Psychological Alterations in Youths with Type I Diabetes: Associations with Salivary Cortisol Concentration
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Psychological Assessments
2.3. Salivary Cortisol Concentration
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic and Clinical Data
3.2. Psychological and Diabetes-Related Quality of Life Assessments
3.3. Relationship between Salivary Cortisol Values and Psychological Assessment
3.4. Relationship between Clinical Parameters and Cortisol Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J.; et al. Global Incidence, Prevalence, and Mortality of Type 1 Diabetes in 2021 with Projection to 2040: A Modelling Study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Barat, P.; Brossaud, J.; Lacoste, A.; Vautier, V.; Nacka, F.; Moisan, M.-P.; Corcuff, J.-B. Nocturnal Activity of 11β-Hydroxy Steroid Dehydrogenase Type 1 Is Increased in Type 1 Diabetic Children. Diabetes Metab. 2013, 39, 163–168. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Acerini, C.L.; Codner, E.; Craig, M.E.; Hofer, S.E.; Pillay, K.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic Control Targets and Glucose Monitoring for Children, Adolescents, and Young Adults with Diabetes. Pediatr. Diabetes 2018, 19, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Herzer, M.; Hood, K.K. Anxiety Symptoms in Adolescents with Type 1 Diabetes: Association with Blood Glucose Monitoring and Glycemic Control. J. Pediatr. Psychol. 2010, 35, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kapellen, T.M.; Reimann, R.; Kiess, W.; Kostev, K. Prevalence of Medically Treated Children with ADHD and Type 1 Diabetes in Germany—Analysis of Two Representative Databases. J. Pediatr. Endocrinol. Metab. 2016, 29, 1293–1297. [Google Scholar] [CrossRef]
- Ai, Y.; Zhao, J.; Liu, H.; Li, J.; Zhu, T. The Relationship between Diabetes Mellitus and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 936813. [Google Scholar] [CrossRef]
- Estrada, C.L.; Danielson, K.K.; Drum, M.L.; Lipton, R.B. Insufficient Sleep in Young Patients with Diabetes and Their Families. Biol. Res. Nurs. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Thakkinstian, A.; Anothaisintawee, T.; Chontong, S.; Borel, A.-L.; Perfect, M.M.; Janovsky, C.C.P.S.; Kessler, R.; Schultes, B.; Harsch, I.A.; et al. Sleep Characteristics in Type 1 Diabetes and Associations with Glycemic Control: Systematic Review and Meta-Analysis. Sleep Med. 2016, 23, 26–45. [Google Scholar] [CrossRef]
- Perfect, M.M.; Elkins, G.R.; Lyle-Lahroud, T.; Posey, J.R. Stress and Quality of Sleep among Individuals Diagnosed with Diabetes. Stress Health 2010, 26, 61–74. [Google Scholar] [CrossRef]
- McDonough, R.J.; Clements, M.A.; DeLurgio, S.A.; Patton, S.R. Sleep Duration and Its Impact on Adherence in Adolescents with Type 1 Diabetes Mellitus. Pediatr. Diabetes 2017, 18, 262–270. [Google Scholar] [CrossRef]
- Çömlek, F.; Çelik, H.; Keskin, B.; Süt, N.; Dilek, E.; Tütüncüler, F. Sleep Quality Assessment in Adolescents with and without Type 1 Diabetes Using the Pittsburg Sleep Quality Index. Indian J. Endocrinol. Metab. 2021, 25, 202. [Google Scholar] [CrossRef] [PubMed]
- Kakleas, K.; Kandyla, B.; Karayianni, C.; Karavanaki, K. Psychosocial Problems in Adolescents with Type 1 Diabetes Mellitus. Diabetes Metab. 2009, 35, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Bargues-Navarro, G.; Ibáñez-del Valle, V.; El Mlili, N.; Cauli, O. Salivary Biomarkers Associated with Psychological Alterations in Patients with Diabetes: A Systematic Review. Medicina 2022, 58, 1091. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M. The Stress Response and the Hypothalamic-Pituitary-Adrenal Axis: From Molecule to Melancholia. QJM 2000, 93, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Aminkeng, F.; Ross, C.J.D.; Rassekh, S.R.; Hwang, S.; Rieder, M.J.; Bhavsar, A.P.; Smith, A.; Sanatani, S.; Gelmon, K.A.; Bernstein, D.; et al. Recommendations for Genetic Testing to Reduce the Incidence of Anthracycline-induced Cardiotoxicity. Br. J. Clin. Pharmacol. 2016, 82, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Cidlowski, J.A. Glucocorticoid-Induced Apoptosis of Healthy and Malignant Lymphocytes. Prog. Brain Res. 2010, 182, 1–30. [Google Scholar] [PubMed]
- Roy, M.S.; Roy, A.; Gallucci, W.T.; Collier, B.; Young, K.; Kamilaris, T.C.; Chrousos, G.P. The Ovine Corticotropin-Releasing Hormone-Stimulation Test in Type I Diabetic Patients and Controls: Suggestion of Mild Chronic Hypercortisolism. Metabolism 1993, 42, 696–700. [Google Scholar] [CrossRef]
- Gaete, X.; Iñiguez, G.; Linares, J.; Avila, A.; Mericq, V. Cortisol Hyporesponsiveness to the Low Dose ACTH Test Is a Frequent Finding in a Pediatric Population with Type 1 Diabetes Mellitus. Pediatr. Diabetes 2013, 14, 429–434. [Google Scholar] [CrossRef]
- Simunkova, K.; Hampl, R.; Hill, M.; Kriz, L.; Hrda, P.; Janickova-Zdarska, D.; Zamrazil, V.; Vrbikova, J.; Vondra, K. Adreno-cortical Function in Young Adults with Diabetes Mellitus Type 1. J. Steroid Biochem. Mol. Biol. 2010, 122, 35–41. [Google Scholar] [CrossRef]
- Korczak, D.J.; Pereira, S.; Koulajian, K.; Matejcek, A.; Giacca, A. Type 1 Diabetes Mellitus and Major Depressive Disorder: Evidence for a Biological Link. Diabetologia 2011, 54, 2483–2493. [Google Scholar] [CrossRef]
- Lebinger, T.G.; Saenger, P.; Fukushima, D.K.; Kream, J.; Wu, R.; Finkelstein, J.W. Twenty-Four-Hour Cortisol Profiles Demonstrate Exaggerated Nocturnal Rise in Diabetic Children. Diabetes Care 1983, 6, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Vanaelst, B.; De Vriendt, T.; Huybrechts, I.; Rinaldi, S.; De Henauw, S. Epidemiological Approaches to Measure Childhood Stress. Paediatr. Perinat. Epidemiol. 2012, 26, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Nygren, M.; Carstensen, J.; Koch, F.; Ludvigsson, J.; Frostell, A. Experience of a Serious Life Event Increases the Risk for Childhood Type 1 Diabetes: The ABIS Population-Based Prospective Cohort Study. Diabetologia 2015, 58, 1188–1197. [Google Scholar] [CrossRef]
- Peuhmond, A. CA-163: Vécu de l’enfant et de l’adolescent Diabétique à Propos de 3 Cas Au Centre Anti-Diabétique d’Abidjan: Difficultés et Perspectives. Diabetes Metab. 2016, 42, A80. [Google Scholar] [CrossRef]
- Suleiman, K.H.; Yates, B.C.; Berger, A.M.; Pozehl, B.; Meza, J. Translating the Pittsburgh Sleep Quality Index into Arabic. West. J. Nurs. Res. 2010, 32, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Al-Haidar, F.; Al-Alim, F.; Al-Hag, O. A Screening Tool for Attention Deficit Hyperactivity Disorder in Children in Saudi Arabia. Ann. Saudi Med. 2009, 29, 294–298. [Google Scholar] [CrossRef]
- DuPaul, G.J.; Anastopoulos, A.D.; Power, T.J.; Reid, R.; Ikeda, M.J.; McGoey, K.E. Parent Ratings of Atten-tion-Deficit/Hyperactivity Disorder Symptoms: Factor Structure and Normative Data. J. Psychopathol. Behav. Assess. 1998, 20, 83–102. [Google Scholar] [CrossRef]
- Al Jabery, M.A.; Arabiat, D.H. Psychometric Properties of the Arabic Translated Version of the RCMAS: Preliminary Indicators from a Jordanian Sample. J. Int. Couns. Educ. 2011, 3, 13–24. [Google Scholar]
- Reynolds, C.R.; Richmond, B.O. Revised Children’s Manifest Anxiety Scales-Second Edition (RCMAS-2): Manual; Western Psychological Services: Los Angeles, CA, USA, 2008. [Google Scholar]
- Al-Qerem, W.; Al-Maayah, B.; Ling, J. Developing and Validating the Arabic Version of the Diabetes Quality of Life Questionnaire. East. Mediterr. Health J. 2021, 27, 414–426. [Google Scholar] [CrossRef]
- The DCCT Research Group. Reliability and Validity of a Diabetes Quality-of-Life Measure for the Diabetes Control and Complications Trial (DCCT). Diabetes Care 1988, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Jena, B.; Yeravdekar, R. Emotional and Psychological Needs of People with Diabetes. Indian J. Endocrinol. Metab. 2018, 22, 696. [Google Scholar] [CrossRef] [PubMed]
- Snoek, R.; van Jaarsveld, R.H.; Nguyen, T.Q.; Peters, E.D.J.; Elferink, M.G.; Ernst, R.F.; Rookmaaker, M.B.; Lilien, M.R.; Spierings, E.; Goldschmeding, R.; et al. Genetics-First Approach Improves Diagnostics of ESKD Patients <50 Years Old. Nephrol. Dial. Transplant. 2022, 37, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Rechenberg, K.; Whittemore, R.; Grey, M. Anxiety in Youth with Type 1 Diabetes. J. Pediatr. Nurs. 2017, 32, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.; Mukerji, P.; Iyengar, S.; Drash, A. Psychiatric Disorder and Metabolic Control Among Youths With IDDM: A Longitudinal Study. Diabetes Care 1996, 19, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Northam, E.A.; Matthews, L.K.; Anderson, P.J.; Cameron, F.J.; Werther, G.A. Psychiatric Morbidity and Health Outcome in Type 1 Diabetes—Perspectives from a Prospective Longitudinal Study. Diabet. Med. 2005, 22, 152–157. [Google Scholar] [CrossRef]
- Jones, J.M.; Lawson, M.L.; Daneman, D.; Olmsted, M.P.; Rodin, G. Eating Disorders in Adolescent Females with and without Type 1 Diabetes: Cross Sectional Study. BMJ 2000, 320, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Hannonen, R.; Eklund, K.; Tolvanen, A.; Komulainen, J.; Riikonen, R.; Delamater, A.M.; Ahonen, T. Psychological Distress of Children with Early-onset Type 1 Diabetes and Their Mothers’ Well-being. Acta Paediatr. 2015, 104, 1144–1149. [Google Scholar] [CrossRef]
- Radobuljac, M.D. Adolescent Risk Behavior Is Less Frequent in Patients with Type 1 Diabetes. J. Diabetes Metab. 2013, 12, 2. [Google Scholar] [CrossRef]
- Sildorf, S.M.; Breinegaard, N.; Lindkvist, E.B.; Tolstrup, J.S.; Boisen, K.A.; Teilmann, G.K.; Skovgaard, A.M.; Svensson, J. Poor Metabolic Control in Children and Adolescents with Type 1 Diabetes and Psychiatric Comorbidity. Diabetes Care 2018, 41, 2289–2296. [Google Scholar] [CrossRef]
- Young, V.; Eiser, C.; Johnson, B.; Brierley, S.; Epton, T.; Elliott, J.; Heller, S. Eating Problems in Adolescents with Type 1 Diabetes: A Systematic Review with Meta-analysis. Diabet. Med. 2013, 30, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.M.; Stockwell, M.S.; Gallagher, M.P.; Rosenthal, S.L.; Soren, K. Mental Health Issues in Adolescents and Young Adults with Type 1 Diabetes. Clin. Pediatr. 2013, 52, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.W.; Mengel, M.; Mengel, L.; Larson, D.; Campbell, R.; Montague, R.B. Psychosocial and Psychopathologic Influences on Management and Control of Insulin-Dependent Diabetes. Int. J. Psychiatry Med. 1992, 22, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Colton, P.A.; Olmsted, M.P.; Daneman, D.; Rodin, G.M. Depression, Disturbed Eating Behavior, and Metabolic Control in Teenage Girls with Type 1 Diabetes. Pediatr. Diabetes 2013, 14, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Engström, I.; Kroon, M.; Arvidsson, C.-G.; Segnestam, K.; Snellman, K.; Åman, J. Eating Disorders in Adolescent Girls with Insulin-Dependent Diabetes Mellitus: A Population-Based Case-Control Study. Acta Paediatr. 1999, 88, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Laffel, L.M.; Domenger, C.; Danne, T.; Phillip, M.; Mazza, C.; Hanas, R.; Waldron, S.; Beck, R.W.; Calvi-Gries, F.; et al. Factors Associated with Diabetes-Specific Health-Related Quality of Life in Youth with Type 1 Diabetes: The Global TEENs Study. Diabetes Care 2017, 40, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Delcher, H.K.; Snitzer, J.; Bianchi, B.; Epstein, S. Personality Variables and Metabolic Control in Children with Diabetes. J. Genet. Psychol. 1985, 146, 19–26. [Google Scholar] [CrossRef]
- Young-Hyman, D.; de Groot, M.; Hill-Briggs, F.; Gonzalez, J.S.; Hood, K.; Peyrot, M. Psychosocial Care for People with Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2126–2140. [Google Scholar] [CrossRef]
- Smith, M.S.; Mauseth, R.; Palmer, J.P.; Pecoraro, R.; Wenet, G. Glycosylated Hemoglobin and Psychological Adjustment in Adolescents with Diabetes. Adolescence 1991, 26, 31–40. [Google Scholar]
- Lawler, M.K.; Volk, R.; Viviani, N.; Mengel, M.B. Individual and Family Factors Impacting Diabetic Control in the Adolescent: A Preliminary Study. Matern. Child. Nurs. J. 1990, 19, 331–345. [Google Scholar]
- Melin, E.O.; Hillman, M.; Thunander, M.; Landin-Olsson, M. Midnight Salivary Cortisol Secretion and the Use of Antidepressants Were Associated with Abdominal Obesity in Women with Type 1 Diabetes: A Cross Sectional Study. Diabetol. Metab. Syndr. 2019, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Brossaud, J.; Corcuff, J.-B.; Vautier, V.; Bergeron, A.; Valade, A.; Lienhardt, A.; Moisan, M.-P.; Barat, P. Altered Cortisol Metabolism Increases Nocturnal Cortisol Bioavailability in Prepubertal Children with Type 1 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 742669. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.H. Emerging Therapeutic Approaches for the Management of Diabetes Mellitus and Macrovascular Complications. Am. J. Cardiol. 2011, 108, 59B–67B. [Google Scholar] [CrossRef]
- Hinds, J.A.; Sanchez, E.R. The Role of the Hypothalamus–Pituitary–Adrenal (HPA) Axis in Test-Induced Anxiety: Assessments, Physiological Responses, and Molecular Details. Stresses 2022, 2, 146–155. [Google Scholar] [CrossRef]
- Maguire, A.M.; Cowell, C.T. Salivary Cortisol to Assess the Hypothalamic-Pituitary-Adrenal Axis in Healthy Children under 3 Years Old. J. Pediatr. 2007, 83, 502–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kristiansen, E.; Wanby, P.; Åkesson, K.; Blomstrand, P.; Brudin, L.; Thegerström, J. Assessing Heart Rate Variability in Type 1 Diabetes Mellitus—Psychosocial Stress a Possible Confounder. Ann. Noninvasive Electrocardiol. 2020, 25, e12760. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.W.; Ein, N.; Peck, K.; Huang, V.; Pruessner, J.C.; Vickers, K. Sex Differences in Salivary Cortisol Reactivity to the Trier Social Stress Test (TSST): A Meta-Analysis. Psychoneuroendocrinology 2017, 82, 26–37. [Google Scholar] [CrossRef]
- Stroud, L.R.; Papandonatos, G.D.; Williamson, D.E.; Dahl, R.E. Sex Differences in Cortisol Response to Corticotropin Releasing Hormone Challenge over Puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology 2011, 36, 1226–1238. [Google Scholar] [CrossRef]
- Zeitoun, M.H.; Abdel Reheem, A.A.; Kharboush, I.F.; Sheshtawy, H.; Assad, D.H.; El Feky, A.Y. Relationship between Depressive and Anxiety Symptoms and Fear of Hypoglycemia among Adolescents and Adults with Type 1 Diabetes Mellitus. Prim. Care Diabetes 2023, 17, 255–259. [Google Scholar] [CrossRef]
- Liakos, A.; Karagiannis, T.; Avgerinos, I.; Tsapas, A.; Bekiari, E. Burden and Coping Strategies of Hypoglycemia in People with Diabetes. Curr. Diabetes Rev. 2023, 20. [Google Scholar] [CrossRef]
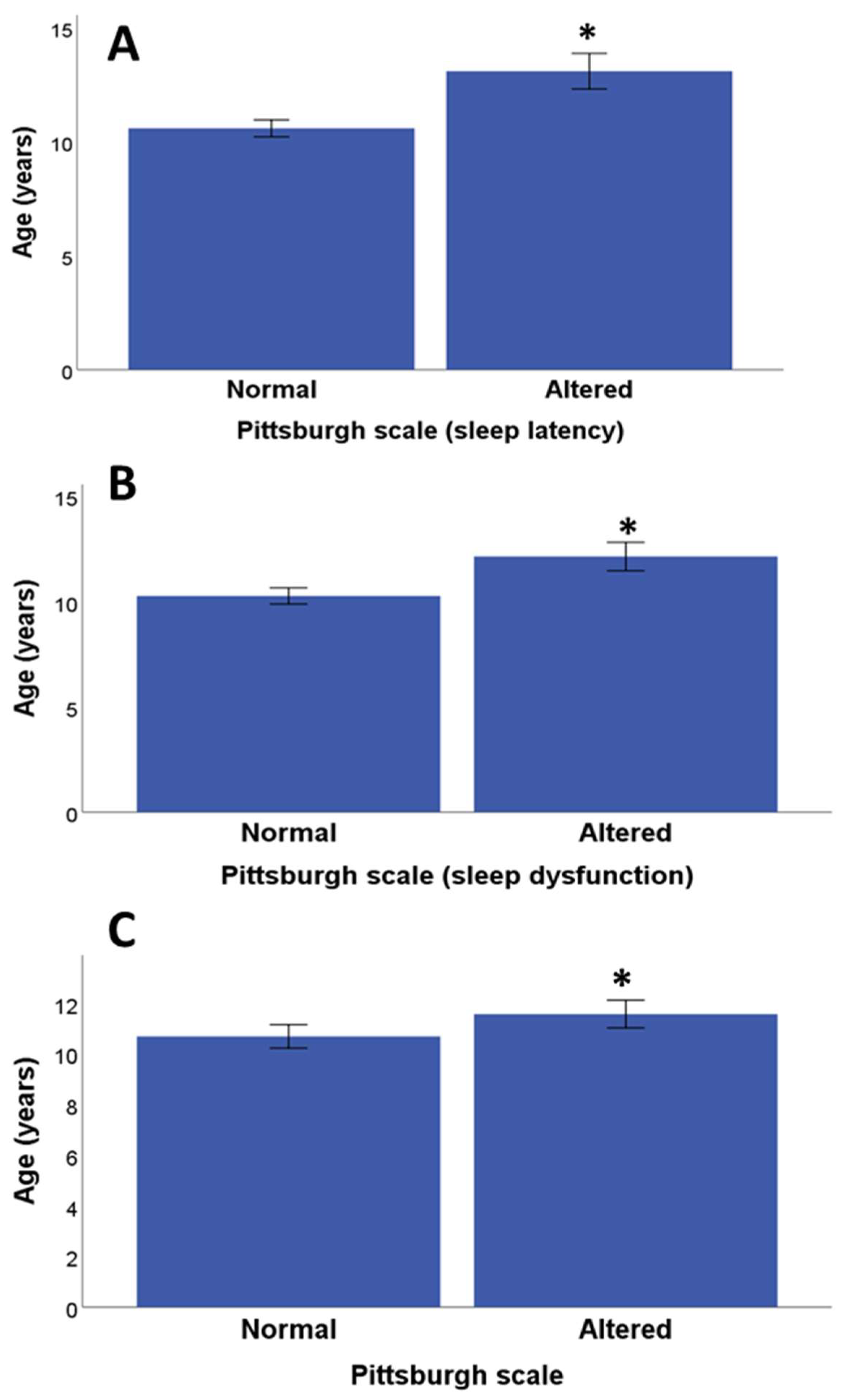
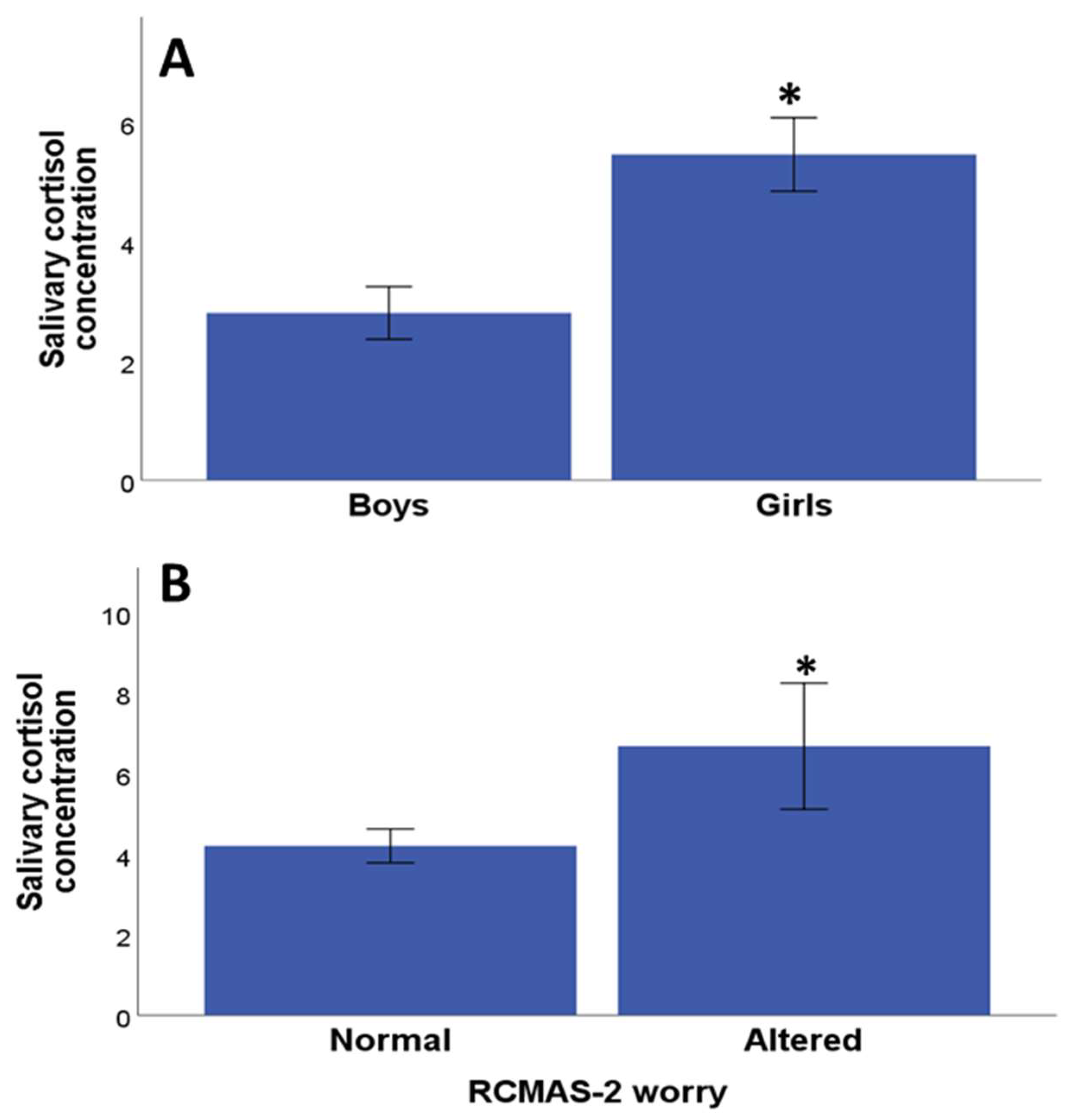
| Variables | Mean and Standard Error of the Mean (Min-Max Range) (Discrete Variables) or Frequencies (Categorical Variables) |
|---|---|
| Age | 11.05 ± 0.35 (6–17) |
| Puberty | Pre-pubertal:58.3% (n = 35) Post-pubertal: 41.7% (n = 25) |
| Number of years elapsed since diabetes diagnosis | 3.40 ± 0.26 (1–9) |
| BMI | 18.5 ± 0.31 (13.9–25.8 kg/m2) |
| BMI categories | Normal weight 71.6% (n = 43) Underweight 5.0% (n = 3) Overweight 23.4% (n = 14) |
| Mean concentration of cortisol in saliva ng/mL | 4.77 ± 0.49 (0.7–20.2) |
| Mean concentration of H1bAC | 9.0 ± 0.25 (5.5–15) |
| Glycemic control | Good (Hb1AC < 7.5%): 21.7% (n = 13) Poor (Hb1AC ≥ 7.5%): 78.3% (n = 47) |
| Family history of diabetes (type 1 and 2) (first-degree relatives) | Yes: 58.3% (n = 35) No:41.7% (n = 25) |
| Variable | Standard B Coefficient | t | p Value | 95% Confidence Interval Lower Limit Upper Limit | |
|---|---|---|---|---|---|
| ADHD score | −0.285 | −1.713 | 0.096 | −0.268 | 0.023 |
| Pittsburg index for “Sleep quality” | −0.043 | −0.274 | 0.786 | −1.265 | 0.965 |
| Pittsburg index for “Sleep latency” | −0.305 | −1.486 | 0.147 | −3.106 | 0.486 |
| Pittsburg index for “Sleep duration” | −0.017 | −0.112 | 0.912 | −8.434 | 7.556 |
| Pittsburg index for “Sleep efficiency” | 0.071 | 0.434 | 0.668 | −6.727 | 10.364 |
| Pittsburg index for “Sleep disturbances” | 0.053 | 0.321 | 0.750 | −1.845 | 2.535 |
| Pittsburg index for “Use of hypnotic medication” | −0.071 | −0.441 | 0.662 | −2.423 | 1.561 |
| Pittsburg index for “Daytime dysfunction” | 0.197 | 1.096 | 0.281 | −0.920 | 3.064 |
| RCMAS2 score for “Physiological anxiety” | 0.160 | 0.824 | 0.416 | −0.353 | 0.833 |
| RCMAS2 score for Worry | 0.537 | 2.117 | 0.042 * | 0.021 | 1.092 |
| RCMAS2 score for social anxiety | 0.009 | 0.046 | 0.964 | −0.440 | 0.460 |
| QoL subdomain “Impact” | −0.017 | −0.087 | 0.931 | −0.246 | 0.226 |
| QoL subdomain “Anxiety” | 0.392 | 1.851 | 0.043 * | 0.017 | 0.766 |
| QoL total score | 0.056 | 0.276 | 0.784 | −0.122 | 0.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mlili, N.; Ahabrach, H.; Bahri, H.; Kerkeb, A.; Mafla-España, M.A.; Cauli, O. Psychological Alterations in Youths with Type I Diabetes: Associations with Salivary Cortisol Concentration. Medicina 2024, 60, 19. https://doi.org/10.3390/medicina60010019
El Mlili N, Ahabrach H, Bahri H, Kerkeb A, Mafla-España MA, Cauli O. Psychological Alterations in Youths with Type I Diabetes: Associations with Salivary Cortisol Concentration. Medicina. 2024; 60(1):19. https://doi.org/10.3390/medicina60010019
Chicago/Turabian StyleEl Mlili, Nisrin, Hanan Ahabrach, Hind Bahri, Abdelilah Kerkeb, Mayra Alejandra Mafla-España, and Omar Cauli. 2024. "Psychological Alterations in Youths with Type I Diabetes: Associations with Salivary Cortisol Concentration" Medicina 60, no. 1: 19. https://doi.org/10.3390/medicina60010019
APA StyleEl Mlili, N., Ahabrach, H., Bahri, H., Kerkeb, A., Mafla-España, M. A., & Cauli, O. (2024). Psychological Alterations in Youths with Type I Diabetes: Associations with Salivary Cortisol Concentration. Medicina, 60(1), 19. https://doi.org/10.3390/medicina60010019







