Redefining the Axillary Aesthetic: Surgical Management of Axillary Tissue Hypertrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Demarcation
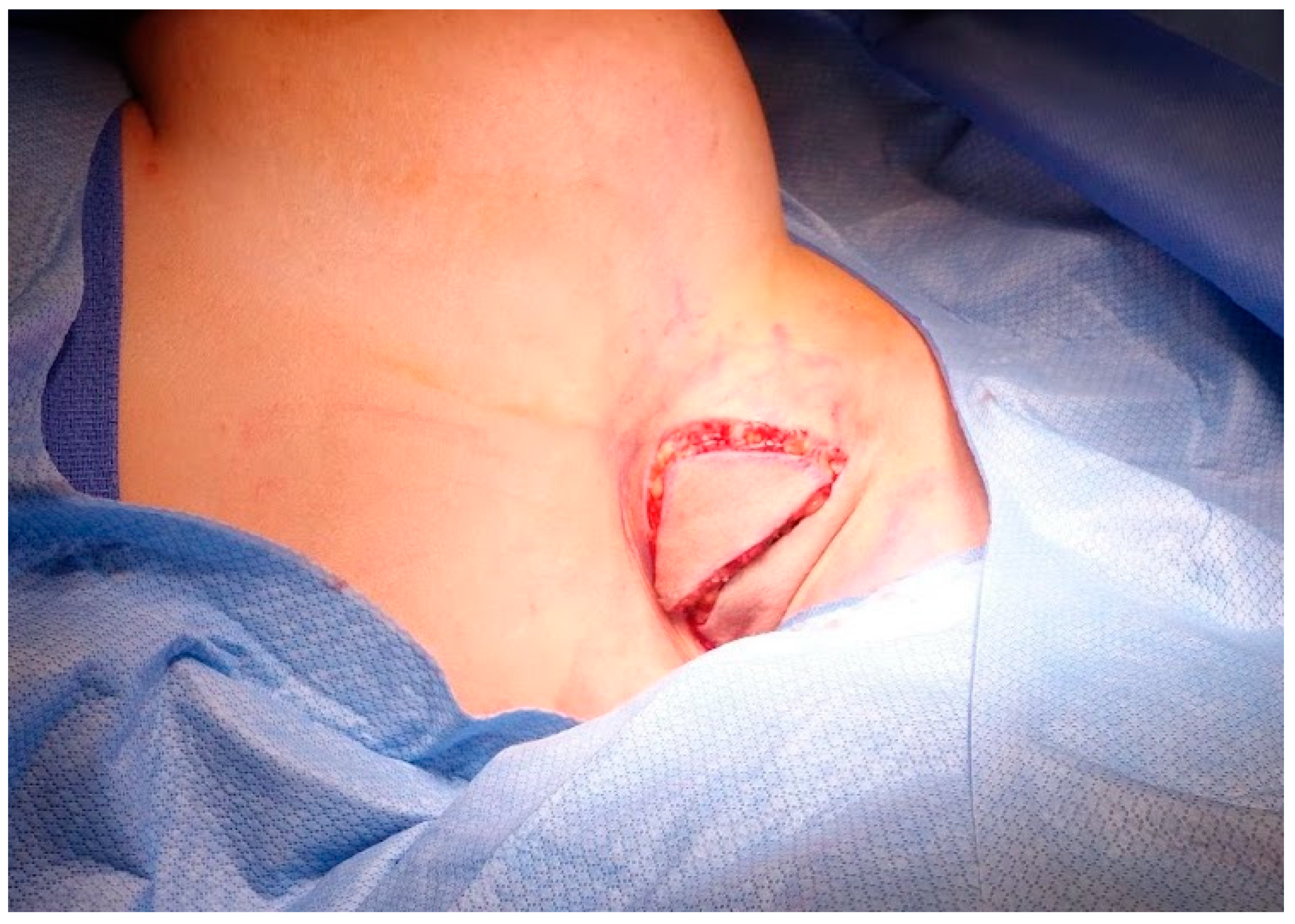
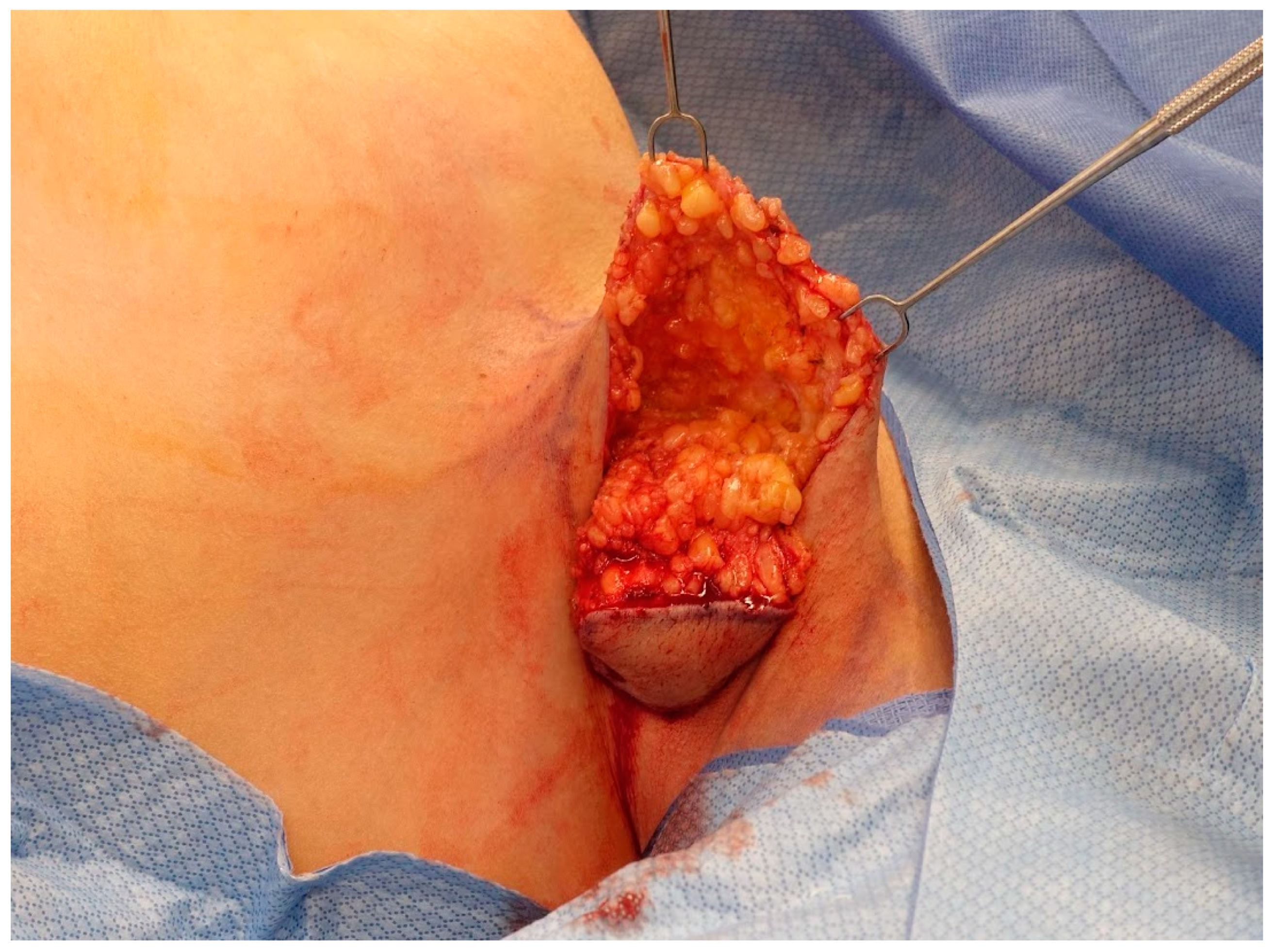
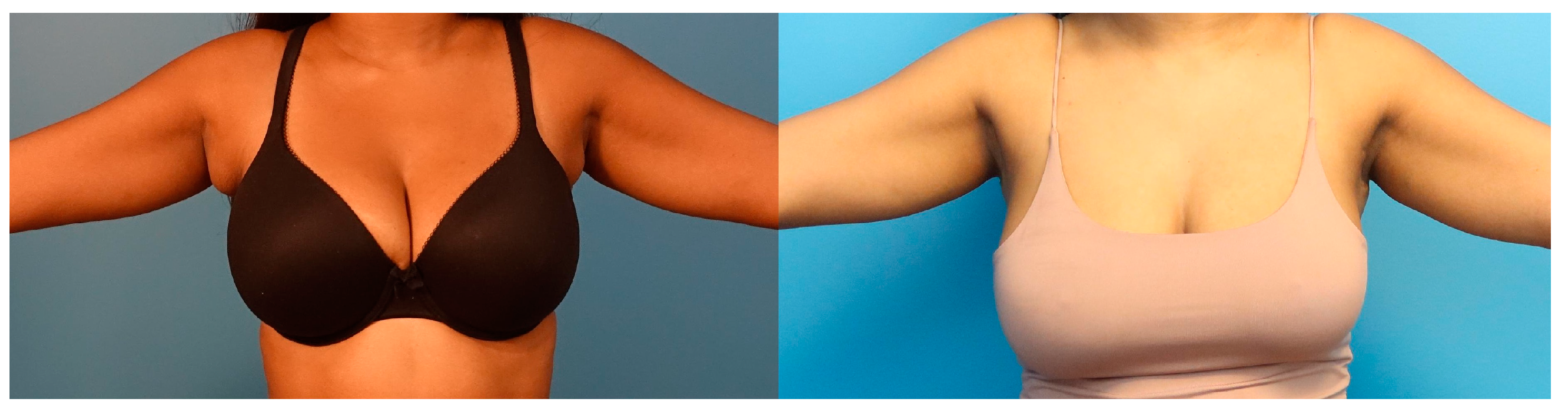
2.2. Operative Technique
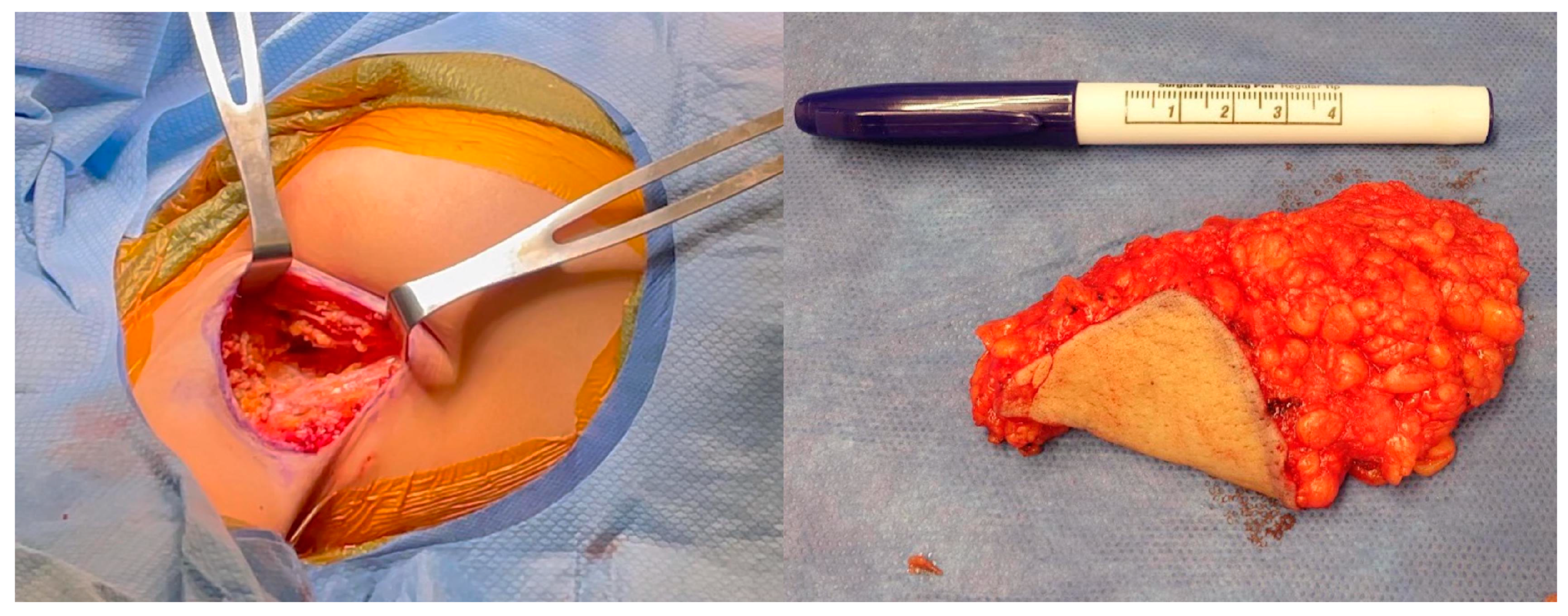
2.3. Pathology
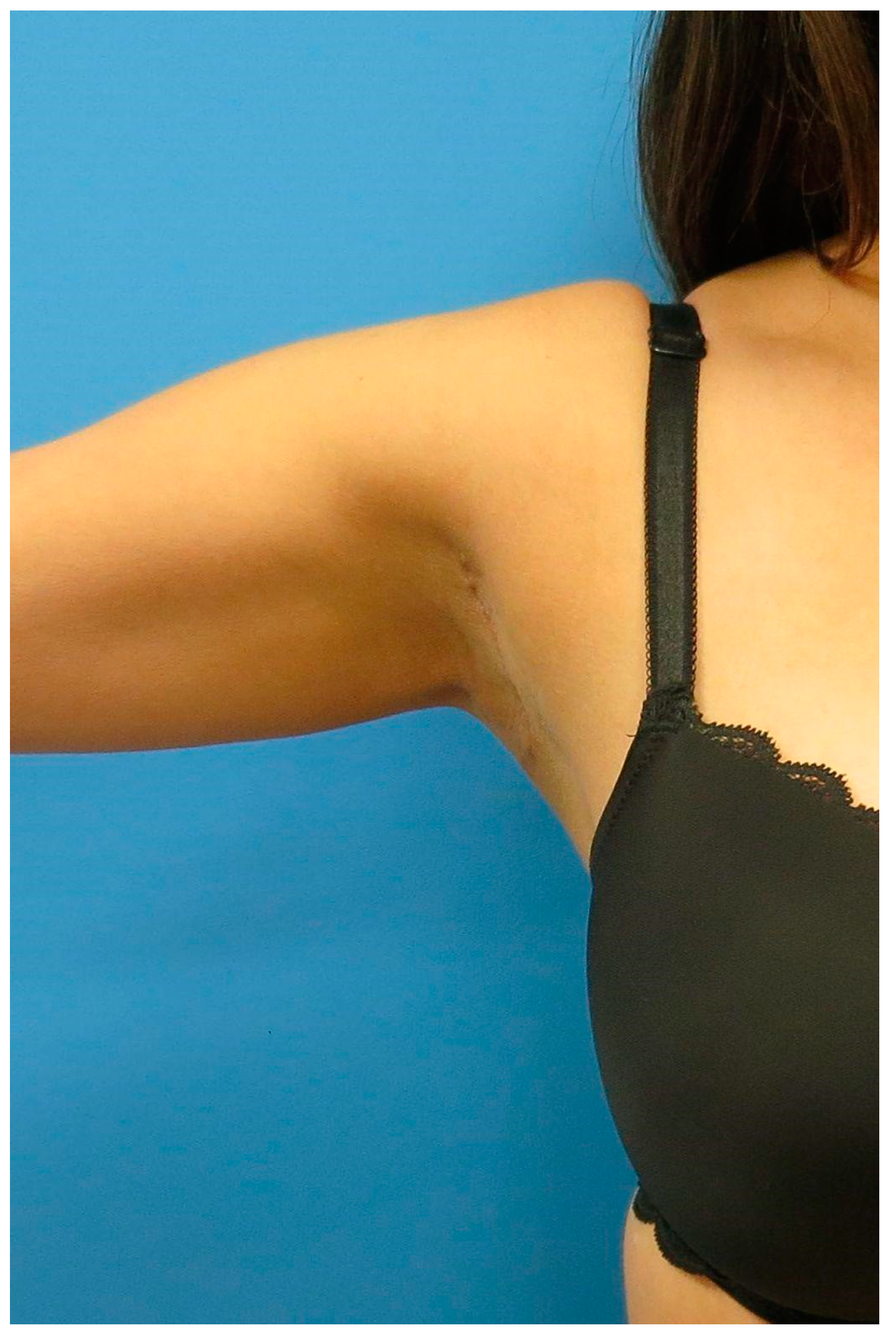
2.4. Postoperative Management
3. Results
3.1. Patient Characteristics
3.2. Pathology Reports
3.3. Complications
3.3.1. Cording
3.3.2. Hypertrophic Scarring
3.3.3. Seroma
| Pt | Age at Surgery | BMI (kg/m2) | Laterality | Complications |
|---|---|---|---|---|
| 1 | 27 | 24.8 | bilateral (L > R) | None |
| 2 | 23 | 21.95 | bilateral (L > R) | None |
| 3 | 23 | 22.08 | bilateral (R > L) | None |
| 4 | 30 | 26.3 | bilateral (R > L) | None |
| 5 | 45 | 29.85 | bilateral (L > R) | Mild axillary cording, bilateral. Hypertrophic scar, left |
| 6 | 49 | 25.4 | bilateral | Seroma, left |
| 7 | 36 | 39.44 | bilateral | Hypertrophic scar, left |
| 8 | 32 | 23.86 | bilateral (R > L) | None |
| 9 | 45 | 25.82 | bilateral (L > R) | None |
| 10 | 36 | 27.26 | left | Axillary cording, left |
| 11 | 17 | 27.46 | bilateral (R > L) | None |
| 12 | 28 | 35.85 | bilateral (R > L) | None |
| 13 | 52 | 31.64 | bilateral (R > L) | None |
| 14 | 53 | 25.61 | bilateral (R > L) | None |
| 15 | 35 | 31.93 | right | None |
| 16 | 45 | 25.69 | bilateral (R > L) | None |
| Pt | Sex | Age at Surgery | BMI (kg/m2) | Laterality | Drain (Y/N) | Complications |
|---|---|---|---|---|---|---|
| 1 | F | 43 | 26.4 | right | N | None |
| 2 | F | 51 | 41.4 | bilateral | N | Bilateral axillary seromas |
| 3 | F | 38 | 18.3 | bilateral | N | Bilateral axillary seroma |
| 4 | F | 49 | 31.32 | bilateral | N | Bilateral axillary seroma |
| 5 | F | 54 | 26.5 | bilateral | N | Bilateral axillary seroma |
| 6 | F | 48 | 27.7 | bilateral | Y | None |
| 7 | F | 34 | 22.7 | bilateral | N | Right axillary seroma |
| 8 | F | 44 | 23 | bilateral | N | Bilateral axillary seroma Bilateral cording Small right axillary wound requiring packing/wound care |
| 9 | F | 34 | 28.97 | bilateral | N | None |
| 10 | F | 56 | 27.2 | bilateral | N | None |
| 11 | F | 59 | 30 | bilateral | N | None |
| 12 | F | 16 | 27.5 | bilateral | N | Small Left axillary seroma |
| 13 | F | 26 | 21 | bilateral | N | None |
| 14 | F | 50 | 26 | bilateral | N | None |
| 15 | F | 31 | 20.8 | bilateral | N | None |
| 16 | F | 36 | 22.3 | left | N | None |
| 17 | F | 27 | 22.1 | bilateral | N | Hypertrophic right axillary scar |
| 18 | F | 42 | 23.6 | bilateral | N | None |
| 19 | F | 42 | 30.8 | bilateral | N | None |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoenwolf, G.C.; Bleyl, S.B.; Brauer, P.R.; Francis-West, P.H. Development of the skin and its derivatives. In Human Embryology, 4th ed.; Larsen, W.J., Ed.; Churchill Livingstone: Philadelphia, PA, USA, 2009; pp. 193–216. [Google Scholar]
- Hwang, S.B.; Choi, B.S.; Byun, G.Y.; Koo, B.H.; Lee, S.R. Accessory Axillary Breast Excision with Liposuction Using Minimal Incision: A Preliminary Report. Aesthetic Plast. Surg. 2016, 41, 10–18. [Google Scholar] [CrossRef]
- Greer, K.E. Accessory axillary breast tissue. Arch. Dermatol. 1974, 109, 88–89. [Google Scholar] [CrossRef]
- Youn, H.J.; Jung, S.H. Accessory Breast Carcinoma. Breast Care 2009, 4, 104–106. [Google Scholar] [CrossRef]
- Sahu, S.K.; Husain, M.; Sachan, P.K. Bilateral accessory breast. Internet J. Surg. 2008. [Google Scholar]
- DeFilippis, E.M.; Arleo, E.K. The ABCs of Accessory Breast Tissue: Basic Information Every Radiologist Should Know. Am. J. Roentgenol. 2014, 202, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, F.; Baghaki, S.; Celik, V.; Kocael, A.; Gokcal, F.; Cetinkale, O.; Unal, H. Surgical Treatment of Axillary Accessory Breasts. Am. Surg. 2010, 76, 270–272. [Google Scholar] [CrossRef]
- Fan, J. Removal of Accessory Breasts: A Novel Tumescent Liposuction Approach. Aesthetic Plast. Surg. 2009, 33, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Tang, X. Mammotome-Assisted Liposuction: A Novel Technique for Accessory Breasts. Aesthetic Plast. Surg. 2017, 41, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, S.M.; Jack, C.S.; Vincent, Y.K.L.; Evan, W.K.Y. The use of microdebrider for the treatment of accessory axillary breast. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, e301–e304. [Google Scholar] [CrossRef]
- Kim, Y.S. Correction of accessory axillary breast tissue without visible scar. Aesthetic Surg. J. 2004, 24, 531–535. [Google Scholar] [CrossRef]
- Down, S.; Barr, L.; Baildam, A.D.; Bundred, N. Management of accessory breast tissue in the axilla. Br. J. Surg. 2003, 90, 1213–1214. [Google Scholar] [CrossRef]
- Bhave, M.A. Axillary breast: Navigating uncharted terrain. Indian J. Plast. Surg. 2015, 48, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Koehler, L.A.; Haddad, T.C.; Hunter, D.W.; Tuttle, T.M. Axillary web syndrome following breast cancer surgery: Symptoms, complications, and management strategies. Breast Cancer Targets Ther. 2018, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies. Mol. Med. 2010, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Boostrom, S.Y.; Throckmorton, A.D.; Boughey, J.C.; Holifield, A.C.; Zakaria, S.; Hoskin, T.L.; Degnim, A.C. Incidence of Clinically Significant Seroma after Breast and Axillary Surgery. J. Am. Coll. Surg. 2009, 208, 148–150. [Google Scholar] [CrossRef]
- Yeung, W.M.; McPhail, S.M.; Kuys, S.S. A systematic review of axillary web syndrome (AWS). J. Cancer Surviv. 2015, 9, 576–598. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Guajardo, I.; Denkler, K.; Sbitany, H. Axillary Web Syndrome. Ann. Plast. Surg. 2016, 76, S227–S231. [Google Scholar] [CrossRef]
- Leidenius, M.; Leppänen, E.; Krogerus, L.; von Smitten, K. Motion restriction and axillary web syndrome after sentinel node biopsy and axillary clearance in breast cancer. Am. J. Surg. 2003, 185, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lacomba, M.; del Moral, O.M.; Zazo, J.L.C.; Sánchez, M.J.Y.; Ferrandez, J.-C.; Goñi, Á.Z. Axillary web syndrome after axillary dissection in breast cancer: A prospective study. Breast Cancer Res. Treat. 2009, 117, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, A.H.; O Anderson, B.; Yeung, R.S.; Byrd, D.R.; Lawton, T.J.; E Moe, R. Axillary web syndrome after axillary dissection. Am. J. Surg. 2001, 181, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Coveney, E.C.; O’Dwyer, P.J.; Geraghty, J.G.; O’Higgins, N.J. Effect of closing dead space on seroma formation after mastectomy—A prospective randomized clinical trial. Eur. J. Surg. Oncol. 1993, 19, 143–146. [Google Scholar] [PubMed]
- Myung, Y.; Heo, C.-Y. Relationship Between Obesity and Surgical Complications After Reduction Mammaplasty: A Systematic Literature Review and Meta-Analysis. Aesthet. Surg. J. 2016, 37, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.; Mendes, V.V.; de Almeida Dias, R.; e Silva, B.D.A.; da Costa Leite Ferreira, M.G.; Fabro, E.A.N. Incidence and risk factors for axillary web syndrome after breast cancer surgery. Breast Cancer Res. Treat. 2011, 131, 987–992. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanna, N.; Barnett, S.; Aiello, C.; Boehm, L.M.; Calobrace, M.B. Redefining the Axillary Aesthetic: Surgical Management of Axillary Tissue Hypertrophy. Medicina 2024, 60, 126. https://doi.org/10.3390/medicina60010126
Tanna N, Barnett S, Aiello C, Boehm LM, Calobrace MB. Redefining the Axillary Aesthetic: Surgical Management of Axillary Tissue Hypertrophy. Medicina. 2024; 60(1):126. https://doi.org/10.3390/medicina60010126
Chicago/Turabian StyleTanna, Neil, Sarah Barnett, Christopher Aiello, Lucas M. Boehm, and M. Bradley Calobrace. 2024. "Redefining the Axillary Aesthetic: Surgical Management of Axillary Tissue Hypertrophy" Medicina 60, no. 1: 126. https://doi.org/10.3390/medicina60010126
APA StyleTanna, N., Barnett, S., Aiello, C., Boehm, L. M., & Calobrace, M. B. (2024). Redefining the Axillary Aesthetic: Surgical Management of Axillary Tissue Hypertrophy. Medicina, 60(1), 126. https://doi.org/10.3390/medicina60010126








