IgA Nephropathy: Beyond the Half-Century
Abstract
:1. Introduction
2. Materials and Methods
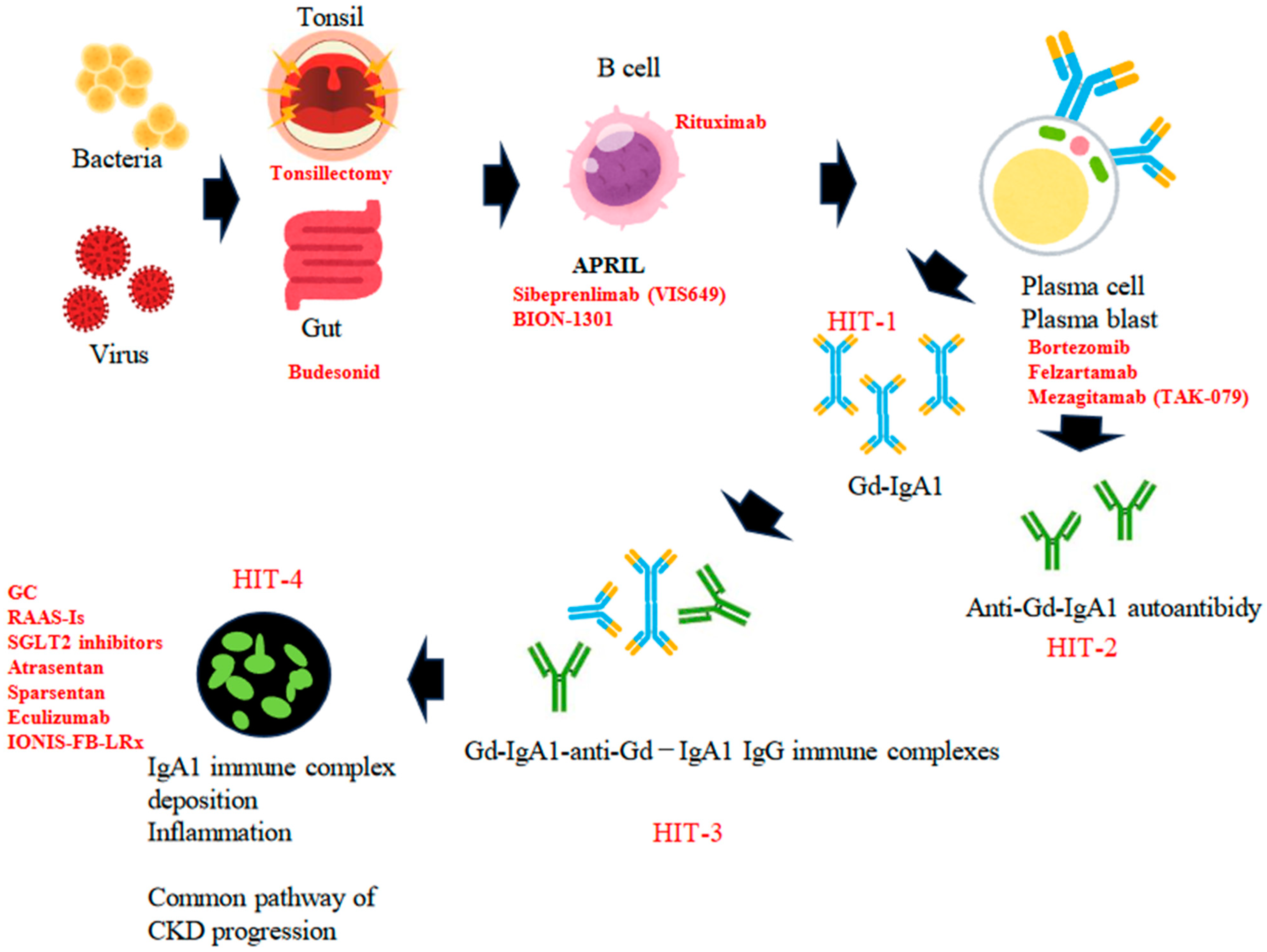
3. Gd-IgA1 and the Multi-Hit Hypothesis
4. Current Therapeutic Options for IgAN
4.1. RAAS-Is
4.2. Tonsillectomies
4.3. SGLT2 Inhibitors
4.4. Glucocorticoids
5. Upcoming Therapeutic Options for IgAN
5.1. Therapeutic Target: APRIL
5.2. Therapeutic Target: Plasma Cells
5.3. Therapeutic Target: Complement Systems
5.4. Therapeutic Target: Endothelin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manno, C.; Strippoli, G.F.; D’altri, C.; Torres, D.; Rossini, M.; Schena, F.P. A novel simpler histological classification for renal survival in IgA nephropathy: A retrospective study. Am. J. Kidney Dis. 2007, 49, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.C.; Mohey, H.; Afiani, A. Natural history of primary IgA nephropathy. Semin. Nephrol. 2008, 28, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Tanaka, K.; Iwasaki, C.; Oshima, Y.; Ochi, A.; Kataoka, H.; Itabashi, M.; Takei, T.; Uchida, K.; Nitta, K. Prognosis in IgA nephropathy: 30-year analysis of 1012 patients at a single center in Japan. PLoS ONE 2014, 9, e91756. [Google Scholar] [CrossRef] [PubMed]
- Rizk, D.V.; Saha, M.K.; Hall, S.; Novak, L.; Brown, R.; Huang, Z.-Q.; Fatima, H.; Julian, B.A.; Novak, J. Glomerular Immunodeposits of Patients with IgA Nephropathy Are Enriched for IgG Autoantibodies Specific for Galactose-Deficient IgA1. J. Am. Soc. Nephrol. 2019, 30, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- KnoRobert, T.; Berthelot, L.; Cambier, A.; Rondeau, E.; Monteiro, R.C. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trends Mol. Med. 2015, 21, 762–775. [Google Scholar]
- Placzek, W.J.; Yanagawa, H.; Makita, Y.; Renfrow, M.B.; Julian, B.A.; Rizk, D.V.; Suzuki, Y.; Novak, J.; Suzuki, H. Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS ONE. 2018, 13, e0190967. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.T.; Leung, C.B.; Chow, K.M.; Cheng, Y.L.; Fung, S.K.; Mak, S.K.; Tang, A.W.; Wong, T.Y.; Yung, C.Y.; Yung, J.C.; et al. HKVIN Study Group. Hong Kong study using valsartan in IgAN (HKVIN): A double-blind, randomized, placebo-controlled study. Am. J. Kidney Dis. 2006, 47, 751–760. [Google Scholar] [CrossRef]
- Praga, M.; Gutiérrez, E.; González, E.; Morales, E.; Hernández, E. Treatment of IgAN with ACE inhibitors: A randomized and controlled trial. J. Am. Soc. Nephrol. 2003, 14, 1578–1583. [Google Scholar] [CrossRef]
- Woo, K.T.; Lau, Y.K.; Zhao, Y.; E Liu, F.; Tan, H.B.; Tan, E.K.; Stephanie, F.C.; Chan, C.M.; Wong, K.S. Disease progression, response to ACEI/ATRA therapy and influence of ACE gene in IgA nephritis. Cell Mol. Immunol. 2007, 4, 227–232. [Google Scholar]
- Kanno, Y.; Okada, H.; Yamaji, Y.; Nakazato, Y.; Suzuki, H. Angiotensin-converting-enzyme inhibitors slow renal decline in IgAN, independent of tubulointerstitial fibrosis at presentation. QJM 2005, 98, 199–203. [Google Scholar] [CrossRef]
- Woo, K.T.; Lau, Y.K.; Wong, K.S.; Chiang, G.S. ACEI/ATRA therapy decreases proteinuria by improving glomerular permselectivity in IgA nephritis. Kidney Int. 2000, 58, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Horita, Y.; Tadokoro, M.; Taura, K.; Ashida, R.; Hiu, M.; Taguchi, T.; Furusu, A.; Kohno, S. Prednisolone co-administered with losartan confers renoprotection in patients with IgA nephropathy. Ren. Fail. 2007, 29, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushiyama, C.; Suzuki, S.; Hara, M.; Shimada, N.; Sekizuka, K.; Ebihara, I.; Koide, H. Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgAN. Am. J. Nephrol. 2000, 20, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Takei, T.; Uchida, K.; Tsuchiya, K.; Nitta, K. Low-dose losartan therapy reduces proteinuria in normotensive patients with immunoglobulin A nephropathy. Hypertens. Res. 2008, 31, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Yoshimura, M.; Miyazaki, Y.; Okamoto, H.; Kimura, K.; Hirano, K.; Matsushima, M.; Utsunomiya, Y.; Ogura, M.; Yokoo, T.; et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol. Dial. Transplant. 2014, 29, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Matsuzaki, K.; Yasuda, T.; Nishikawa, M.; Yasuda, Y.; Koike, K.; Maruyama, S.; Yokoo, T.; Matsuo, S.; Kawamura, T.; et al. Association between tonsillectomy and outcomes in patients with immunoglobulin A nephropathy. JAMA Netw. Open 2019, 2, e194772. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Vas, T.; Kövesdy, C.P.; Degrell, P.; Nagy, G.; Rékási, Z.; Wittmann, I.; Nagy, J. Effect of tonsillectomy and its timing on renal outcomes in Caucasian IgA nephropathy patients. Int. Urol. Nephrol. 2014, 46, 2175–2182. [Google Scholar] [CrossRef]
- Feehally, J.; Coppo, R.; Troyanov, S.; Bellur, S.S.; Cattran, D.; Cook, T.; Roberts, I.S.; Verhave, J.C.; Camilla, R.; Vergano, L.; et al. Tonsillectomy in a European Cohort of 1147 Patients with IgA Nephropathy. Nephron 2016, 132, 15–24. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Toto, R.D.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Pecoits-Filho, R.; Correa-Rotter, R.; et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021, 100, 215–224. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, H.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Lv, J.; Zhang, H.; Chen, Y.; Li, G.; Jiang, L.; Singh, A.K.; Wang, H. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgAN: A randomized controlled trial. Am. J. Kidney Dis. 2009, 53, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Torres, D.D.; Rossini, M.; Pesce, F.; Schena, F.P. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol. Dial. Transplant. 2009, 24, 3694–3701. [Google Scholar] [CrossRef] [PubMed]
- Tesar, V.; Troyanov, S.; Bellur, S.; Verhave, J.C.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Cattran, D.; Coppo, R.; VALIGA study of the ERA-EDTA Immunonephrology Working Group. Corticosteroids in IgA Nephropathy: A retrospective Analysis from the VALIGA Study. J. Am. Soc. Nephrol. 2015, 26, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T.; Eitner, F.; Fitzner, C.; Sommerer, C.; Zeier, M.; Otte, B.; Panzer, U.; Peters, H.; Benck, U.; Mertens, P.R.; et al. STOP-IgAN Investigators. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N. Engl. J. Med. 2015, 373, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, H.; Wong, M.G.; Jardine, M.J.; Hladunewich, M.; Jha, V.; Monaghan, H.; Zhao, M.; Barbour, S.; Reich, H.; et al. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients with IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2017, 318, 432–442. [Google Scholar] [CrossRef]
- Lv, J.; Wong, M.G.; Hladunewich, M.A.; Jha, V.; Hooi, L.S.; Monaghan, H.; Zhao, M.; Barbour, S.; Jardine, M.J.; Reich, H.N.; et al. Effect of Oral Methylprednisolone oEdsn Decline in Kidney Function or Kidney Failure in Patients with IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2022, 327, 1888–1898. [Google Scholar] [CrossRef]
- Fellström, B.C.; Barratt, J.; Cook, H.; Coppo, R.; Feehally, J.; de Fijter, J.W.; Floege, J.; Hetzel, G.; Jardine, A.G.; Locatelli, F.; et al. Targeted-release budesonide versus placebo in patients with IgAN (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 2017, 389, 2117–2127. [Google Scholar] [CrossRef]
- Barratt, J.; Lafayette, R.; Kristensen, J.; Stone, A.; Cattran, D.; Floege, J.; Tesar, V.; Trimarchi, H.; Zhang, H.; Eren, N.; et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023, 103, 391–402. [Google Scholar] [CrossRef]
- Mathur, M.; Barratt, J.; Suzuki, Y.; Engler, F.; Pasetti, M.F.; Yarbrough, J.; Sloan, S.; Oldach, D. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VIS649 (Sibeprenlimab), an APRIL-Neutralizing IgG2 Monoclonal Antibody, in Healthy Volunteers. Kidney Int. Rep. 2022, 7, 993–1003. [Google Scholar] [CrossRef]
- Barratt, J.; Hour, B.; Sibley, C.; Mittan, A.; Roy, S.; Stromatt, C.; Aaron Endsley, A.; Lo, J.; Glicklich, A. FC 040 Interim results of phase 1 and 3 trials to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and clinical activity of BION-1301 in patients with IgAN. Nephrol. Dial. Transplant. 2021, 36 (Suppl. S1), gfab117.004. [Google Scholar] [CrossRef]
- Hartono, C.; Chung, M.; Perlman, A.S.; Chevalier, J.M.; Serur, D.; Seshan, S.V.; Muthukumar, T. Bortezomib for Reduction of Proteinuria in IgA Nephropathy. Kidney Int. Rep. 2018, 3, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Maixnerova, D.; Tesar, V. Emerging role of monoclonal antibodies in the treatment of IgAN. Expert Opin. Biol. Ther. 2023, 23, 419–427. [Google Scholar] [CrossRef] [PubMed]
- A Study of Mezagitamab in Adults with Primary Immunoglobulin A Nephropathy Receiving Stable Background Therapy. Available online: https://www.clinicaltrials.gov/ClinicalTrials.gov(NCT05174221) (accessed on 5 December 2023).
- IONIS Pharma Press Releases: Ionis presents positive Phase 2 data in patients with IgAN at American Society of Nephrology’s Kidney Week 2022. Available online: https://ir.ionispharma.com/news-releases/news-release-details/ionis-presents-positive-phase-2-data-patients-IgAN (accessed on 5 December 2023).
- Heerspink, L.; Kohan, D.; Lafyette, R.; Levin, A.; Zhang, H.; Glicklich, A.; King, A.; Camargo, M.; Barratt, J. A phase 3, randomized, double-blind placebo-controlled study of atrasentan in patients with IgA nephropathy. KI Rep. 2021, 6, S164–S165. [Google Scholar]
- Heerspink, H.J.L.; Radhakrishnan, J.; Alpers, C.E.; Barratt, J.; Bieler, S.; Diva, U.; Inrig, J.; Komers, R.; Mercer, A.; Noronha, I.L.; et al. Sparsentan in patients with IgAN: A prespecified interim analysis from a randomized, double-blind, active-controlled clinical trial. Lancet 2023, 401, 1584–1594. [Google Scholar] [CrossRef]
- Knoppova, B.; Reily, C.; King, R.G.; Julian, B.A.; Novak, J.; Green, T.J. Pathogenesis of IgA Nephropathy: Current Understanding and Implications for Development of Disease-Specific Treatment. Clin. Med. 2021, 10, 4501. [Google Scholar] [CrossRef]
- Suzuki, H.; Novak, J. IgA glycosylation and immune complex formation in IgAN. Semin. Immunopathol. 2021, 43, 669–678. [Google Scholar] [CrossRef]
- Gesualdo, L.; Di Leo, V.; Coppo, R. The mucosal immune system and IgA nephropathy. Semin. Immunopathol. 2021, 43, 657–668. [Google Scholar] [CrossRef]
- Hall, J.E. The renin-angiotensin system: Renal actions and blood pressure regulation. Compr. Ther. 1991, 17, 8. [Google Scholar]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef]
- Fernández-Fernandez, B.; Sarafidis, P.; Soler, M.J.; Ortiz, A. EMPA-KIDNEY: Expanding the range of kidney protection by SGLT2 inhibitors. Clin. Kidney J. 2023, 16, 1187–1198. [Google Scholar] [CrossRef]
- Nuffield Department of Population Health Renal Studies Group. SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves First Drug to Decrease Urine Protein in IgA Nephropathy, A Rare Kidney Disease|FDA. Available online: https://www.fda.gov (accessed on 5 December 2023).
- Edsbäcker, S.; Andersson, T. Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn’s disease. Clin. Pharmacokinet. 2004, 43, 803–821. [Google Scholar] [PubMed]
- Rizk, D.V.; Maillard, N.; Julian, B.A.; Knoppova, B.; Green, T.J.; Novak, J.; Wyatt, R.J. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front. Immunol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, C.J.; Kuo, K.H.M. Eculizumab and Beyond: The Past, Present, and Future of Complement Therapeutics. Transfus. Med. Rev. 2019, 33, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Nakazawa, H.; Kurasawa, Y.; Sakai, H.; Nishina, S.; Senoo, N.; Senoo, Y.; Ishida, F. Severe Infection of Pseudomonas aeruginosa during Eculizumab Therapy for Paroxysmal Nocturnal Hemoglobinuria. Int. Med. 2018, 57, 127–130. [Google Scholar] [CrossRef]
- Hawkins, K.L.; Hoffman, M.; Okuyama, S.; Rowan, S.E. A Case of Fulminant Meningococcemia: It Is All in the Complement. Case Rep. Infect. Dis. 2017, 2017, 6093695. [Google Scholar] [CrossRef]
- Khedraki, R.; Noor, Z.; Rick, J. The Most Expensive Drug in the World: To Continue or Discontinue, That Is the Question. Fed. Pract. 2016, 33, 22–28. [Google Scholar]
- Martínez-Díaz, I.; Martos, N.; Llorens-Cebrià, C.; Álvarez, F.J.; Bedard, P.W.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Endothelin Receptor Antagonists in Kidney Disease. Int. J. Mol. Sci. 2023, 24, 3427. [Google Scholar] [CrossRef]
- Available online: https://www.chinooktx.com/file.cfm/52/docs/asn-2022-atrasentan-affinity-igan-update-th-po497.pdf (accessed on 5 December 2023).
| Author (Year), Location | Design | Population (Sample Size, Age, Health) | Methods | Health Outcomes | Main Findings |
|---|---|---|---|---|---|
| Li et al. (2006), Hong Kong [7] | Multicenter RCT | n = 109, age > 18 years Group 1: UP > 1 g/day and S-Cr < 2.8 mg/dL. Group 2: S-Cr 1.4–2.8 mg/dL | Treatment group (n = 54): valsartan 80–160 mg/day. Control group (n = 55): placebo for 104 weeks. | GFR and UP | Treatment group: decreased 33% of UP. Mean decrease rate of GFR was less than that of control group. Control group: no significant change in UP |
| Praga et al. (2003), Spain [8] | Single-center RCT | n = 44. Age: treatment group, 27.8 ± 12 years; control group, 29.9 ± 12.3 years. UP < or = 0.5 g/day and S-Cr < 1.5 mg/dL. | Treatment group (n = 23): dose arrangement of for BP under 140/90 mmHg with enalapril. Control group (n = 21): dose arrangement for BP under 140/90 mmHg with other than ACE-I or ARB. Mean follow-up period: 78 ± 28 months. | Primary endpoint: 50% increase in basal s-Cr. Secondary endpoint: s-Cr > 1.5 mg/dL and increase in UP. | Treatment group: decreased 33% of UP. Mean decrease rate of GFR was less than that of control group. Control group: No significant change in UP. In total, 13% of Pt in enalapril group reached primary endpoint and 52% in control group. Proteinuria significantly decreased in enalapril group. |
| Woo et al. (2007), Singapore [9] | Single-center RCT | n = 75. Age: 62 ± 5 years. UP > 1 g/day and/or S-Cr > 1.6 mg/dL. | Treatment group (n = 37): enalapril 5–10 mg/day or losartan 50–100 mg/day. Control group: no treatment. Follow-up: 5 years. | UP, S-Cr, progression to ESKD | The study suggests that the common mechanism of therapy is likely through lower levels of ACE, glomerular pressure, and proteinuria, resulting in reduced renal damage and slowed progression to ESKD. |
| Kanno et al. (2005), Japan [10] | Prospective 3-year follow-up study | n = 49. Age: ACE-I group, 35 ± 2 years; CCB group, 35 ± 1 years. | ACE-I group (n = 26): dose arrangement BP < 130/85 mmHg with trandolapril or temocapril. CCB group (n = 23): dose arrangement with BP < 130/85 mmHg with amlodipine 2.5–5 mg/day | CCr | In the ACEI group, the rate of decline in CCr was lower. |
| Woo et al. (2000), Singapore [11] | Case control trial | n = 41. Age: treatment group, 39 ± 10 years; control group, 37 ± 6 years. UP > 1 g/day and/or S-Cr > 1.4 mg/dL. | Treatment group (n = 21): dose arrangement BP < 150/90 mmHg with enalapril 5–10 mg/day or losartan 50–100 mg/day. Control group (n = 20): dose arrangement BP < 150/90 mmHg with other than ACE-I, ARB or CCB. | UP, Selectivity Index (SI), Ccr | Among the 21 patients in the treatment group, 10 responded to ACEI/ATRA therapy with a decrease in proteinuria by 30% (responders). Among the responders, SI improved. Eight out of twenty-one patients in the treatment group who had documented renal impairment had improved renal function compared with two in the control group. |
| Horita et al. (2007), Japan [12] | Single-center RCT | n = 38. Age: 33 ± 11 years. BP < 140/90 mmHg. UP, 1.0–1.6 g/day; and CCr > 50 mL/min/1.73 m2. | Treatment group (n = 20): prednisolone 30 mg/day (2 mo), 20 mg/day (2 mo), 15 mg/day (6 mo), 10 mg/day (12 mo) and 5 mg/day (1 mo) and losartan 50 mg. Control group (n = 18): prednisolone as treatment group. Follow-up period: 2 years. | UP, Ccr | Both groups showed a significant decrease in proteinuria compared to baseline. However, the combination of PSL plus LST was more effective than PSL alone. The group treated with PSL plus LST maintained a similar creatinine clearance level, while the group treated with PSL alone showed a decrease. |
| Nakamura et al. (2000), Japan [13] | Non-blinded controlled trial Trial | n = 32. Age: mean 32.6 years (18–54), normotensive (BP < 140/90 mmHg), UP < 3 g/day and Ccr > 80 mL/min. | Verapamil group (n = 8): 120 mg/day, trandolapril group (n = 8): 2 mg/day, candesartan group (n = 8): 8 mg/day. Control group (n = 8): placebo. Follow-up period: 3 mo. | UP, urinary podocytes | The antiproteinuric response in the trandolapril group was similar to that in the candesartan cilexetil group, and both were greater than that of verapamil. The reduction in the number of urinary podocytes from baseline was significantly greater in patients treated with trandolapril or candesartan cilexetil than in patients treated with verapamil. |
| Shimizu et al. (2008), Japan [14] | Single-center RCT | n = 36. Normotensive (BP < 140/90 mmHg), mild proteinuria. | Treatment group: losartan (n = 18) 12.5 mg/day Control group: anti-platelet agent | UP, S-Cr, urinary NAG | Low-dose losartan significantly reduced proteinuria from 0.8 +/− 0.5 g/d at baseline to 0.4 +/− 0.4 g/d at 12 months (p = 0.006). Proteinuria was significantly lower at 12 months in the losartan group than in the control group (p = 0.04). Urinary N-acetyl-beta-D-glucosaminidase (NAG) levels at 12 months were significantly lower in the losartan group than in the control group (p = 0.009). |
| Author (Year), Location | Design | Population (Sample Size, Age, Health) | Methods | Health Outcomes | Main Findings |
|---|---|---|---|---|---|
| Kawamura et al. (2014), Japan [15] | Multicenter RCT | n = 72. Age: 10–69 years. UP: 1.0–3.5 g/day and S-Cr < 1.5 mg/dL. | Group A (n = 33): tonsillectomy and steroid pulse therapy. Group B (n = 39): tonsillectomy. Observation period: 12 mo. | UP, hematuria, clinical remission | UP was significantly reduced in the patients with tonsillectomy and steroid pulse therapy. No significant difference in the attenuation of hematuria and the rate of clinical remission. AE: n = 54 (total), death (n = 6) in Group B due to malignancy (n = 2), COLD (n = 1), aortic dissection (n = 1) |
| Hirano et al. (2016), Japan [16] | Observational study | n = 1065. Age (median): 35 (25–52) years. Propensity score-matched analysis. | Propensity-matched patiemts. Tonsillectomy group (n = 153). Control group (n = 153): RAAS-Is and/or steroids. Observation period: 3.6 years. | 1.5-fold increase in serum creatinine level from baseline or dialysis initiation. | In a matching analysis, tonsillectomy was associated with primary outcome reduction (hazard ratio, 0.34; 95% CI, 0.13–0.77; p = 0.009). |
| Kovács et al. (2014), Hungary [17] | Retrospective cohort study | n = 264. Age: group without tonsillectomy, 38 ± 113.9 years; group with tonsillectomy, 30.2 ± 10.1 years. CKD stage G1-G3. | Group without tonsillectomy (n = 166): RAAS-Is and/or statin and/or steroid. Mean observation period: 133 ± 102 mo. Group with tonsillectomy (n = 98): tonsillectomy, RAAS-Is, statin, steroid. Mean observation period: 171 ± 114 mo. | Renal survival time | The mean renal survival time was significantly longer in patients who underwent tonsillectomy. Tonsillectomy, baseline eGFR, and 24-h proteinuria were identified as independent risk factors for renal end points. |
| Feehally et al. (2016), Europe [18] | Retrospective cohort study (sub-analysis of VALIGA study) | n = 1147. Age: tonsillectomy group, 31.1 ± 17.9 years; control group (without tonsillectomy), 36.0 ± 16.5 years. Propensity-matched analysis. | Tonsillectomy group (n = 41) and control group (n = 41): RAAS-Is and/or steroids. Observation period > 4.7 years. | Impact of tonsillectomy on the progression to ESKD and/or a 50% loss of estimated glomerular filtration rate (eGFR). | No significant difference in outcomes was seen between the two groups. This was also the case when pairing patients who underwent tonsillectomy after the diagnosis of IgAN with those who did not have the procedure. |
| Author (Year), Location | Design | Population (Sample Size, Age, Health) | Methods | Health Outcomes | Main Findings |
|---|---|---|---|---|---|
| Wheeler et al. (2021), worldwide [19] | Multicenter RCT (sub-analysis of DAPA-CKD trial) | n = 270. Age: 51.2 years (mean). UACR, 900 mg/gCr; and mean eGFR, 43.8 mL/min/1.73 m2 | Dapagliflozin group (n = 137): dapagliflozin 10 mg/day Placebo group (n = 133) Follow-up period: 2.4 years (median) | Primary outcome: sustained decline in eGFR of 50% or more, end-stage kidney disease, or death from a kidney disease-related or cardiovascular cause. UCR, decline rate of eGFR, | The primary outcome occurred in 4% of participants on dapagliflozin and 15% on placebo. Dapagliflozin also reduced the UACR by 26% relative to placebo. The mean rates of eGFR decline with dapagliflozin and placebo were −3.5 and −4.7 mL/min/1.73 m2/year, respectively. AE leading to discontinuation of study drug: dapagliflozin group, 6/137; placebo group, 7/133. Severe AEs, including death: dapagliflozin group, 22(16.1%); placebo group, 34(25.6%). |
| Nuffield et al. (2022), worldwide [20] | Multicenter RCT (EMPA-KIDNEY trial) | n = 6609, CKD patients. Age (mean): 63.9 years (empagliflozin group) and 63.8 (placebo group), eGFR 25 ≤ 45 mL/min/1.73 m2, UACR > 200 mg/gCr 853 (26%) and 816 (25%) patients with glomerular disease in empagliflozin group and placebo group, respectively. | Empagliflozin group (n = 1097): empagliflozin 10 mg/day. Placebo group (n = 1095). Follow-up period: 2.0 years (median). | Primary outcome: end-stage kidney disease, a sustained eGFR < 10 mL/minute/1.73 m2, a sustained decline in eGFR of ≥40%, or a renal death) or death from cardiovascular causes. | The 13.1% of patients in the empagliflozin group experienced progression of kidney disease or death from cardiovascular causes, compared to 16.9% in the placebo group. This result was consistent among patients with or without diabetes and across different levels of kidney function. AE: Ketoacidosis: 6/1 (0.09/0.02 patient-years, lower-limb amputation: 28/19 (0.43/0.29 patient-years) in empagliflozin/placebo group, respectively. |
| Author (Year), Location | Design | Population (Sample Size, Age, Health) | Methods | Health Outcomes | Main Findings |
|---|---|---|---|---|---|
| Lv et al. (2009), China [21] | Single-center RCT | n = 63, age > 18–65 years. Group 1: UP 1–5 g/day and eGFR > 30 mL/min/1.73 m2 | Combination group (n = 33): cilazapril 2.5→5 mg/day and 6-to-8-month course of prednisone was started with oral prednisone, 0.8-to-1.0 mg/kg/d, for 8 weeks; the dose was tapered by 5 to 10 mg every 2 weeks. Cilazapril group (n = 30): 2.5→5 mg/day for 6–8 mo. Follow-up period: 48 mo. | Primary endpoint: defined as a 50% increase in baseline serum creatinine level UP. | The 7 patients in the cilazapril group (24.1%) reached the primary end point compared with 1 patient (3%) in the combination group. UP was significantly decreased in patients in the combination group compared with the cilazapril group (time-average proteinuria, 1.04 ± 0.54 versus 1.57 ± 0.86 g/d of protein; p = 0.01). Severe AEs, including hyperkalemia, were not observed. |
| Manno et al. (2009), Italy [22] | Single-center RCT | n = 97. Age: ramipril-alone group, 34.9 ± 11.2 years; prednisone plus-ramipril group: 31.8 ± 11.3 years. Histological grade: moderate. UP > or = 1.0 g/day, and eGFR > or = 5 mL/min/1.73 m2 | Ramipril-alone group (n = 23): dose arrangement for BP under 120/80 mmHg with ramipril (starting with 2.5 mg/day). Control group (n = 21): dose arrangement for BP under 120/80 mmHg with ramipril (starting at 2.5 mg/day). 6-month course of prednisone began with oral prednisone 1.0 mg/kg/day for 2 months and then the dose was tapered by 0.2 mg/kg/day every mo. The maximal prednisone dose was fixed at 75 mg/day. Follow-up period: 96 mo. | Primary endpoint: Combination of doubling of baseline serum creatinine or ESKD, defined as a need for dialysis or renal transplantation. Secondary endpoint: Decline by means of eGFR slope over time and UP. | The13/49 (26.5%) patients in the ramipril alone group reached the primary outcome compared with 2/48 (4.2%) in the prednisone plus-ramipril group. The mean rate of eGFR decline was higher in the ramipril alone group than in the combination therapy group (−6.17 ± 13.3 vs. −0.56 ± 7.62 mL/min/1.73 m2). Prednisone plus-ramipril treatment reduced 24-h proteinuria more than ramipril alone during the first 2 years. Severe AEs were not observed in both groups. |
| Tesar et al. (2015), Europe [23] | Retrospective cohort study (sub-analysis of VALIGA study, propensity matched) | n = 1147. Age: 36 ± 16 years (mean). UP, 1.3 g/day (0.6–2.6). eGFR,, 73 ± 30 mL/min/1.73 m2. Caucasian: 97%. MEST score, mesangial hypercellularity (M1) was present in 28%, endocapillary hypercellularity (E1) was present in 11%, segmental glomerulosclerosis (S1) was present in 70%, and 21% of the patients had >25% tubular atrophy and interstitial fibrosis (T1–2). | Supportive care group (n = 80): corticosteroid and RAAS-Is. Corticosteroid plus supportive-care group: (n = 82) RAAS-Is. Observation period: 4.7 years. | 50% decrease in eGFR or ESKD | Corticosteroid reduced proteinuria and the rate of renal function decline and increased renal survival. These benefits extended to those with an eGFR </= 50, and the benefits increased proportionally with the level of proteinuria. Corticosteroid reduced the risk of progression regardless of initial eGFR and in direct proportion to the extent of proteinuria in this cohort. |
| Rauen et al. (2015), Europe [24] | Multicenter RCT (STOP-IgAN study) | n = 162, Age: 43.7 ± 12.8 years (mean), UP > 0.75 g/day | Supportive care group (n = 26): dose arrangement BP < 125/75 mmHg with RAAS-Is. Immunosuppression group (n = 23): Dose arrangement with BP < 125/75/85 mmHg with RAAS-Is and glucocorticoid monotherapy for 6 mo (methylprednisolone, administered intravenously at a dose of 1 g per day for 3 days at the start of months 1, 3, and 5; and oral prednisolone at a dose of 0.5 mg per kilogram per 48 h on the other days). Follow-up period: 3 years. | Primary endpoints: Full clinical remission at the end of the trial (protein-to-creatinine ratio < 0.2 [with both protein and creatinine measured in grams] and a decrease in the estimated glomerular filtration rate (eGFR) of <5 mL per minute per 1.73 m2 | The 4 patients (5%) in the supportive-care group, as compared with 14 (17%) in the immunosuppression group, had a full clinical remission (p = 0.01). A total of 22 patients (28%) in the supportive-care group and 21 (26%) in the immunosuppression group had a decrease in the eGFR of at least 15 mL per minute per 1.73 m2 (p = 0.75). More patients in the immunosuppression group than in the supportive-care group had severe infections, impaired glucose tolerance, and weight gain of more than 5 kg in the first year of treatment. One patient in the immunosuppression group died of sepsis. |
| Lv et al. (2017), worldwide [25] | Multicenter RCT (Testing trial) | n = 272. Age: 38.6 ± 11.1 years. (mean). UP > 1 g/day. eGFR: 20-to-120 mL/min/1.73 m2 after at least 3 mo of blood pressure control with RAAS-Is. | Treatment group (n = 136): oral methylprednisolone (0.6–0.8 mg/kg/d; maximum, 48 mg/day for 4–6 mo. Control group (n = 126): placebo for 4–6 mo. The mean required follow-up period: 5 years. | Primary renal outcome: ESKD, death due to kidney failure, or a 40% decrease in eGFR. | The study stopped early (after 28 of the 335 planned events) due to a significantly increased risk of serious adverse events with oral methylprednisolone vs. placebo (14.7% vs. 3.2% primarily excess infections). The primary renal outcome occurred in 8 participants (5.9%) in the methylprednisolone group vs. 20 (15.9%) in the placebo group (hazard ratio, 0.37 (95% CI, 0.17–0.85); risk difference, 10.0% (95% CI, 2.5–17.9%); p = 0.02). |
| Lv et al. (2022), worldwide [26] | Multicenter RCT (Testing 2.0 trial) | n = 503. Age: 38 years (mean), UP > 1 g/day. eGFR: 20-to-120 mL/min/1.73 m2 after at least 3 mo of blood pressure control with RAAS-Is | Participants were randomized in a 1:1 ratio to receive oral methylprednisolone (initially 0.6–0.8 mg/kg/d, maximum 48 mg/d, weaning by 8 mg/d/mo; n = 136) or placebo (n = 126). After 262 participants were randomized, an excess of serious infections was identified, leading to dose reduction (0.4 mg/kg/d, maximum 32 mg/d, weaning by 4 mg/d/mo) and addition of antibiotic prophylaxis for pneumocystis pneumonia for subsequent participants (121 in the oral methylprednisolone group and 120 in the placebo group). The mean required follow-up: 5 years. | The primary composite outcome: ESKD, death due to kidney failure, or a 40% decrease in eGFR. Predefined safety outcomes were serious infection, new diabetes, gastrointestinal hemorrhage, fracture/osteonecrosis, and cardiovascular events. | In this randomized clinical trial that included 503 participants, a 6-to-9-month course of oral methylprednisolone, compared with the placebo, significantly reduced the risk of the composite outcome of kidney function decline, kidney failure, or death due to kidney disease (hazard ratio, 0.53); however, the risk of serious adverse events was increased. Severe AEs were observed in 28 (10.9%) patients in methylprednisolone group and 7 (2.8%) in placebo group. |
| Fellström et al. (2017), Europe [27] | Multicenter RCT Phase 2b (NEFIGAN trial) | n = 149. Age: mean 39 ± 12.3. years. Persistent proteinuria despite optimized renin–angiotensin system (RAS) blockade. | TRF-budesonide 8 mg/day (n = 51), TRF-budesonide 16 mg/day (n = 48), Placebo (n = 50). Follow-up period: 9 mo. | Primary outcome: mean change from baseline in UPCR. Safety was assessed in all patients who received the intervention. | Mean UPCR decreased by 27.3% in 48 patients who received 16 mg/day (0.71; 0.53–0.94; p = 0.0092) and 21.5% in the 51 patients who received 8 mg/day (0.76; 0.58–1.01; p = 0.0290); 50 patients who received placebo had an increase in mean UPCR of 2.7%. The incidence of adverse events was similar in all groups (43 [88%] of 49 in the TRF-budesonide 16 mg/day group, 48 [94%] of 51 in the TRF-budesonide 8 mg/day, and 42 [84%] of 50 controls). AEs: 13 cases of AEs were recorded. Deep vein thrombosis (16 mg/day) and unexplained deterioration in renal function in follow-up were considered to be TRF-budesonide related. |
| Barratt et al. (2023), worldwide [28] | Multicenter RCT Phase 3 (NefIgArd trial) | n = 199. Age 44 years (Nefecon group), 43 (placebo group) (mean), UPCR >/= 0.8 g/gCr, or UP > 1 g/day, eGFR >/= 35, </= 90 mL/min/1.73 m2 | Treatment group: (n = 97) Nefecon 16 mg/day. Control group: (n = 100) placebo. | Primary outcome: UPCR after 9 mo. Secondary outcome: eGFR after 9 and 12 mo. | At nine months, UPCR was 27% lower in the Nefecon group compared with placebo, along with a benefit in eGFR preservation corresponding to a 3.87 mL/min/1.73 m2 difference versus placebo (both significant). Nefecon was well tolerated, and treatment-emergent adverse events were mostly mild-to-moderate in severity and reversible. |
| Author (Year), Location | Drug (Target) | Design | Population (Sample Size, Age, Health) | Methods | Health Outcomes | Main Findings |
|---|---|---|---|---|---|---|
| Mathur et al. (2022), USA [29] | VIS649 (APRIL) | Phase 1 randomized, placebo-controlled, single ascending-dose, first-in-human study | n = 45, age: 18–55 years, BMI: 18–32, IgG > 750 mg/dL, IgA > 80 mg/dL, IgM > 55 mg/dL, healthy volunteer. | Placebo (n = 8): Single injection VIS649 0.5 mg/Kg (n = 7), 2 mg/Kg (n = 7), 6 mg/Lg (n = 7), 12 mg/Kg (n = 7): single injection | Standard safety assessments, including AEs, clinical laboratory tests, vital signs, electrocardiograms, and physical examinations, were performed at regular intervals. The PK profile of VIS649, the effect of VIS649 on various PD parameters. | There were no serious adverse events (AEs). Half-life increased with dose, and drug exposure increased in a greater than dose-proportional manner. Serum APRIL, IgA, galactose-deficient (Gd) IgA1, IgG, and IgM were reversibly suppressed in a dose-dependent manner, with a dose–response in time to recovery. Treatment-emergent AEs (TEAEs) were experienced by 4 of 8 (50.0%) participants who received placebo and 11 of 28 (39.3%) who received VIS649 (all doses). |
| Barratt et al. (2021), UK [30] | BION-1301 (APRIL) | Phase1/2, Part 1: double-blind, randomized, placebo-controlled, single ascending dose (SAD) in healthy volunteers (HVs). Part 2: double-blind, randomized, placebo-controlled multiple ascending dose (MAD) in HVs. | n = ?, Age > 18 (18–55) years. eGFR >/= 30 mL/min/1.73 m2, UP > 0.5/day or UPCR >/= 0.5 g/gCr, On stable and optimized RAAS-Is treatment | Cohrt 1: BION-1301: 400 mg, iv/every 2 weeks→600 mg, sc/every 2 weeks Cohort2: BION-1301: 600 mg, sc/every 2 weeks | Incidence of Treatment Emergent Adverse Events (TEAEs) and Severity of TEAEs up to 76 weeks. APRIL, UP, UPCR, Immunoglobulin, Gd-IgA1. | No SAEs or terminations due to AEs. Durable reductions in serum levels of free APRIL and immunoglobulins were observed. In Cohort 1, clinically meaningful reductions in proteinuria were seen as early as 12 weeks (30.4% geometric mean UPCR reduction, n = 7) and were sustained through 24 weeks (48.8% geometric mean UPCR reduction, n = 8) and 52 weeks (66.9% geometric mean UPCR reduction, n = 8). Reductions in proteinuria were consistent in Cohort 2 (53.8% geometric mean UPCR reduction, n = 9) at 24 weeks. Significant and durable reductions in serum Gd-IgA1 concentrations were observed. |
| Hartono et al. (2018), USA [31] | Bortezomib (Plasma cell, CD38) | Pilot trial | n = 8, Age: 35 ± 12 (22–53) years, UP > 1 g/day and/or CCr > 30 mL/min, stable-dose RAAS-Is. | Bortezomib 1.3 mg/m2 (BSA), 4 doses/every 2 weeks Follow-up period: 1 yr | Primary endpoint: clinical remission, UP, S-Cr, UPCR | At 1-year follow-up, 3 subjects (38%) had achieved the primary endpoint. Four patients (50%) did not have any response or had progression of disease. All of the participants tolerated 4 doses of bortezomib without any serious AEs. |
| Maixnerova et al. (2023), worldwide [32] | Felzartamab (plasma cell, CD38) | Phase 2a, Multicenter RCT | n = 48, Age: 18–80 years, UP > 1 g/day, Treatment with RAAS-Is | #1 Placebo, #2 Experimental arm 1: felzartamab, arm 2: felzartamab, arm 3: felzartamab (detail not shown). Follow-up period: 9 mo. | Safety: determined by the frequency, incidence and severity of TEAEs, UP, Complete response, Pharmacokinetic: serum concentrations of Felzartamab over time | Not shown. |
| Takeda Pharmaceuticals (2023), Japan [33] | Mezagitamab (plasma cell, CD38) | Phase 1b, multicenter, open-label study | n = 41, Age: UP > 1 g/day or UPCR > 1 g/gCr, eGFR >/= 45 mL/min/1.73 m2 | Mezagitamab, subcutaneous injection, once weekly for 8 weeks then once every 2 weeks for 16 weeks in the main study. Follow-up period: 48 weeks | Main Study: Percentage of Participants With one or More Treatment-emergent Adverse Events (TEAEs), Grade 3 or Higher TEAEs, Serious Adverse Events (SAEs), and Adverse Events (AEs) Leading to Mezagitamab Discontinuation. | Not shown. |
| IONIS Pharma (2022), USA, Asia, Oceania [34] | IONIS-FB-LRx (Complement system, Factor B) | Phase 2, single-arm, open-label clinical study | n = 25, Age: 25–62 years. Proteinuria > 1.5 g/day hematuria despite maximum tolerated RAAS blockade. eGFR > 40 mL/min/1.73 m2 | Participants will receive IONIS-FB-LRx, by subcutaneous injection (SC) at Week 1 and every 4 weeks through Week 25. Optional 48-week Extension, with drug dosing continuing every 4 weeks. | Percent reduction in 24-h urine protein excretion (time frame: baseline to week 29, UP, UACR, Factor B, AH50) | IONIS-FB-LRx met its primary endpoint of change in 24-h urinary protein, demonstrating a 44% mean reduction in proteinuria from baseline to week 29. Kidney function, as measured by estimated glomerular filtration rate (eGFR), was maintained in all patients in the study. IONIS-FB-LRx achieved robust and sustained reductions in plasma complement Factor B (CFB), alternative pathway activity (AH50), and urinary complement fragment Ba (Factor Ba). AE: a reversible elevation of ALT (n = 1). All patients completed the study. |
| Heerspink et al. (2021), worldwide [35] | Atrasentan (endothelin, ETA receptor) | Phase 3 multicenter RCT | n = 340. Age: UP > 1 g/day despite taking RAAS-Is, eGFR >/= 30 mL/min/1.73 m2, SGLT2 inhibitors: available. | Atrasentan group (n = ?): 0.75 mg/day for 132 weeks. Placebo group (n = ?): placebo for 132-weeks follow-up period: 3 mo. | UP, UPCR (at week 24), eGFR (at 2.6 years), second composite endpoint: 40% reduction in eGFR, dialysis, transplantation, and all-cause mortality. | Atrasentan demonstrated mean proteinuria reductions of 38.1% proteinuria at six weeks of treatment, 48.3% at 12 weeks of treatment, and 54.7% at 24 weeks of treatment. Atrasentan was generally well tolerated. Treatment-emergent AEs observed in 16 patients were mild or moderate in severity. |
| Heespink HJL et al. (2023), worldwide [36] | Sparsentan (endothelin, angiotensin receptor) | Multicenter RCT | n = 404. Age: sparsentan group, 46.6; irbesartan group, 45.4 years (mean). UP > 1 g/day despite maximum RAAS blockade. | Sparsentan group: (n = 202) 400 mg/day for 36 weeks irbesartan group: (n = 202) irbesartan 300 mg/day for 36 weeks. | UP, UPCR (at week 36), TEAEs. | Mean percent change from baseline in UPCR was statistically significantly greater in the sparsentan group (–49.8%) than the irbesartan group (–15.1%), resulting in a between-group relative reduction of 41% (least squares mean ratio = 0.59; 95% CI, 0.51–0.69; p < 0.0001). TEAEs with sparsentan were similar to irbesartan. There were no cases of severe oedema, heart failure, hepatotoxicity, or oedema-related discontinuations. |
| Treatment Option | Therapeutic Target | RCT | UP Reduction | Reno-Protection | AEs | Remarks |
|---|---|---|---|---|---|---|
| RAAS-Is | Intraglomerular pressure | Performed | Shown | Shown | Mild | |
| Common pathway of CKD | ||||||
| Progression | ||||||
| Tonsillectomy | Tonsillar lymphoid tissues | Performed | Shown (Japan); | Shown (Japan) | Mild | Available in Japan |
| not shown (Europe) | Not shown (Europe) | |||||
| Dapagliflozin | Tubular SGLT2 | Performed | shown | Shown | Mild | |
| Common pathway of CKD | ||||||
| Progression | ||||||
| Empagliflozin | Tubular SGLT2 | Performed | Not performed | Shown | Mild | |
| Common pathway of CKD | Data from CKD patients | |||||
| Progression | IgAN (25%) | |||||
| Cellular immunity | Performed | Shown | Shown | * Infection, diabetes, death | ||
| Glucocorticoid | Humoral immunity | Moderate–severe | ||||
| (prednisolone) | Inflammation | With high dose * | ||||
| TRF-budesonide | Gut lymphoid tissues | Performed | Shown | Shown | Severe with high dose * | * Infection, deep vein thrombosis |
| VIS649 | APRIL (B cell) | Performed | Shown | Not shown | Mild | |
| BION-1301 | APRIL (B cell) | Performed | Confirmed | Not shown | Mild | |
| Bortezomib | Plasma cell (CD38) | Not performed | Shown * | Not shown | Mild | * Patients with T score of 0 on the Oxford classification |
| Felzartamab | Plasma cell (CD38) | Ongoing * | Not shown | Not shown | Not shown | * Phase 2 study |
| Mezagitamab | Plasma cell (CD38) | Not performed * | Not shown | not shown | Not shown | * Phase 1 study |
| Eculizumab | Complement C5 | Not performed | Not performed | Not performed | Not performed | Too expensive |
| IONIS-FB-LRx | Complement Factor B | Ongoing * | Shown | Not performed | Mild | * Phase 2 study interlim results |
| Atrasentan | Endothelin (ETA receptor) | Performed | Shown | Not performed | Mild–moderate | |
| Sparsentan | Endothelin (ETA receptor) | Performed * | Shown | Not performed | Mild | * Phase 3 study |
| Angiotensin receptor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, Y.; Tomino, Y.; Suzuki, Y. IgA Nephropathy: Beyond the Half-Century. Medicina 2024, 60, 54. https://doi.org/10.3390/medicina60010054
Shimizu Y, Tomino Y, Suzuki Y. IgA Nephropathy: Beyond the Half-Century. Medicina. 2024; 60(1):54. https://doi.org/10.3390/medicina60010054
Chicago/Turabian StyleShimizu, Yoshio, Yasuhiko Tomino, and Yusuke Suzuki. 2024. "IgA Nephropathy: Beyond the Half-Century" Medicina 60, no. 1: 54. https://doi.org/10.3390/medicina60010054
APA StyleShimizu, Y., Tomino, Y., & Suzuki, Y. (2024). IgA Nephropathy: Beyond the Half-Century. Medicina, 60(1), 54. https://doi.org/10.3390/medicina60010054







