Association between Ranolazine, Ischemic Preconditioning, and Cardioprotection in Patients Undergoing Scheduled Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Electrocardiography and Echocardiography
3.3. Medical Treatment
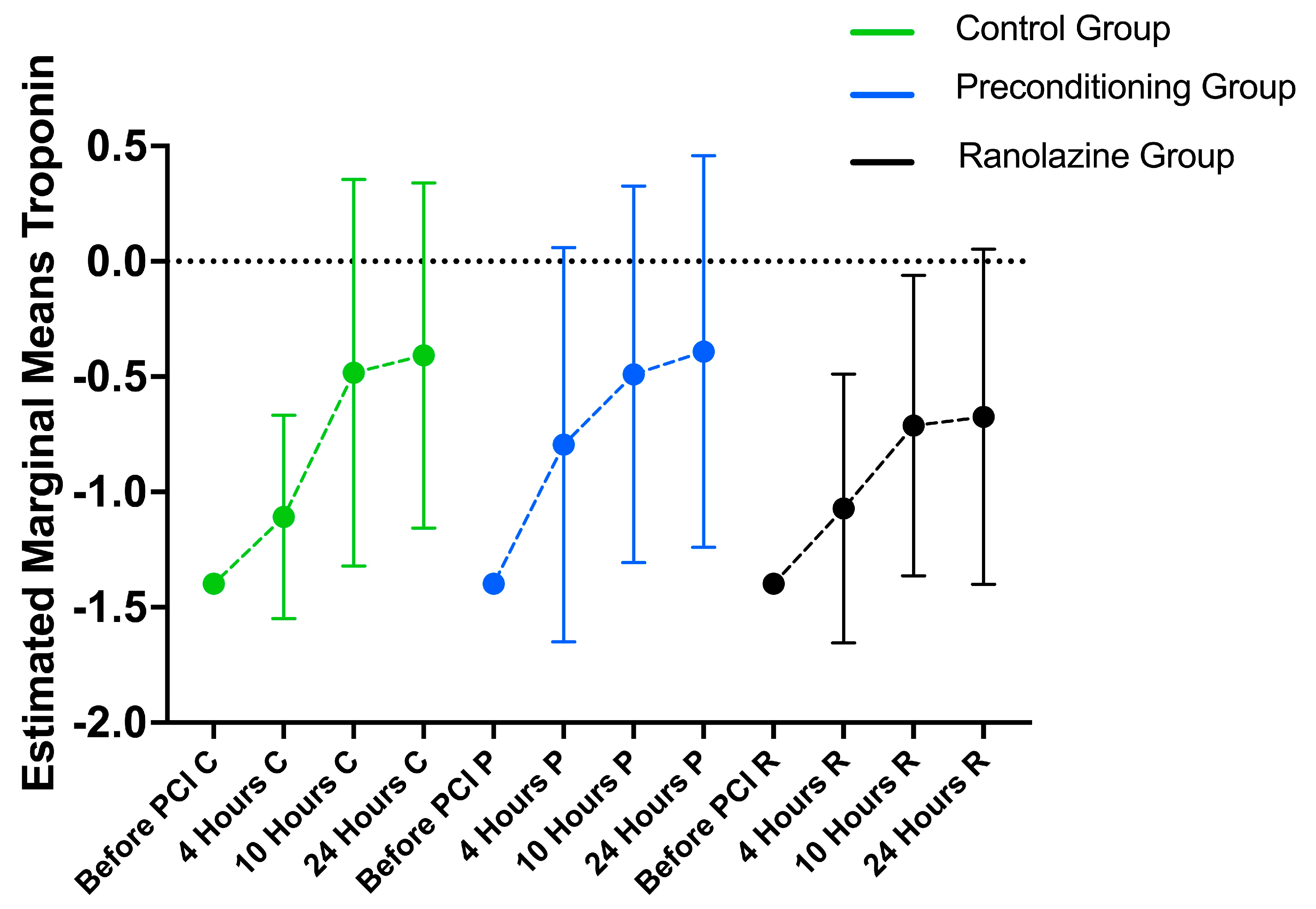
3.4. Hematology Markers’ Longitudinal Changes
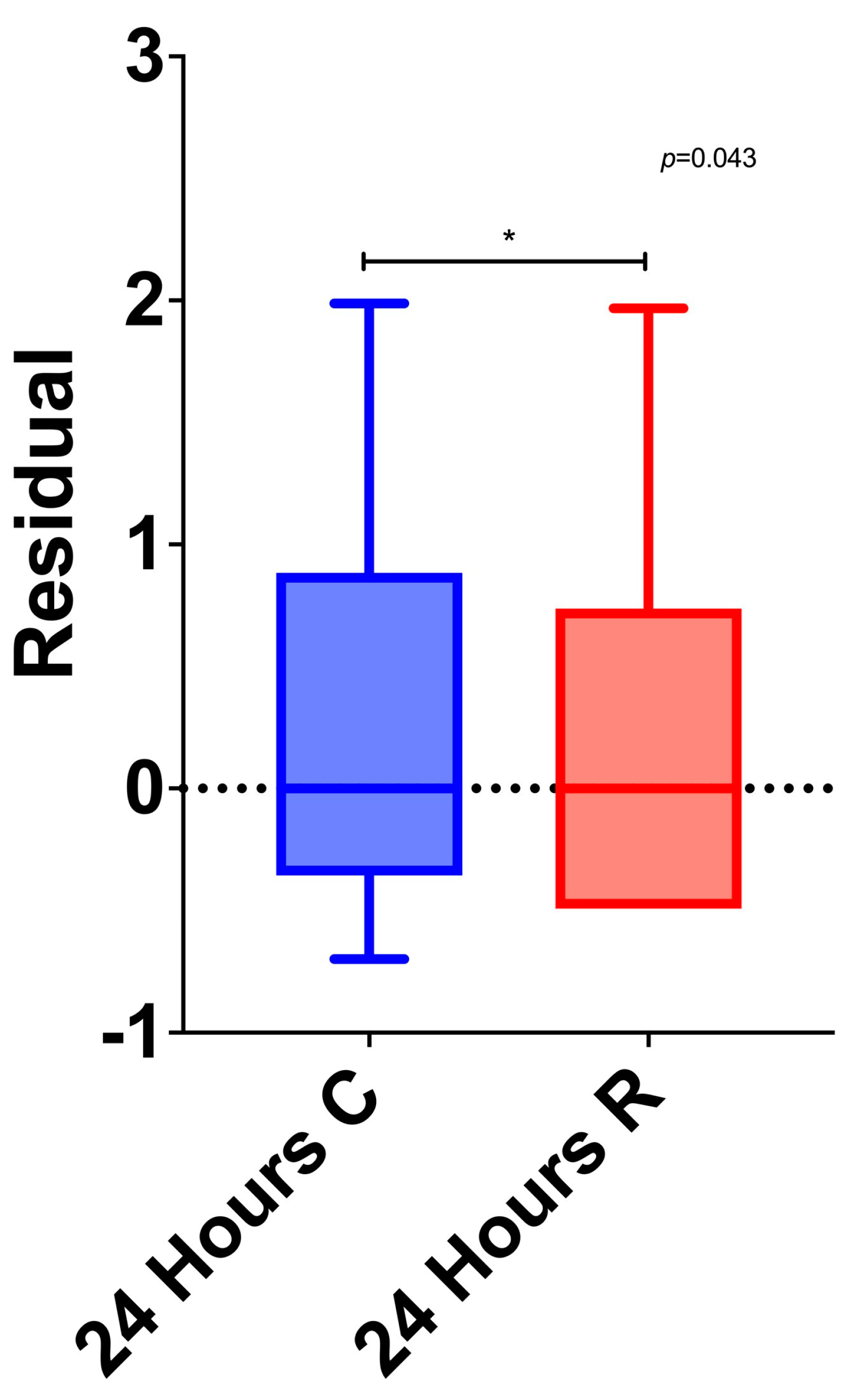
3.5. Between-Group Differences
3.6. Multivariable Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tapuria, N.; Kumar, Y.; Habib, M.M.; Abu Amara, M.; Seifalian, A.M.; Davidson, B.R. Remote ischemic preconditioning: A novel protective method from ischemia reperfusion injury—A review. J. Surg. Res. 2008, 150, 304–330. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, K.; Miyoshi, T.; Kohno, K.; Nakahama, M.; Doi, M.; Munemasa, M.; Murakami, M.; Takaishi, A.; Nakamura, K.; Ito, H.; et al. Protective Effect of Remote Ischemic Preconditioning on Myocardial Damage After Percutaneous Coronary Intervention in Stable Angina Patients with Complex Coronary Lesions—Subanalysis of a Randomized Controlled Trial. Circ. J. 2018, 82, 1788–1796. [Google Scholar] [CrossRef]
- Lau, J.K.; Roy, P.; Javadzadegan, A.; Moshfegh, A.; Fearon, W.F.; Ng, M.; Lowe, H.; Brieger, D.; Kritharides, L.; Yong, A.S. Remote Ischemic Preconditioning Acutely Improves Coronary Microcirculatory Function. J. Am. Heart Assoc. 2018, 7, e009058. [Google Scholar] [CrossRef] [PubMed]
- Przyklenk, K.; Whittaker, P. Remote ischemic preconditioning: Current knowledge, unresolved questions, and future priorities. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Karwatowska-Prokopczuk, E.; Murphy, S.A.; Belardinelli, L.; Farzaneh-Far, R.; Walker, G.; Morrow, D.A.; Scirica, B.M. Effects of Ranolazine in Patients with Chronic Angina in Patients with and without Percutaneous Coronary Intervention for Acute Coronary Syndrome: Observations from the MERLIN-TIMI 36 Trial. Clin. Cardiol. 2015, 38, 469–475. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, Q.; Guo, Q.; Peng, L.; Li, X.; Rao, L.; Li, M. Remote ischemic preconditioning can extend the tolerance to extended drug-coated balloon inflation time by reducing myocardial damage during percutaneous coronary intervention. Int. J. Cardiol. 2022, 353, 3–8. [Google Scholar] [CrossRef]
- Stone, P.H.; Chaitman, B.R.; Stocke, K.; Sano, J.; DeVault, A.; Koch, G.G. The anti-ischemic mechanism of action of ranolazine in stable ischemic heart disease. J. Am. Coll. Cardiol. 2010, 56, 934–942. [Google Scholar] [CrossRef]
- Reed, M.; Kerndt, C.C.; Gopal, S.; Nicolas, D. Ranolazine. In StatPearls [Internet]; Updated 16 May 2023; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507828/ (accessed on 1 November 2023).
- White, S.K.; Frohlich, G.M.; Sado, D.M.; Maestrini, V.; Fontana, M.; Treibel, T.A.; Tehrani, S.; Flett, A.S.; Meier, P.; Ariti, C.; et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2015, 8, 178–188. [Google Scholar] [CrossRef]
- Pelliccia, F.; Pasceri, V.; Marazzi, G.; Rosano, G.; Greco, C.; Gaudio, C. A pilot randomized study of ranolazine for reduction of myocardial damage during elective percutaneous coronary intervention. Am. Heart J. 2012, 163, 1019–1023. [Google Scholar] [CrossRef]
- Botker, H.E.; Kharbanda, R.; Schmidt, M.R.; Bottcher, M.; Kaltoft, A.K.; Terkelsen, C.J.; Munk, K.; Andersen, N.H.; Hansen, T.M.; Trautner, S.; et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet 2010, 375, 727–734. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Moretti, C.; Omede, P.; Cerrato, E.; Cavallero, E.; Er, F.; Presutti, D.G.; Colombo, F.; Crimi, G.; Conrotto, F.; et al. Cardiac remote ischaemic preconditioning reduces periprocedural myocardial infarction for patients undergoing percutaneous coronary interventions: A meta-analysis of randomised clinical trials. EuroIntervention 2014, 9, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.G.; Walker, J.M.; Yellon, D.M.; Hausenloy, D.J. Peri-procedural myocardial injury during percutaneous coronary intervention: An important target for cardioprotection. Eur. Heart J. 2011, 32, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.; Selvanayagam, J.B.; Van Gaal, W.J.; Prati, F.; Cheng, A.; Channon, K.; Neubauer, S.; Banning, A.P. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: Insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation 2006, 114, 662–669. [Google Scholar] [CrossRef]
- Prasad, A.; Herrmann, J. Myocardial infarction due to percutaneous coronary intervention. N. Engl. J. Med. 2011, 364, 453–464. [Google Scholar] [CrossRef]
- Selvanayagam, J.B.; Porto, I.; Channon, K.; Petersen, S.E.; Francis, J.M.; Neubauer, S.; Banning, A.P. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: Insights from cardiovascular magnetic resonance imaging. Circulation 2005, 111, 1027–1032. [Google Scholar] [CrossRef]
- Anderson, S.E.; Murphy, E.; Steenbergen, C.; London, R.E.; Cala, P.M. Na-H exchange in myocardium: Effects of hypoxia and acidification on Na and Ca. Am. J. Physiol. 1990, 259, C940–C948. [Google Scholar] [CrossRef] [PubMed]
- Imahashi, K.; Pott, C.; Goldhaber, J.I.; Steenbergen, C.; Philipson, K.D.; Murphy, E. Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ. Res. 2005, 97, 916–921. [Google Scholar] [CrossRef]
- Steenbergen, C.; Perlman, M.E.; London, R.E.; Murphy, E. Mechanism of preconditioning. Ionic alterations. Circ. Res. 1993, 72, 112–125. [Google Scholar] [CrossRef]
- Thireau, J.; Pasquie, J.L.; Martel, E.; Le Guennec, J.Y.; Richard, S. New drugs vs. old concepts: A fresh look at antiarrhythmics. Pharmacol. Ther. 2011, 132, 125–145. [Google Scholar] [CrossRef]
- Rouhana, S.; Virsolvy, A.; Fares, N.; Richard, S.; Thireau, J. Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning. Pharmaceuticals 2021, 15, 31. [Google Scholar] [CrossRef]
- Weisz, G.; Généreux, P.; Iñiguez, A.; Zurakowski, A.; Shechter, M.; Alexander, K.P.; Dressler, O.; Osmukhina, A.; James, S.; Ohman, E.M.; et al. Ranolazine in patients with incomplete revascularisation after percutaneous coronary intervention (RIVER-PCI): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2016, 387, 136–145. [Google Scholar] [CrossRef] [PubMed]
| Control Group | Preconditioning Group | Ranolazine Group | p | |
|---|---|---|---|---|
| (1) N = 50; 33.3% | (2) N = 50; 33.3% | (3) N = 50; 33.3% | ||
| N (%) | N (%) | N (%) | ||
| Gender | ||||
| Men | 41 (82.0) | 39 (78.0) | 39 (78.0) | 0.850 + |
| Women | 9 (18.0) | 11 (22.0) | 11 (22.0) | |
| Age, mean (SD) | 65.5 (11.3) | 66.3 (9.7) | 66.3 (13.4) | 0.913 ‡ |
| Heart rate, mean (SD) | 63.1 (8.5) 2 | 68.5 (9.3) 1 | 65.4 (8.0) | 0.009 ‡ |
| Systolic blood pressure, mean (SD) | 135.8 (8.8) | 133.9 (12.8) | 133.6 (10.0) | 0.553 ‡ |
| Diastolic blood pressure, mean (SD) | 79.2 (7.2) | 77 (9.0) | 78.1 (9.2) | 0.440 ‡ |
| Nationality | ||||
| Greek | 48 (96.0) | 45 (90.0) | 49 (98.0) | 0.277 ++ |
| Other | 2 (4.0) | 5 (10.0) | 1 (2.0) | |
| Βody mass index, mean (SD) | 28.5 (4) | 29.3 (4.1) | 29.4 (4.8) | 0.543 ‡ |
| Βody mass index | ||||
| Normal | 5 (10.0) | 7 (14.0) | 10 (20.0) | 0.289 + |
| Overweight | 30 (60.0) | 21 (42.0) | 23 (46.0) | |
| Obese | 15 (30.0) | 22 (44.0) | 17 (34.0) | |
| Body surface area, mean (SD) | 2.01 (0.17) | 1.98 (0.22) | 2.02 (0.24) | 0.673 ‡ |
| Smoking | ||||
| No | 17 (34.0) | 16 (32.0) | 22 (44.0) | 0.052 + |
| Yes, in the past | 26 (52.0) | 17 (34.0) | 14 (28.0) | |
| Yes, in the present | 7 (14.0) | 17 (34.0) | 14 (28.0) | |
| Packs/day, median (IQR) | 0 (0–1) | 0 (0–1) | 1 (0–1.3) | 0.424 ‡‡ |
| Alcohol consumption | 2 (4.0) | 1 (2.0) | 5 (10.0) | 0.277 ++ |
| Glomerular filtration rate, median (IQR) | 91.3 (79.1–124.7) | 84.8 (67.7–104.2) | 90.3 (70.3–110.5) | 0.211 ‡‡ |
| Comorbidities | ||||
| Hypertention | 44 (88.0) | 43 (86.0) | 44 (88.0) | 0.942 + |
| Dyslipidemia | 49 (98.0) | 47 (94.0) | 43 (86.0) | 0.083 ++ |
| Diabetes | 24 (48.0) | 22 (44.0) | 21 (42.0) | 0.828 + |
| Coronary artery disease | 33 (67.3) | 28 (57.1) | 30 (60.0) | 0.563 + |
| Atrial Fibrillation | 6 (12.0) | 5 (10.0) | 4 (8.0) | 0.801 + |
| Chronic obstructive pulmonary disease | 2 (4.0) | 5 (10.0) | 7 (14.3) | 0.202 ++ |
| Renal failure | 18 (36.0) | 10 (20.0) | 16 (32.0) | 0.188 + |
| Peripheral vascular disease | 7 (14.0) | 3 (6.0) | 5 (10.0) | 0.411 + |
| Sleep disorder | 5 (10.0) | 3 (6.0) | 6 (12.0) | 0.686 ++ |
| Previous stroke/transient ischemic Attack | 0 (0.0) | 1 (2.0) | 3 (6.0) | 0.324 ++ |
| Myocardial infarction | 27 (54.0) | 21 (42.0) | 24 (48.0) | 0.486 + |
| Cognitive impairment | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0.329 ++ |
| Thyroid dysfuction | 8 (16.0) | 6 (12.0) | 5 (10.0) | 0.656 + |
| Heart failure | 11 (22.4) | 14 (29.2) | 16 (32.7) | 0.521 + |
| PCI characteristics * | ||||
| Number of vessels | ||||
| 1 | 32 (66.7) | 38 (76.0) | 31 (62.0) | 0.268 + |
| 2 | 12 (25.0) | 12 (24.0) | 17 (34.0) | |
| 3 | 2 (4.2) | 0 (0.0) | 2 (4.0) | |
| 4 | 2 (4.2) | 0 (0.0) | 0 (0.0) | |
| Number of DESs, median (IQR) | 2 (1–3) | 1 (1–2) | 1 (1–2) | 0.420 ‡ |
| Medical treatment * | ||||
| Acetylsalicylic acid | 50 (100.0) | 50 (100.0) | 50 (100.0) | - |
| Clopidogrel | 14 (28.0) | 12 (24.5) | 17 (34.0) | 0.572 + |
| Prasugrel | 19 (38.0) | 21 (42.0) | 11 (22.0) | 0.082 + |
| Ticagrelor | 18 (36.0) | 17 (34.0) | 23 (46.0) | 0.418 + |
| IIBIIIA during PCI | 0 (0.0) | 2 (4.1) | 0 (0.0) | 0.107 ++ |
| IIBIIIA after PCI | 0 (0.0) | 4 (8.0) | 0 (0.0) | 0.034 ++ |
| Coumarin anticoagulant | 2 (4.0) | 1 (2.0) | 3 (6.0) | 0.871 ++ |
| Dabigatran | 1 (2.0) | 1 (2.0) | 0 (0.0) | >0.999 ++ |
| Rivaroxaban | 1 (2.0) | 1 (2.0) | 0 (0.0) | >0.999 ++ |
| Apixaban | 2 (4.0) | 2 (4.0) | 1 (2.0) | >0.999 ++ |
| Statin | 50 (100.0) | 50 (100.0) | 46 (93.9) | 0.034 ++ |
| Ezetimibe | 5 (10.0) | 5 (10.0) | 10 (20.4) | 0.216 + |
| Control Group (1) | Preconditioning Group (2) | Ranolizine Group (3) | p ++ | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| TROPONIN I | |||||||
| Before PCI | 0.04 (0) | 0.04 (0.04–0.04) | 0.04 (0) | 0.04 (0.04–0.04) | 0.04 (0) | 0.04 (0.04–0.04) | >0.999 |
| 4 h | 0.16 (0.29) | 0.04 (0.04–0.11) | 0.46 (1.21) | 0.05 (0.04–0.32) | 0.5 (1.93) | 0.04 (0.04–0.08) | 0.202 |
| 10 h | 1.91 (5.02) | 0.19 (0.07–1.13) | 1.09 (1.90) | 0.21 (0.06–1.18) | 0.74 (1.65) | 0.13 (0.06–0.42) | 0.366 |
| 24 h | 1. 92 (4.11) | 0.27 (0.09–2.04) | 2.55 (6.18) | 0.33 (0.06–2.1) | 0.95 (2.08) | 0.13 (0.04–0.68) | 0.159 |
| p + | <0.001 | <0.001 | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourtis, K.; Bourazana, A.; Xanthopoulos, A.; Skoularigkis, S.; Papadakis, E.; Patsilinakos, S.; Skoularigis, J. Association between Ranolazine, Ischemic Preconditioning, and Cardioprotection in Patients Undergoing Scheduled Percutaneous Coronary Intervention. Medicina 2024, 60, 166. https://doi.org/10.3390/medicina60010166
Kourtis K, Bourazana A, Xanthopoulos A, Skoularigkis S, Papadakis E, Patsilinakos S, Skoularigis J. Association between Ranolazine, Ischemic Preconditioning, and Cardioprotection in Patients Undergoing Scheduled Percutaneous Coronary Intervention. Medicina. 2024; 60(1):166. https://doi.org/10.3390/medicina60010166
Chicago/Turabian StyleKourtis, Konstantinos, Angeliki Bourazana, Andrew Xanthopoulos, Spyridon Skoularigkis, Emmanouil Papadakis, Sotirios Patsilinakos, and John Skoularigis. 2024. "Association between Ranolazine, Ischemic Preconditioning, and Cardioprotection in Patients Undergoing Scheduled Percutaneous Coronary Intervention" Medicina 60, no. 1: 166. https://doi.org/10.3390/medicina60010166
APA StyleKourtis, K., Bourazana, A., Xanthopoulos, A., Skoularigkis, S., Papadakis, E., Patsilinakos, S., & Skoularigis, J. (2024). Association between Ranolazine, Ischemic Preconditioning, and Cardioprotection in Patients Undergoing Scheduled Percutaneous Coronary Intervention. Medicina, 60(1), 166. https://doi.org/10.3390/medicina60010166







