition of colonic inertia. World J. Gastroenterol. 2004, 10, 2465–2467. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Huizinga, J.D.; Hussain, A.; Chen, J.H. Interstitial cells of Cajal and human colon motility in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G552–G575. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Friedman, L.S.; Brandt, L.J. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management, 11th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Sharma, A.; Rao, S. Constipation: Pathophysiology and Current Therapeutic Approaches. Handb. Exp. Pharmacol. 2017, 239, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Tornblom, H.; Lang, B.; Clover, L.; Knowles, C.H.; Vincent, A.; Lindberg, G. Autoantibodies in patients with gut motility disorders and enteric neuropathy. Scand. J. Gastroenterol. 2007, 42, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Sadeghi, P.; Beaty, J.; Kavlock, R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am. J. Gastroenterol. 2004, 99, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Thayalasekeran, S.; Ali, H.; Tsai, H.H. Novel therapies for constipation. World J. Gastroenterol. 2013, 19, 8247–8251. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Wald, A. Chronic Constipation. Mayo Clin. Proc. 2019, 94, 2340–2357. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.e3. [Google Scholar] [CrossRef]
- Wlodarczyk, J.; Wasniewska, A.; Fichna, J.; Dziki, A.; Dziki, L.; Wlodarczyk, M. Current Overview on Clinical Management of Chronic Constipation. J. Clin. Med. 2021, 10, 1738. [Google Scholar] [CrossRef]
- Tian, H.; Chen, Q.; Yang, B.; Qin, H.; Li, N. Analysis of Gut Microbiome and Metabolite Characteristics in Patients with Slow Transit Constipation. Dig. Dis. Sci. 2021, 66, 3026–3035. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Philips, S.F. Slow-transit Constipation. Curr. Treat Options Gastroenterol. 2001, 4, 309–315. [Google Scholar] [CrossRef]
- Roszkowska, A.; Pawlicka, M.; Mroczek, A.; Balabuszek, K.; Nieradko-Iwanicka, B. Non-Celiac Gluten Sensitivity: A Review. Medicina 2019, 55, 222. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.J.; Rao, S.S.; Koch, K.L.; Kuo, B.; Parkman, H.P.; McCallum, R.W.; Sitrin, M.D.; Wilding, G.E.; Semler, J.R.; Chey, W.D. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am. J. Gastroenterol. 2010, 105, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Tantiphlachiva, K.; Rao, P.; Attaluri, A.; Rao, S.S. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin. Gastroenterol. Hepatol. 2010, 8, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Southwell, B.R.; Clarke, M.C.; Sutcliffe, J.; Hutson, J.M. Colonic transit studies: Normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr. Surg. Int. 2009, 25, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Yu, Y.J.; Fei, F.; Zheng, M.Y.; Zhang, S.W. High-resolution colonic manometry and its clinical application in patients with colonic dysmotility: A review. World J. Clin. Cases 2019, 7, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.M.; Pedersen, H.E.; Clemmensen, K.K.B.; Ekblond, T.S.; Ried-Larsen, M.; Faerch, K.; Brock, C.; Quist, J.S. Associations Between Physical Activity and Gastrointestinal Transit Times in People with Normal Weight, Overweight, and Obesity. J. Nutr. 2023, in press. [Google Scholar] [CrossRef]
- Song, B.K.; Cho, K.O.; Jo, Y.; Oh, J.W.; Kim, Y.S. Colon transit time according to physical activity level in adults. J. Neurogastroenterol. Motil. 2012, 18, 64–69. [Google Scholar] [CrossRef]
- Oettle, G.J. Effect of moderate exercise on bowel habit. Gut 1991, 32, 941–944. [Google Scholar] [CrossRef]
- Robertson, G.; Meshkinpour, H.; Vandenberg, K.; James, N.; Cohen, A.; Wilson, A. Effects of exercise on total and segmental colon transit. J. Clin. Gastroenterol. 1993, 16, 300–303. [Google Scholar] [CrossRef]
- Suares, N.C.; Ford, A.C. Systematic review: The effects of fibre in the management of chronic idiopathic constipation. Aliment. Pharmacol. Ther. 2011, 33, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Slow Transit Constipation. Curr. Treat Options Gastroenterol. 2002, 5, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef]
- Chang, L.; Chey, W.D.; Imdad, A.; Almario, C.V.; Bharucha, A.E.; Diem, S.; Greer, K.B.; Hanson, B.; Harris, L.A.; Ko, C.; et al. American Gastroenterological Association-American College of Gastroenterology Clinical Practice Guideline: Pharmacological Management of Chronic Idiopathic Constipation. Gastroenterology 2023, 164, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Lee-Robichaud, H.; Thomas, K.; Morgan, J.; Nelson, R.L. Lactulose versus Polyethylene Glycol for Chronic Constipation. Cochrane Database Syst. Rev. 2010, CD007570. [Google Scholar] [CrossRef]
- Morishita, D.; Tomita, T.; Mori, S.; Kimura, T.; Oshima, T.; Fukui, H.; Miwa, H. Senna Versus Magnesium Oxide for the Treatment of Chronic Constipation: A Randomized, Placebo-Controlled Trial. Am. J. Gastroenterol. 2021, 116, 152–161. [Google Scholar] [CrossRef]
- Kamm, M.A.; Mueller-Lissner, S.; Wald, A.; Richter, E.; Swallow, R.; Gessner, U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin. Gastroenterol. Hepatol. 2011, 9, 577–583. [Google Scholar] [CrossRef]
- Mueller-Lissner, S.; Kamm, M.A.; Wald, A.; Hinkel, U.; Koehler, U.; Richter, E.; Bubeck, J. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am. J. Gastroenterol. 2010, 105, 897–903. [Google Scholar] [CrossRef]
- Muller-Lissner, S.A.; Kamm, M.A.; Scarpignato, C.; Wald, A. Myths and misconceptions about chronic constipation. Am. J. Gastroenterol. 2005, 100, 232–242. [Google Scholar] [CrossRef]
- Wald, A. Is chronic use of stimulant laxatives harmful to the colon? J. Clin. Gastroenterol. 2003, 36, 386–389. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M. Elobixibat and its potential role in chronic idiopathic constipation. Ther. Adv. Gastroenterol. 2014, 7, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Taniguchi, S.; Kurosu, S.; Gillberg, P.G.; Mattsson, J.P.; Camilleri, M. Efficacy, long-term safety, and impact on quality of life of elobixibat in more severe constipation: Post hoc analyses of two phase 3 trials in Japan. Neurogastroenterol. Motil. 2019, 31, e13571. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Piessevaux, H.; Yiannakou, Y.; Tack, J.; Kerstens, R.; Quigley, E.M.M.; Ke, M.; Da Silva, S.; Levine, A. Efficacy and Safety of Prucalopride in Chronic Constipation: An Integrated Analysis of Six Randomized, Controlled Clinical Trials. Dig. Dis. Sci. 2016, 61, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lv, B.; Zhang, S.; Fan, Y.H.; Meng, L.N. Itopride therapy for functional dyspepsia: A meta-analysis. World J. Gastroenterol. 2012, 18, 7371–7377. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Suares, N.C. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: Systematic review and meta-analysis. Gut 2011, 60, 209–218. [Google Scholar] [CrossRef]
- Emmanuel, A.V.; Roy, A.J.; Nicholls, T.J.; Kamm, M.A. Prucalopride, a systemic enterokinetic, for the treatment of constipation. Aliment. Pharmacol. Ther. 2002, 16, 1347–1356. [Google Scholar] [CrossRef]
- Tack, J.; Coremans, G.; Janssens, J. A risk-benefit assessment of cisapride in the treatment of gastrointestinal disorders. Drug Saf. 1995, 12, 384–392. [Google Scholar] [CrossRef]
- Ueno, N.; Inui, A.; Satoh, Y. The effect of mosapride citrate on constipation in patients with diabetes. Diabetes Res. Clin. Pract. 2010, 87, 27–32. [Google Scholar] [CrossRef]
- Liu, Z.; Sakakibara, R.; Odaka, T.; Uchiyama, T.; Uchiyama, T.; Yamamoto, T.; Ito, T.; Asahina, M.; Yamaguchi, K.; Yamaguchi, T.; et al. Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov. Disord. 2005, 20, 680–686. [Google Scholar] [CrossRef]
- Morrow, A. ZELNORM® (Tegaserod) Notice of Withdrawal from Market. Alfasigma USA, Inc. 2022. Available online: https://www.myzelnorm.com/assets/pdfs/Press%20Release%20on%20Notice%20of%20Withdrawal.pdf (accessed on 2 November 2023).
- Taghavi, S.A.; Shabani, S.; Mehramiri, A.; Eshraghian, A.; Kazemi, S.M.; Moeini, M.; Hosseini-Asl, S.M.; Saberifiroozi, M.; Alizade-Naeeni, M.; Mostaghni, A.A. Colchicine is effective for short-term treatment of slow transit constipation: A double-blind placebo-controlled clinical trial. Int. J. Colorectal Dis. 2010, 25, 389–394. [Google Scholar] [CrossRef]
- Roarty, T.P.; Weber, F.; Soykan, I.; McCallum, R.W. Misoprostol in the treatment of chronic refractory constipation: Results of a long-term open label trial. Aliment. Pharmacol. Ther. 1997, 11, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Chiarioni, G.; Vantini, I.; Morelli, A.; Whitehead, W.E. Effect of different doses of erythromycin on colonic motility in patients with slow transit constipation. Z. Gastroenterol. 1998, 36, 209–213. [Google Scholar] [PubMed]
- Sen, A.; Chokshi, R. Update on the Diagnosis and Management of Acute Colonic Pseudo-obstruction (ACPO). Curr. Gastroenterol. Rep. 2023, 25, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, B.D.; Noori, J.; Johnston, M.; Woods, R.; Keck, J.O.; Behrenbruch, C. Pyridostigmine in chronic intestinal pseudo-obstruction—A systematic review. ANZ J. Surg. 2023, 93, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, C.J.; Brookes, J.H.; Wattchow, D.A. The efficacy of treatment of patients with severe constipation or recurrent pseudo-obstruction with pyridostigmine. Colorectal Dis. 2010, 12, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Law, N.M.; Bharucha, A.E.; Undale, A.S.; Zinsmeister, A.R. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1228–G1237. [Google Scholar] [CrossRef] [PubMed]
- Soufi-Afshar, I.; Moghadamnia, A.; Bijani, A.; Kazemi, S.; Shokri-Shirvani, J. Comparison of pyridostigmine and bisacodyl in the treatment of refractory chronic constipation. Casp. J. Intern Med. 2016, 7, 19–24. [Google Scholar]
- Bharucha, A.E.; Low, P.; Camilleri, M.; Veil, E.; Burton, D.; Kudva, Y.; Shah, P.; Gehrking, T.; Zinsmeister, A.R. A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation. Gut 2013, 62, 708–715. [Google Scholar] [CrossRef]
- Ahuja, N.K.; Mische, L.; Clarke, J.O.; Wigley, F.M.; McMahan, Z.H. Pyridostigmine for the treatment of gastrointestinal symptoms in systemic sclerosis. Semin. Arthritis Rheum. 2018, 48, 111–116. [Google Scholar] [CrossRef]
- Ly, A.; Rahman, M.; Song, D. Pyridostigmine as an Effective Treatment for Atonic Colon in Parkinson’s Disease (P13-11.007). Neurology 2022, 98, 1539. [Google Scholar] [CrossRef]
- Tian, H.; Ge, X.; Nie, Y.; Yang, L.; Ding, C.; McFarland, L.V.; Zhang, X.; Chen, Q.; Gong, J.; Li, N. Fecal microbiota transplantation in patients with slow-transit constipation: A randomized, clinical trial. PLoS ONE 2017, 12, e0171308. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Fan, W.; Gu, L.; Tian, H.; Ge, X.; Gong, J.; Nie, Y.; Li, N. Outcomes and prognostic factors of fecal microbiota transplantation in patients with slow transit constipation: Results from a prospective study with long-term follow-up. Gastroenterol. Rep. 2018, 6, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dinning, P.G.; Hunt, L.; Patton, V.; Zhang, T.; Szczesniak, M.; Gebski, V.; Jones, M.; Stewart, P.; Lubowski, D.Z.; Cook, I.J. Treatment efficacy of sacral nerve stimulation in slow transit constipation: A two-phase, double-blind randomized controlled crossover study. Am. J. Gastroenterol. 2015, 110, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Patton, V.; Stewart, P.; Lubowski, D.Z.; Cook, I.J.; Dinning, P.G. Sacral Nerve Stimulation Fails to Offer Long-term Benefit in Patients with Slow-Transit Constipation. Dis. Colon Rectum 2016, 59, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Zerbib, F.; Siproudhis, L.; Lehur, P.A.; Germain, C.; Mion, F.; Leroi, A.M.; Coffin, B.; Le Sidaner, A.; Vitton, V.; Bouyssou-Cellier, C.; et al. Randomized clinical trial of sacral nerve stimulation for refractory constipation. Br. J. Surg. 2017, 104, 205–213. [Google Scholar] [CrossRef]
- Yiannakou, Y.; Etherson, K.; Close, H.; Kasim, A.; Mercer-Jones, M.; Plusa, S.; Maier, R.; Green, S.; Cundall, J.; Knowles, C.; et al. A randomized double-blinded sham-controlled cross-over trial of tined-lead sacral nerve stimulation testing for chronic constipation. Eur. J. Gastroenterol. Hepatol. 2019, 31, 653–660. [Google Scholar] [CrossRef]
- Ng, R.T.; Lee, W.S.; Ang, H.L.; Teo, K.M.; Yik, Y.I.; Lai, N.M. Transcutaneous electrical stimulation (TES) for treatment of constipation in children. Cochrane Database Syst. Rev. 2016, 11, CD010873. [Google Scholar] [CrossRef]
- Yang, Y.; Yim, J.; Choi, W.; Lee, S. Improving slow-transit constipation with transcutaneous electrical stimulation in women: A randomized, comparative study. Women Health 2017, 57, 494–507. [Google Scholar] [CrossRef]
- Martellucci, J.; Valeri, A. Colonic electrical stimulation for the treatment of slow-transit constipation: A preliminary pilot study. Surg. Endosc. 2014, 28, 691–697. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.; Zheng, Q.; Zhang, W.; Li, Y. The Effectiveness of Acupuncture in Management of Functional Constipation: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2020, 2020, 6137450. [Google Scholar] [CrossRef]
- Peng, W.N.; Wang, L.; Liu, Z.S.; Guo, J.; Cai, H.J.; Ni, J.N.; Duan, J.X.; Yang, D.L. Analysis on follow-up efficacy and safety of slow transit constipation treated with individualized deep puncture at Tianshu (ST 25): A multi-central randomized controlled trial. Zhongguo Zhen Jiu 2013, 33, 865–869. [Google Scholar] [PubMed]
- Emmett, C.D.; Close, H.J.; Yiannakou, Y.; Mason, J.M. Trans-anal irrigation therapy to treat adult chronic functional constipation: Systematic review and meta-analysis. BMC Gastroenterol. 2015, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Lees, N.P.; Hodson, P.; Hill, J.; Pearson, R.C.; MacLennan, I. Long-term results of the antegrade continent enema procedure for constipation in adults. Colorectal Dis. 2004, 6, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Rongen, M.J.; van der Hoop, A.G.; Baeten, C.G. Cecal access for antegrade colon enemas in medically refractory slow-transit constipation: A prospective study. Dis. Colon Rectum 2001, 44, 1644–1649. [Google Scholar] [CrossRef]
- Chu, D.I.; Balsara, Z.R.; Routh, J.C.; Ross, S.S.; Wiener, J.S. Experience with glycerin for antegrade continence enema in patients with neurogenic bowel. J. Urol. 2013, 189, 690–693. [Google Scholar] [CrossRef]
- Knowles, C.H.; Grossi, U.; Horrocks, E.J.; Pares, D.; Vollebregt, P.F.; Chapman, M.; Brown, S.; Mercer-Jones, M.; Williams, A.B.; Yiannakou, Y.; et al. Surgery for constipation: Systematic review and practice recommendations: Graded practice and future research recommendations. Colorectal Dis. 2017, 19 (Suppl. S3), 101–113. [Google Scholar] [CrossRef]
- Mollen, R.M.; Hopman, W.P.; Oyen, W.J.; Kuijpers, H.H.; Edelbroek, M.A.; Jansen, J.B. Effect of subtotal colectomy on gastric emptying of a solid meal in slow-transit constipation. Dis. Colon Rectum 2001, 44, 1189–1195. [Google Scholar] [CrossRef]

| Chronic Idiopathic Constipation |
| Dyssynergic defecation (with or without delayed colonic transit) |
| Slow transit constipation |
| Normal transit constipation † |
| Constipation predominant irritable bowel syndrome † |
| Neurologic Disorders |
| Parkinson’s disease |
| Multiple sclerosis |
| Stroke |
| Spinal cord injury |
| Diabetic enteric neuropathy |
| Myopathies |
| Systemic sclerosis |
| Amyloidosis |
| Metabolic disorders |
| Hypothyroidism |
| Hypercalcaemia |
| Uraemia |
| Diabetes mellitus |
| Medications |
| Opiates |
| Anticholinergics (e.g., antidepressants, antispasmodics, antipsychotics) |
| Dopaminergics (e.g., levodopa, dopamine agonists, antipsychotics) |
| Calcium channel blockers |
| 5-HT3 antagonists |
| Medication | Mechanism | Recommended Regimen | Comments |
|---|---|---|---|
| Prokinetics | |||
| Prucalopride | 5-HT4 agonist | 1–2 mg daily, oral Maximum 4 mg/day | Typical first line prokinetic in STC. |
| Cisapride | Cholinergic; 5-HT4 agonist | 10 mg QID, oral | May be preferred in patients with co-existing gastroparesis. |
| Mosapride | 5-HT4 agonist | 5 mg TDS, oral | Evidence for use in secondary causes of STC but limited in idiopathic STC. |
| Colchicine | Uncertain | 1 mg daily, oral | Limited evidence in STC, but available evidence suggests benefit. |
| Misoprostol | Prostaglandin analogue | 200 µg TDS, oral Maximum 2400 µg/day | May be limited by abdominal discomfort. Limited evidence in STC. |
| Erythromycin | Motilin receptor agonist | 40 mg TDS, oral or IV Maximum 2 g/day | Conflicting data for benefit in STC. |
| Pyridostigmine | Cholinesterase inhibitor | 60 mg TDS, oral Maximum 720 mg/day | Physiologically plausible and beneficial in similar conditions (pseudo-obstruction and secondary STC), but limited evidence in idiopathic STC. |
| Bile acid transporter inhibitors | |||
| Elobixibat | Bile acid transporter antagonist | 5–15 mg daily, oral | Limited evidence in STC, but available evidence suggests benefit. |
| Secretagogues | |||
| Lubiorostone | Chloride channel agonist | 24 µg BD, oral | Limited evidence in STC, but effective in severe CIC. |
| Linaclotide | CFTR agonist | 72–145 µg daily, oral Maximum 290 µg/day | Limited evidence in STC, but effective in severe CIC. |
| Plecanatide | CFTR agonist | 3 mg daily, oral | Limited evidence in STC, but effective in severe CIC. |
| Author, Year, Article Type | Treatment | Population | Study Characteristics | Outcomes |
|---|---|---|---|---|
| Emmanuel et al. [47] 2002 RCT | Prucalopride 1 mg | Females aged over 18 with functional constipation. Whole gut transit was performed on all participants, and subgroup analysis on those with STC was performed. | 74 (all female) participants, 43 classified with STC. Overall, 37 treatment, 37 placebo. Of those with STC, 22 treatment, 21 placebo. | Prucalopride reduced the number of retained markers in all patients when compared with placebo by 11.2 vs. 1.1 (p < 0.05), respectively. Prucalopride significantly reduced the number of retained markers in those with STC by 17.3 (p < 0.05), but the change in baseline by 1.6 in NTC was not significant. |
| Taghavi et al. [52] 2010 RCT | Colchicine 1 mg daily | Patients with chronic constipation who had STC confirmed with colon transit time. | 60 participants (47 female). 30 treatment, 30 placebo. | Colchicine significantly improved symptom scores and increased frequency of spontaneous bowel movements. 26/30 participants treated with colchicine had an acceptable symptomatic response. |
| Roarty et al. [53] 1997 Open-label trial | Misoprostol 200 µg TDS. Dose titration based on response and tolerance was allowed, with a range of 400–2400µg/day. | Adults with chronic constipation refractory to available medical therapy, who had STC confirmed with colonic transit time. | 18 participants (15 females). All received treatment. | Intolerance to misoprostol due to abdominal discomfort was common, with 6/18 patients dropping out prior to completion of the study period. 10/12 participants who tolerated misoprostol had improved frequency of bowel movements. Of the patients who tolerated the medication, mean bowel movement frequency improved from 11.25 to 4.8 days (p = 0.0004). |
| Bassotti et al. [54] 1998 Open-label | Erythromycin 50, 200, and 500 mg IV | Females with severe constipation with confirmed STC with colonoscopically positioned manometric probe, and effects of treatment on motility were assessed. | 18 participants (all female). All received placebo infusion followed by treatment. | Erythromycin had little prokinetic effects in the colon, although some increased activity in the distal colon was demonstrated at a low dose. |
| Bharucha et al. [60] 2013 RCT | Pyridostigmine 60 mg TDS initially. Increased every three days to a maximum of 120 mg TDS, based on effect and tolerance. | Diabetic patients with CIC. All patients had scintigraphy to determine colonic transit time, and 13/30 participants had confirmed slow transit. | 30 patients (22 female) 16 received treatment, and 14 received placebo. Of the 13 participants with STC, eight received pyridostigmine and five placebo. | Significantly increased colonic transit overall (p < 0.01), as well as improved stool frequency and consistency (p = 0.04). 7/8 vs. 2/5 patients with STC had normalisation of colonic transit times with pyridostigmine vs. placebo, respectively. |
| O’Dea et al. [57] 2010 Open-label | Pyridostigmine 10 mg BD initially, increased if required. | Adults with refractory STC or recurrent pseudo-obstruction who were being considered for colectomy. | 13 overall, six with STC. All patients received treatment. | Of those with STC, 1/6 participants had improved symptoms. 4/5 who had no benefit ultimately underwent colectomy. |
| Tian et al. [63] 2017 RCT | FMT 100 mL by nasointestinal tube daily for six days, in addition to conventional therapy. Compared unblinded to conventional therapy alone. | Adults with refractory STC. | 60 participants (40 female). 30 received FMT plus conventional therapy, 30 received conventional therapy. | FMT plus conventional therapy resulted in a clinical cure rate of 36.7% vs. 13.3% (p = 0.04) compared with conventional therapy alone. Treatment compared with control was also associated with an increased number of CSBMs per week (3.2 vs. 2.1, p = 0.001) and colonic transit time (58.5 vs. 73.6 h, p < 0.00001). |
| Dinning et al. [65] 2015 RCT | SNS | Adults with medically refractory STC confirmed by scintigraphy. | Of 59 participants who underwent peripheral nerve evaluation to assess for suitability for permanent SNS, 55 participants (51 females) proceeded with permanent SNS insertion and were included. All patients received both actual and sham stimulations in a cross-over design. | There was no significant difference with either supraseonsory or subsensory stimulation compared with sham stimulations in any of the outcome measures. |
| Zerbib et al. [67] 2017 RCT | SNS | Adults with medically refractory CIC. All patients underwent assessment of colonic transit times using radio-opaque marker test. 28/36 of the initial participants, and 16/20 of those who progressed to permanent SNS, were classified as STC. | Of 36 participants (34 female) who underwent peripheral nerve evaluation to assess for suitability for permanent SNS, 20 responded and received a permanent SNS and were included. All patients received both actual and sham stimulations in a cross-over design. | There was no significant difference between on- and off- periods of stimulation in any of the outcomes measured. |
| Yiannakou et al. [68] 2019 RCT | SNS | Adults with medically refractory CIC. All patients underwent assessment of colonic transit times. 30/45 of initial participants were classified as STC. | Of the 45 participants (43 female) who underwent peripheral nerve evaluation to assess for suitability for permanent SNS, 29 were responders, 2/29 did not proceed, and 27 ultimately received a permanent SNS and were included. All patients received both actual and sham stimulations in a cross-over design. | There was no significant difference between on- and off- periods of stimulation in any of the outcomes measured. Additionally, there was no difference between those who were discriminate and indiscriminate responders during the peripheral nerve evaluation. |
| Ng et al. [69] 2016 Systematic review | TES | Children with STC confirmed by scintigraphy. | 10 studies reporting on a single RCT cohort of 42 children (18 girls) aged 8–18 years, with additional data from their subsequent long-term studies. 21 received TES, 21 received sham stimulation. | TES was associated with a significantly reduced colonic transit time compared with sham stimulation (mean difference 1.05, 95%CI 0.36–1.74). There was no statistical difference between TES and sham stimulation in terms of CSBM/week, soiling or QOL. |
| Yang et al. [70] 2017 RCT | TES | Women with STC. | 28 participants (all female). 14 received TES, 14 received sham stimulation. | TES improved symptoms scores and frequency of SBMs compared with sham stimulation (p < 0.05). |
| Martellucci and Valeri. [71] 2013 Pilot study | Colonic pacing | Adults with medically refractory STC. | Two participants (both female). Both underwent intramuscular electrode placement for colonic pacing. | Number of SBM/week improved from 0.3 to 3.5 in one patient, and 0.5 to 2.5 in the other. Both patients were able to subsequently cease all conventional therapy for constipation and there were no complications. |
| Peng et al. [73] 2013 RCT | Acupuncture | 128 participants. 64 received deep puncture, 33 shallow puncture, and 31 western medication. | Defecation frequency improved from 1.8 to 3.9 SBMs/week with deep puncture acupuncture but did not meet statistical significance (p > 0.05). Deep puncture acupuncture was significantly associated with improved defecation frequency to 3.5 SBMs/week at the six month follow up visit (p < 0.05). | |
| Lees et al. [75] 2004 Cohort | ACE | Medically refractory CIC (combination of STC, DD, mixed STC/DD patients) | 32 participants (26 female) Median age 35. All received ACE. | 28/32 required further conduit procedure (19/32 reversed). Satisfactory ACE function achieved in 47%. 12 ultimately went on to surgery (colectomy/ileostomy). Further surgical interventions not affected by prior caecostomy. |
| Rongen et al. [76] 2001 Cohort | ACE | Medically refractory STC | 12 participants (8 female) Mean age 43. All received ACE. | Median defecation frequency improved from 1/week to 1/day. 4/12 ultimately required colectomy. Further surgery not compromised by preceding caecostomy. |
| Knowles et al. [78] 2017 Systematic review | Surgery | Patients undergoing colectomy for medically refractory STC. | 40 studies including 2045 participants. All patients received surgery. | Colectomy resulted in a global satisfaction rate of 86% (range 81–89%). Peri-operative complications occurred in 24.4% (range 17.8–31.7%), with a mortality rate of 0.4%. Abdominal pain and bloating present in 20–50%. Persistent constipation present in 10–30%. Diarrhoea and/or incontinence in 5–15%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlismas, L.J.; Wu, W.; Ho, V. Idiopathic Slow Transit Constipation: Pathophysiology, Diagnosis, and Management. Medicina 2024, 60, 108. https://doi.org/10.3390/medicina60010108
Vlismas LJ, Wu W, Ho V. Idiopathic Slow Transit Constipation: Pathophysiology, Diagnosis, and Management. Medicina. 2024; 60(1):108. https://doi.org/10.3390/medicina60010108
Chicago/Turabian StyleVlismas, Luke J., William Wu, and Vincent Ho. 2024. "Idiopathic Slow Transit Constipation: Pathophysiology, Diagnosis, and Management" Medicina 60, no. 1: 108. https://doi.org/10.3390/medicina60010108
APA StyleVlismas, L. J., Wu, W., & Ho, V. (2024). Idiopathic Slow Transit Constipation: Pathophysiology, Diagnosis, and Management. Medicina, 60(1), 108. https://doi.org/10.3390/medicina60010108






