The Characteristics and Laboratory Findings of SARS-CoV-2 Infected Patients during the First Three COVID-19 Waves in Portugal—A Retrospective Single-Center Study
Abstract
:1. Introduction
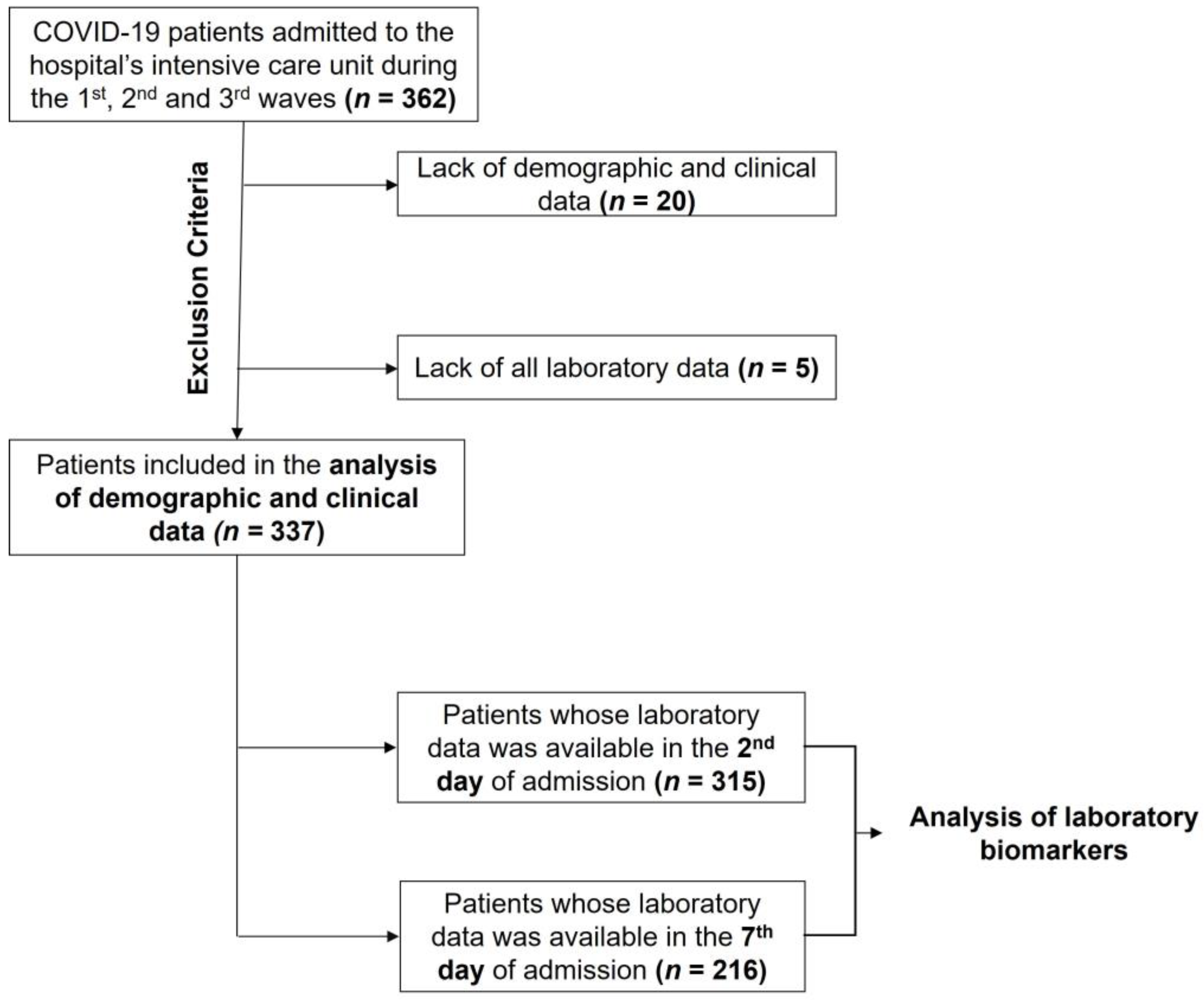
2. Materials and Methods
2.1. Study Design and Sample
2.2. Study Definitions
2.3. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics during the COVID-19 Waves
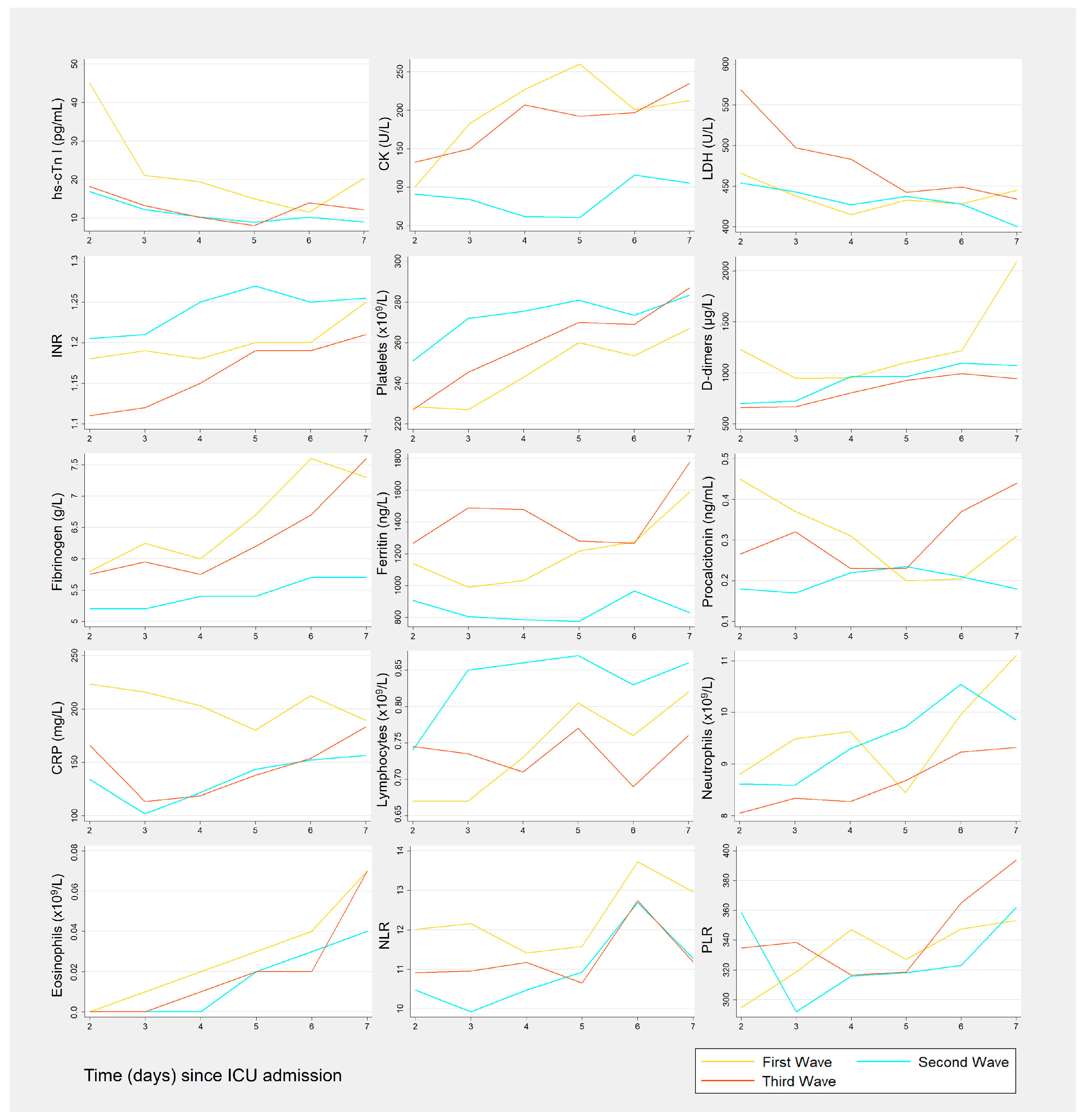
3.2. Biomarkers Results by Wave
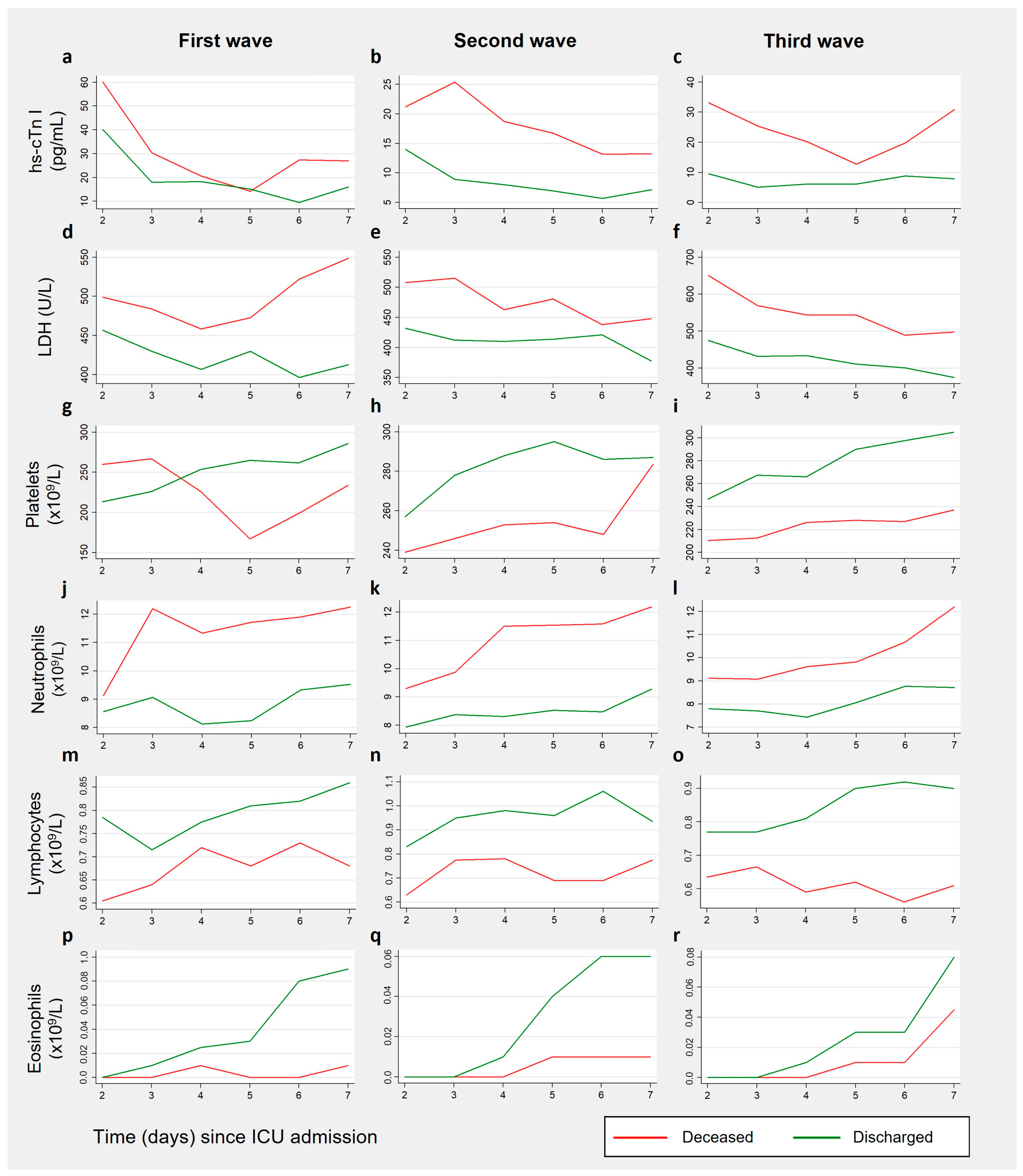
3.3. Biomarkers Results by Patient Outcome, for Each Wave
3.3.1. Cardiac Biomarkers
3.3.2. Coagulation Biomarkers
3.3.3. Inflammation-Related Biomarkers
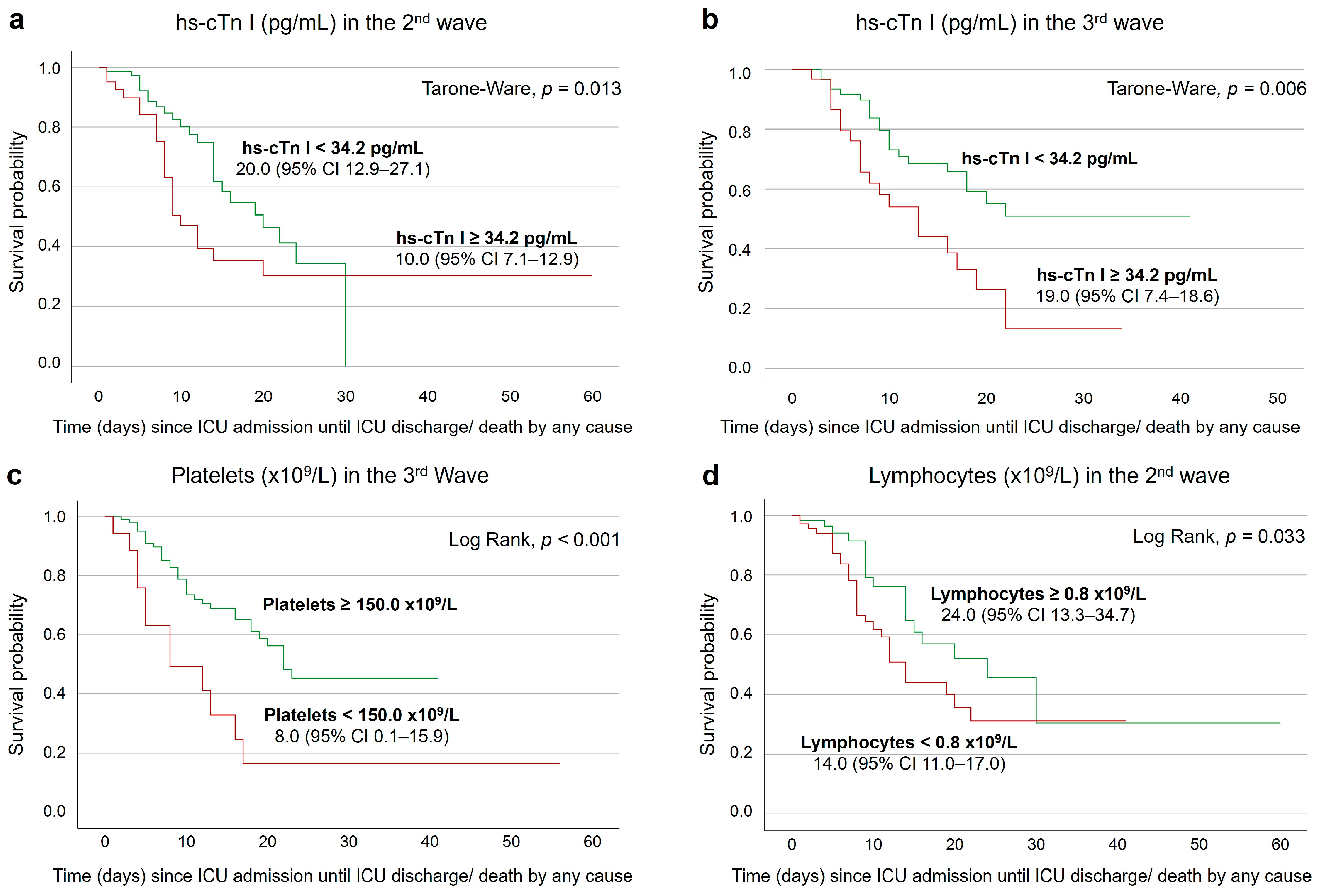
3.4. Survival Analysis on the Second and Seventh Days after ICU Admission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 15 September 2022).
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.M.; McGovern, A.P.; Thomas, N.J.; Wilde, H.; Vollmer, S.J.; Mateen, B.A. Trends in 28-Day Mortality of Critical Care Patients With Coronavirus Disease 2019 in the United Kingdom: A National Cohort Study, March 2020 to January 2021. Crit. Care Med. 2021, 49, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Auld, S.C.; Harrington, K.R.V.; Adelman, M.W.; Robichaux, C.J.; Overton, E.C.; Caridi-Scheible, M.; Coopersmith, C.M.; Murphy, D.J.; Arno, S.; Barnes, T.; et al. Trends in ICU Mortality From Coronavirus Disease 2019: A Tale of Three Surges. Crit. Care Med. 2022, 50, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Ge-rotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef]
- Deng, Q.; Hu, B.; Zhang, Y.; Wang, H.; Zhou, X.; Hu, W.; Cheng, Y.; Yan, J.; Ping, H.; Zhou, Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020, 311, 116–121. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M.; Silva, P.L.; Cruz, F.F.; Battaglini, D.; Robba, C.; Pelosi, P.; Morales, M.M.; Caruso Neves, C.; Rocco, P.R.M. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021, 12, 593223. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; Brodie, D.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Stringer, D.; Braude, P.; Myint, P.K.; Evans, L.; Collins, J.T.; Verduri, A.; Quinn, T.J.; Vilches-Moraga, A.; Stechman, M.J.; Pearce, L.; et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021, 50, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chim. Acta 2020, 505, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intensive Care 2020, 8, 36. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.S.; Kim, T.Y.; Lee, D.G.; Kim, D.W. Lymphopenia as a Biological Predictor of Outcomes in COVID-19 Patients: A Nationwide Cohort Study. Cancers 2021, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Khourssaji, M.; Chapelle, V.; Evenepoel, A.; Belkhir, L.; Yombi, J.C.; Van Dievoet, M.A.; Saussoy, P.; Coche, E.; Fillée, C.; Constantinescu, S.N.; et al. A biological profile for diagnosis and outcome of COVID-19 patients. Clin. Chem. Lab. Med. 2020, 58, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Fumagalli, L.A.M.; D’angelo, L.; Cerino, M.; Bonfanti, G.; Fumagalli, R.M.; Schiavo, G.; Lorini, C.; Lainu, E.; Terragni, S.; et al. Eosinopenia is a reliable marker of severe disease and unfavourable outcome in patients with COVID-19 pneumonia. Int. J. Clin. Pract. 2021, 75, e14047. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.Z.; Shou, Z.X.; Zheng, D.M.; Jin, X. The most important biomarker associated with coagulation and inflammation among COVID-19 patients. Mol. Cell. Biochem. 2021, 476, 2877–2885. [Google Scholar] [CrossRef]
- Palladino, M. Complete blood count alterations in COVID-19 patients: A narrative review. Biochem. Med. 2021, 31, 30501. [Google Scholar] [CrossRef]
- Simadibrata, D.M.; Pandhita, B.A.W.; Ananta, M.E.; Tango, T. Platelet-to-lymphocyte ratio, a novel biomarker to predict the severity of COVID-19 patients: A systematic review and meta-analysis. J. Intensive Care Soc. 2020, 23, 20–26. [Google Scholar] [CrossRef]
- Qu, R.; Ling, Y.; Zhang, Y.H.Z.; Wei, L.Y.; Chen, X.; Li, X.M.; Liu, X.Y.; Liu, H.M.; Guo, Z.; Ren, H.; et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020, 92, 1533–1541. [Google Scholar] [CrossRef]
- Hashem, M.K.; Khedr, E.M.; Daef, E.; Mohamed-Hussein, A.; Mostafa, E.F.; Hassany, S.M.; Galal, H.; Hassan, S.A.; Galal, I.; Amin, M.T.; et al. Prognostic biomarkers in COVID-19 infection: Value of anemia, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and D-dimer. Egypt. J. Bronchol. 2021, 15, 29. [Google Scholar] [CrossRef]
- Sarkar, S.; Kannan, S.; Khanna, P.; Singh, A.K. Role of platelet-to-lymphocyte count ratio (PLR), as a prognostic indicator in COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Eslamijouybari, M.; Heydari, K.; Maleki, I.; Moosazadeh, M.; Hedayatizadeh-Omran, A.; Vahedi, L.; Ghasemian, R.; Sharifpour, A.; Alizadeh-Navaei, R. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in COVID-19 Patients and Control Group and Relationship with Disease Prognosis. Casp. J. Intern. Med. 2020, 11, 531. [Google Scholar] [CrossRef]
- Direção-Geral da Saúde. Resposta Sazonal em Saúde—Vigilância e Monitorização. 2023. Available online: https://covid19.min-saude.pt/resposta-sazonal-em-saude-vigilancia-e-monitorizacao/ (accessed on 13 November 2023).
- Sorensen, R.; Barber, R.; Pigott, D.; Carter, A.; Spencer, C. Variation in the COVID-19 infection-fatality ratio by age, time, and geography during the pre-vaccine era: A systematic analysis. Lancet 2022, 399, 1469–1488. [Google Scholar] [CrossRef]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; van Pel, M.C.; de Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge Academic: Oxford, UK, 1988. [Google Scholar]
- Santos, A.P.; Leite, P.P.; Casaca, P.; Moreno, J.; Dias, C.M.; Nunes, B.; Gomes, J.P.; Silva, S.; Rodrigues, A.P.; Antunes, L.; et al. Monitorização das Linhas Vermelhas para a COVID-19—Relatório nº 11. 2021. Available online: https://covid19.min-saude.pt/relatorio-linhas-vermelhas/ (accessed on 7 May 2022).
- Hodcroft, E.B.; Zuber, M.; Nadeau, S.; Vaughan, T.G.; Crawford, K.H.D.; Althaus, C.L.; Reichmuth, M.L.; Bowen, J.E.; Walls, A.C.; Corti, D.; et al. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature 2021, 595, 707–712. [Google Scholar] [CrossRef]
- National Institute of Health Doutor Ricardo Jorge. Genomic Epidemiology of Novel Coronavirus in Portugal from March 1st 2020 up to March 31st 2021. Available online: https://insaflu.insa.pt/ncov/E-Mar2020-Mar2021 (accessed on 20 February 2023).
- National Institute of Health (INSA) Doutor Ricardo Jorge. Genomic Epidemiology of Novel Coronavirus in Portugal from February 1st 2021 up to November 30th 2021. Available online: https://insaflu.insa.pt/ncov/D-Fev2021-Nov2021 (accessed on 20 February 2023).
- Iftimie, S.; López-Azcona, A.F.; Lozano-Olmo, M.J.; Hernández-Aguilera, A.; Sarrà-Moretó, S.; Joven, J.; Camps, J.; Castro, A. Characteristics of hospitalized patients with SARS-CoV-2 infection during successive waves of the COVID-19 pandemic in a reference hospital in Spain. Sci. Rep. 2022, 12, 17384. [Google Scholar] [CrossRef]
- Contou, D.; Fraissé, M.; Pajot, O.; Tirolien, J.A.; Mentec, H.; Plantefève, G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: No prognostic improvement during the second wave? Crit. Care 2021, 25, 3. [Google Scholar] [CrossRef]
- Kabbaha, S.; Al-Azzam, S.; Karasneh, R.A.; Khassawneh, B.Y.; Al-Mistarehi, A.H.; Lattyak, W.J.; Aldiab, M.; Hasan, S.S.; Conway, B.R.; Aldeyab, M.A. Predictors of invasive mechanical ventilation in hospitalized COVID-19 patients: A retrospective study from Jordan. Expert Rev. Respir. Med. 2022, 16, 945–952. [Google Scholar] [CrossRef]
- Imam, Z.; Odish, F.; Gill, I.; O’Connor, D.; Armstrong, J.; Vanood, A.; Ibironke, O.; Hanna, A.; Ranski, A.; Halalau, A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J. Intern. Med. 2020, 288, 469–476. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Rhee, J.-W.; Cheng, P.; Waliany, S.; Chang, A.; Witteles, R.M.; Maecker, H.; Davis, M.M.; Nguyen, P.K.; Wu, S.M. Cardiovascular Complications in Patients with COVID-19: Consequences of Viral Toxicities and Host Immune Response. Curr. Cardiol. Rep. 2020, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Rathore, S.; Shannon, J.; Clarkson, P.; Faircloth, M.; Achan, V. Effect of the COVID-19 pandemic on ST-elevation myocardial infarction presentation and survival. Br. J. Cardiol. 2022, 29, 36–40. [Google Scholar] [CrossRef]
- Rodríguez-Leor, O.; Cid-Álvarez, B.; de Prado, A.P.; Rossello, X.; Ojeda, S.; Serrador, A.; López-Palop, R.; Martín-Moreiras, J.; Rumoroso, J.R.; Cequier, Á.; et al. Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience. Rev. Esp. Cardiol. 2020, 73, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zhu, H.; Zhao, J.; Zhuang, L.; Zhang, H.; Xie, H.; Zhang, R.; Granada, J.F.; Xiang, X.; Hu, W.; et al. Risk factors for myocardial injury in patients with coronavirus disease 2019 in China. ESC Heart Fail. 2020, 7, 4108–4117. [Google Scholar] [CrossRef]
- Mollinedo-Gajate, I.; Villar-Álvarez, F.; de los Ángeles Zambrano-Chacón, M.; Núñez-García, L.; de la Dueña-Muñoz, L.; López-Chang, C.; Górgolas, M.; Cabello, A.; Sánchez-Pernaute, O.; Romero-Bueno, F.; et al. First and Second Waves of Coronavirus Disease 2019 in Madrid, Spain: Clinical Characteristics and Hematological Risk Factors Associated With Critical/Fatal Illness. Crit. Care Explor. 2021, 3, e0346. [Google Scholar] [CrossRef]
- Orsucci, D.; Trezzi, M.; Anichini, R.; Blanc, P.; Barontini, L.; Biagini, C.; Capitanini, A.; Comeglio, M.; Corsini, P.; Gemignani, F.; et al. Increased Creatine Kinase May Predict A Worse COVID-19 Outcome. J. Clin. Med. 2021, 10, 1734. [Google Scholar] [CrossRef]
- de Rosa, A.; Verrengia, E.P.; Merlo, I.; Rea, F.; Siciliano, G.; Corrao, G.; Prelle, A. Muscle manifestations and CK levels in COVID infection: Results of a large cohort of patients inside a Pandemic COVID-19 Area. Acta Myol. 2021, 40, 1–7. [Google Scholar] [CrossRef]
- Akbar, M.R.; Pranata, R.; Wibowo, A.; Lim, M.A.; Sihite, T.A.; Martha, J.W. The prognostic value of elevated creatine kinase to predict poor outcome in patients with COVID-19—A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 529. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Gu, Y.; Yu, H.; Li, Z.; Wang, Y. Thrombocytopenia Is Associated with COVID-19 Severity and Outcome: An Updated Meta-Analysis of 5637 Patients with Multiple Outcomes. Lab. Med. 2021, 52, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Beni, F.; Vahedian-Azimi, A.; Shojaei, S.; Rahimi-Bashar, F.; Shahriary, A.; Johnston, T.P.; Sahebkar, A. The Level of Procalcitonin in Severe COVID-19 Patients: A Systematic Review and Meta-Analysis. Adv. Exp. Med. Biol. 2021, 1321, 277–286. [Google Scholar] [CrossRef] [PubMed]
- George, J.A.; Mayne, E.S. The Novel Coronavirus and Inflammation. Adv. Exp. Med. Biol. 2021, 1321, 127–138. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; de Pauw, I.; Stessel, B.; Dubois, J. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect Dis 2022, 22, 207. [Google Scholar] [CrossRef]
- Chen, R.; Sang, L.; Jiang, M.; Yang, Z.; Jia, N.; Fu, W.; Xie, J.; Guan, W.; Liang, W.; Ni, Z.; et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J. Allergy Clin. Immunol. 2020, 146, 89. [Google Scholar] [CrossRef]
- Li, C.; Jiang, J.; Wang, F.; Zhou, N.; Veronese, G.; Moslehi, J.J.; Ammirati, E.; Wang, D.W. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell Cardiol. 2020, 147, 74. [Google Scholar] [CrossRef]
- Cortés-Vieyra, R.; Gutiérrez-Castellanos, S.; Álvarez-Aguilar, C.; Baizabal-Aguirre, V.M.; Nuñez-Anita, R.E.; Rocha-López, A.G.; Gómez-García, A. Behavior of eosinophil counts in recovered and deceased covid-19 patients over the course of the disease. Viruses 2021, 13, 1675. [Google Scholar] [CrossRef]

| Features | First Wave (n = 62) | Second Wave (n = 136) | Third Wave (n = 139) | p Value a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deceased | p Value | Deceased | p Value | Deceased | p Value | |||||
| No (n = 42) | Yes (n = 20) | No (n = 83) | Yes (n = 53) | No (n = 88) | Yes (n = 51) | |||||
| Age | ||||||||||
| Age, years | 63 (50–74) | 75 (68–79) | 0.005 | 58 (47–70) | 71 (63–79) | <0.001 | 56 (49–64) | 69 (63–74) | <0.001 | 0.021 b |
| Sex | ||||||||||
| Female | 9 (21) | 8 (40) | 0.125 | 25 (30) | 15 (28) | 0.820 | 32 (36) | 21 (41) | 0.573 | 0.190 |
| Male | 33 (79) | 12 (60) | 58 (70) | 38 (72) | 56 (64) | 30 (59) | ||||
| BMI | ||||||||||
| BMI, Kg/m2 | 28 (24–31) | 28 (26–31) | 0.649 | 27 (25–29) | 26 (24–28) | 0.106 | 28 (25–31) | 29 (26–33) | 0.206 | 0.013 c |
| Comorbidities | ||||||||||
| Arterial hypertension | 23 (55) | 11 (55) | 0.986 | 38 (46) | 41 (77) | <0.001 | 41(47) | 38 (75) | 0.001 | 0.912 |
| Diabetes | 15 (36) | 7 (35) | 0.956 | 30 (36) | 23 (43) | 0.398 | 27 (31) | 23 (45) | 0.088 | 0.840 |
| Dyslipidemia | 12 (29) | 3 (15) | 0.346 | 22 (27) | 16 (30) | 0.641 | 18 (21) | 17 (33) | 0.092 | 0.812 |
| Obesity * | 10/37 (27) | 5/16 (31) | 0.751 | 16/72 (22) | 6/42 (14) | 0.300 | 27/77 (35) | 19/45 (42) | 0.431 | 0.008 |
| COPD | 5 (12) | 0 (0) | 0.165 | 1 (1) | 7 (13) | 0.006 | 2 (2) | 2 (4) | 0.624 | 0.254 |
| Myocardial ischemia | 3 (7) | 0 (0) | 0.545 | 2 (2) | 4 (8) | 0.208 | 9 (10) | 5 (10) | 0.936 | 0.140 |
| Chronic Kidney Disease | 2 (5) | 3 (15) | 0.317 | 3 (4) | 5 (9) | 0.261 | 4 (5) | 4 (8) | 0.465 | 0.803 |
| Solid Cancer | 3 (7) | 3 (15) | 0.377 | 1 (1) | 2 (4) | 0.560 | 7 (8) | 3 (6) | 0.746 | 0.062 |
| Hematologic Cancer | 1 (2) | 0 (0) | NR | 2 (2) | 0 (0) | NR | 1 (1) | 1 (2) | NR | NR |
| Respiratory Support | ||||||||||
| IMV | 34 (81) | 20 (100) | 0.046 | 47 (57) | 47 (89) | <0.001 | 62 (71) | 44 (86) | 0.035 | 0.023 |
| Days with IMV | 7 (2–20) | 6 (1–15) | 0.398 | 6 (3–15) | 7 (2–11) | 0.928 | 8 (5–16) | 8 (5–12) | 0.583 | 0.091 |
| ECMO | 10 (24) | 4 (20) | 0.737 | 11 (13) | 7 (13) | 0.994 | 3 (3) | 2 (4) | 0.607 | <0.001 |
| Days with ECMO | 8 (3–18) | 8 (2–31) | NR | 15 (7–31) | 5 (3–15) | 0.085 | NR | NR | NR | 0.791 |
| HFO | 13 (31) | 4 (20) | 0.366 | 45 (54) | 10 (19) | <0.001 | 26 (30) | 7 (14) | 0.035 | 0.009 |
| ICU and hospital length of stay | ||||||||||
| Days in the ICU | 8 (4–24) | 11 (2–16) | 0.751 | 8 (4–15) | 8 (5–13) | 0.719 | 9.0 (4–17) | 8 (4–13) | 0.329 | 0.619 |
| Days in the hospital | 27 (12–50) | 16 (7–26) | 0.023 | 20 (15–32) | 11 (7–17) | <0.001 | 24 (13–40) | 9 (5–14) | <0.001 | 0.062 |
| Features | First Wave (n = 59) | Second Wave (n = 133) | Third Wave (n = 133) | p Value a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deceased | p Value | Deceased | p Value | Deceased | p Value | |||||
| No (n = 41) | Yes (n = 18) | No (n = 83) | Yes (n = 50) | No (n = 85) | Yes (n = 48) | |||||
| hs-cTn I (pg/mL) | 40.1 (10.8; 248.0) | 60.1 (26.2–213.0) | 0.374 | 14.0 (4.5–45.3) | 21.2 (8.2–275.7) | 0.042 | 9.5 (4.8–39.2) | 33.1 (15.1–147.7) | 0.003 | 0.029 b |
| Increased hs-cTn I | 21/39 (53.8%) | 10/15 (66.7%) | 0.393 | 19/69 (27.5%) | 22/46 (47.8%) | 0.026 | 14/55 (25.5%) | 20/41 (48.8%) | 0.018 | 0.014 |
| CK (U/L) | 113.5 (56.0; 388.5) | 82.5 (31.8–209.0) | 0.260 | 80.0 (34.0–170.0) | 101.5 (53.8–275.8) | 0.172 | 115.0 (42.0–342.0) | 221.0 (60.0–393.0) | 0.083 | 0.125 |
| Increased CK | 16/40 (40.0%) | 4/18 (22.2%) | 0.188 | 17/75 (22.7%) | 13/36 (28.3%) | 0.489 | 25/67 (37.3%) | 23/43 (53.5%) | 0.095 | 0.010 |
| Myoglobin (ng/mL) | 98.30 (32.3; 846.2) | 143.4 (103.9–1150.2) | 0.445 | 76.7 (42.8–398.7) | 234.9 (58.9–721.8) | 0.491 | 101.0 (68.3–226.1) | 207.0 (74.8–260.5) | 0.520 | 0.644 |
| Increased Myoglobin | 10 (62.5%) | 5 (100%) | 0.262 | 7 (41.2%) | 5 (55.6%) | 0.683 | 11 (73.3%) | 11 (68.8%) | 1.000 | 0.098 |
| LDH (U/L) | 457.0 (357.0–583.0) | 499.0 (389.5–590.5) | 0.593 | 432.0 (352.3–523.8) | 508.0 (407.5–627.3) | 0.010 | 475.0 (379.5–611.8) | 650.5 (534.0–826.3) | <0.001 | <0.001 c,d |
| Increased LDH | 38/39 (97.4%) | 17/17 (100%) | 1.000 | 73/76 (96.1%) | 43/44 (97.7%) | 1.000 | 66/68 (97.1%) | 44/44 (100.0%) | 0.519 | 0.702 |
| INR | 1.2 (1.1–1.3) | 1.2 (1.1–1.4) | 0.857 | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.455 | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 0.429 | <0.001 c,d |
| Increased INR | 14/39 (35.9%) | 7/18 (38.9%) | 0.828 | 39/79 (49.4%) | 24/47 (51.1%) | 0.854 | 26/83 (31.3%) | 9/48 (18.8%) | 0.117 | 0.001 |
| Platelets (×109/L) | 213.5 (166.0–270.0) | 260.0 (156.8–337.5) | 0.187 | 257.0 (196.0–308.0) | 239.0 (191.0–297.0) | 0.541 | 246.5 (198.8–326.5) | 210.5 (147.0–266.8) | 0.006 | 0.240 |
| Decreased platelets | 8/40 (20.0%) | 3/18 (16.7%) | 1.000 | 11/83 (13.3%) | 5/47 (10.6%) | 0.663 | 6/84 (7.1%) | 12/48 (25.0%) | 0.004 | 0.471 |
| D-dimers (µg/L) | 1127.0 (549.0–3679.0) | 1305.0 (493.0–5997.8) | 0.747 | 629.0 (330.0–2660.0) | 853.0 (390.0–3652.0) | 0.243 | 479.0 (268.5–1024.5) | 795.0 (420.5–1922.5) | 0.024 | 0.002 b,c |
| Increased D-dimers | 36/37 (97.3%) | 18/18 (100.0%) | 1.000 | 68/79 (86.1%) | 43/45 (95.6%) | 0.131 | 60/73 (82.2%) | 42/45 (93.3%) | 0.086 | 0.057 |
| Fibrinogen (g/L) | 5.8 (3.8–7.5) | 5.9 (4.9–7.5) | 0.608 | 5.2 (4.4–6.5) | 5.1 (3.8–6.1) | 0.321 | 6.1 (5.0–7.0) | 5.6 (4.7–6.8) | 0.349 | 0.041 d |
| Increased fibrinogen | 20/28 (71.4%) | 14/16 (87.5%) | 0.283 | 49/62 (79.0%) | 26/36 (72.2%) | 0.443 | 39/43 (90.7%) | 27/29 (93.1%) | 1.000 | 0.028 |
| Ferritin (ng/mL) | 1191.2 (731.7–2421.8) | 947.6 (557.5–3173.3) | 0.924 | 928.8 (584.5–1901.5) | 770.3 (348.9–1798.0) | 0.180 | 1265.6 (692.2–2508.2) | 1271.0 (662.0–3639.8) | 0.634 | 0.032 d |
| Increased ferritin | 32/35 (91.4%) | 14/15 (93.3%) | 0.654 | 53/59 (89.8%) | 29/34 (85.3%) | 0.216 | 45/45 (93.8%) | 23/26 (88.5%) | 0.691 | 0.671 |
| Procalcitonin (ng/mL) | 0.4 (0.1–1.1) | 0.7 (0.2–1.4) | 0.299 | 0.1 (0.1–1.0) | 0.3 (0.1–0.7) | 0.238 | 0.2 (0.1–0.5) | 0.6 (0.2–1.7) | 0.002 | 0.010 b |
| Increased procalcitonin | 32/32 (100.0%) | 16/16 (100.0%) | NR | 46/58 (79.3%) | 36/41 (87.8%) | 0.270 | 60/67 (89.6%) | 41/43 (95.3%) | 0.478 | 0.004 |
| CRP (mg/L) | 208.3 (117.5–254.3) | 242.3 (176.1–283.1) | 0.122 | 117.7 (63.5– 206.3) | 148.1 (94.5– 225.3) | 0.223 | 151.9 (67.4– 232.9) | 199.9 (129.0–256.3) | 0.024 | <0.001 b |
| Increased CRP | 41/41 (100.0%) | 18/18 (100.0%) | NR | 83/83 (100.0%) | 47/47 (100.0%) | NR | 84/84 (100.0%) | 48/48 (100.0%) | NR | NR |
| WBC (×109/L) | 10.7 (8.6–13.1) | 10.8 (9.3–17.3) | 0.290 | 9.6 (7.2–13.9) | 10.8 (7.8–13.6) | 0.453 | 9.0 (7.3–13.6) | 10.0 (7.4–13.7) | 0.488 | 0.254 |
| Increased WBC | 18/40 (45.0%) | 9/18 (50.0%) | 0.724 | 32/83 (38.6%) | 23/47 (48.9%) | 0.250 | 32/84 (38.1%) | 23/48 (47.9%) | 0.271 | 0.166 |
| Lymphocytes (×109/L) | 0.8 (0.5–1.2) | 0.6 (0.5–1.1) | 0.501 | 0.8 (0.6–1.2) | 0.6 (0.4–1.0) | 0.014 | 0.8 (0.6–1.1) | 0.6 (0.5–0.9) | 0.019 | 0.900 |
| Decreased lymphocytes | 25/40 (62.5%) | 12/18 (66.7%) | 0.760 | 47/83 (56.6%) | 33/47 (70.2%) | 0.126 | 55/84 (65.5%) | 33/48 (68.8%) | 0.701 | 0.578 |
| Neutrophils (×109/L) | 8.6 (6.7–11.3) | 9.1 (7.9–14.1) | 0.243 | 7.9 (5.3–12.5) | 9.3 (6.9–12.4) | 0.323 | 7.8 (5.8–11.6) | 9.1 (6.4–12.5) | 0.289 | 0.534 |
| Increased neutrophils | 20/40 (50.0%) | 12/18 (66.7%) | 0.238 | 39/83 (47.0%) | 27/47 (57.4%) | 0.252 | 38/84 (45.2%) | 25/48 (52.1%) | 0.449 | 0.112 |
| Eosinophils (×109/L) | 0.00 (0.00–0.01) | 0.00 (0.00–0.04) | 0.915 | 0.00 (0.00–0.01) | 0.00 (0.00–0.00) | 0.022 | 0.00 (0.00–0.01) | 0.00 (0.00–0.00) | 0.011 | 0.032 b |
| Decreased eosinophils | 32/40 (80.0%) | 13/18 (72.2%) | 0.516 | 73/83 (88.0%) | 44/47 (93.6%) | 0.374 | 68/84 (81.0%) | 45/48 (93.5%) | 0.044 | 0.077 |
| PLR | 256.1 (193.9–414.6) | 435.3 (250.1–681.5) | 0.064 | 330.6 (235.6– 429.1) | 431.4 (269.8– 576.5) | 0.008 | 324.7 (236.1–461.1) | 365.7 (203.3–581.3) | 0.601 | 0.424 |
| Increased PLR | 31/40 (77.5%) | 14/18 (77.8%) | 1.000 | 67/83 (80.7%) | 43/47 (91.5%) | 0.102 | 68/84 (81.0%) | 38/48 (79.2%) | 0.804 | 0.460 |
| NLR | 10.7 (5.5–15.8) | 14.7 (9.2–29.0) | 0.122 | 8.9 (5.6–15.5) | 15.9 (8.9–23.5) | 0.003 | 9.9 (6.7–15.3) | 12.7 (8.5–17.8) | 0.032 | 0.924 |
| Increased NLR | 37/40 (92.5%) | 16/18 (88.9%) | 0.641 | 79/83 (95.2%) | 45/47 (95.7%) | 1.000 | 81/84 (96.4%) | 47/48 (97.9%) | 1.000 | 0.244 |
| Features | First Wave (n = 39) | Second Wave (n = 87) | Third Wave (n = 90) | p Value a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deceased | p Value | Deceased | p Value | Deceased | p Value | |||||
| No (n = 27) | Yes (n = 12) | No (n = 52) | Yes (n = 35) | No (n = 58) | Yes (n = 32) | |||||
| hs-cTn I (pg/mL) | 16.1 (8.9–33.0) | 27.0 (12.5–118.5) | 0.133 | 7.2 (3.0–23.6) | 13.3 (7.2–37.4) | 0.038 | 7.9 (3.6–22.8) | 30.8 (11.7–145.6) | 0.045 | 0.061 |
| Increased hs-cTn I | 5/21 (23.8%) | 5/11 (45.5%) | 0.252 | 7/37 (18.9%) | 7/28 (25.0%) | 0.555 | 6/29 (20.7%) | 9/19 (47.4%) | 0.051 | 0.423 |
| CK (U/L) | 220.0 (55.0–479.5) | 195.0 (69.0–373.0) | 0.919 | 103.5 (20.8–331.5) | 105.5 (50.3–243.5) | 0.371 | 186.0 (71.0–483.0) | 459.5 (206.0–849.8) | 0.011 | 0.008 d |
| Increased CK | 14/25 (56.0%) | 5/11 (45.5%) | 0.559 | 13/38 (34.2%) | 9/26 (34.6%) | 0.973 | 17/39 (43.6%) | 19/24 (79.2%) | 0.006 | 0.040 |
| LDH (U/L) | 413.0 (336.0–470.5) | 548.5 (392.0–631.8) | 0.013 | 377.5 (323.0–515.0) | 448.0 (333.8–564.5) | 0.194 | 374.5 (312.5–501.5) | 498.0 (410.3–561.0) | 0.005 | 0.557 |
| Increased LDH | 25/25 (100.0%) | 12/12 (100.0%) | NR | 46/48 (95.8%) | 29/30 (96.7%) | 1.000 | 48/40 (100.0%) | 30/30 (100.0%) | NR | NR |
| INR | 1.3 (1.2–1.4) | 1.2 (1.1–1.3) | 0.505 | 1.2 (1.2–1.3) | 1.3 (1.2–1.4) | 0.105 | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.216 | 0.012 d |
| Increased INR | 16/26 (61.5%) | 6/12 (50.0%) | 0.503 | 31/52 (59.6%) | 26/34 (76.5%) | 0.106 | 25/57 (43.9%) | 21/32 (65.6%) | 0.049 | 0.146 |
| Platelets (×109/L) | 286.0 (222.0–407.0) | 234.0 (178.3–296.3) | 0.168 | 287.0 (223.0–358.8) | 283.5 (212.3–373.5) | 0.856 | 305.0 (237.5–385.5) | 237.0 (184.5–316.5) | 0.005 | 0.858 |
| Decreased platelets | 3/27 (11.1%) | 2/12 (16.7%) | NR | 4/52 (7.7%) | 3/34 (8.8%) | NR | 2/57 (3.5%) | 5/32 (15.6%) | NR | 0.631 |
| D-dimers (µg/L) | 1340.0 (435.0–2812.0) | 3888.0 (1542.3–8718.8) | 0.018 | 1052.0 (481.0–2473.0) | 1693.0 (706.0–3175.0) | 0.465 | 715.0 (479.5–1300.5) | 1817.5 (941.0–2977.5) | <0.001 | 0.059 |
| Increased D-dimers | 27/27 (100.0%) | 12/12 (100.0%) | NR | 45/47 (95.7%) | 30/31 (96.8%) | 1.000 | 46/49 (93.9%) | 30/30 (100.0%) | 0.284 | NR |
| Fibrinogen (g/L) | 7.3 (6.5–9.7) | 6.8 (3.7–8.4) | 0.210 | 5.5 (4.8–7.2) | 6.0 (4.4–8.2) | 0.593 | 7.4 (5.2–9.4) | 7.7 (5.8–9.3) | 0.729 | 0.004 d |
| Increased Fibrinogen | 12/13 (92.3%) | 6/8 (75.0%) | 0.531 | 23/26 (88.5%) | 15/17 (88.2%) | 1.000 | 19/19 (100.0%) | 18/19 (94.7%) | 1.000 | NR |
| Ferritin (ng/mL) | 1592.3 (837.8–2053.8) | 1450.6 (1134.5–2325.7) | 0.685 | 776.9 (359.8–1993.5) | 935.2 (249.5–2722.6) | 0.949 | 1330.2 (640.2–2431.2) | 2073.0 (1283.2–3610.0) | 0.057 | 0.010 d |
| Increased ferritin | 21/22 (95.5%) | 9/9 (100.0%) | 1.000 | 20/26 (76.9%) | 15/21 (71.4%) | 0.668 | 23/24 (95.8%) | 17/18 (94.4%) | 1.000 | 0.002 |
| Procalcitonin (ng/mL) | 0.2 (0.1–1.1) | 0.4 (0.2–1.9) | 0.313 | 0.2 (0.1–0.5) | 0.3 (0.1–0.8) | 0.126 | 0.4 (0.1–0.8) | 1.2 (0.3–5.9) | 0.008 | 0.010 d |
| Increased procalcitonin | 22/22 (100.0%) | 9/9 (100.0%) | NR | 21/31 (67.7%) | 24/26 (92.3%) | 0.023 | 40/42 (95.2%) | 27/27 (100.0%) | 0.517 | <0.001 |
| CRP (mg/L) | 189.8 (83.6–258.1) | 154.4 (52.3–260.7) | 0.499 | 104.6 (35.9–226.0) | 219.0 (119.6–267.9) | 0.003 | 169.2 (74.5–260.7) | 205.6 (154.5–283.5) | 0.019 | 0.111 |
| Increased CRP | 27/27 (100.0%) | 12/12 (100.0%) | NR | 51/52 (98.1%) | 34/34 (100%) | 1.000 | 56/57 (98.2%) | 32/32 (100.0%) | 1.000 | NR |
| WBC (×109/L) | 12.8 (8.6–14.2) | 13.5 (10.7–19.7) | 0.159 | 10.8 (7.9–14.3) | 14.2 (10.6–18.4) | 0.014 | 10.3 (8.9–13.2) | 13.5 (9.5–18.8) | 0.043 | 0.558 |
| Increased WBC | 17/27 (63.0%) | 10/12 (83.3%) | 0.276 | 32/52 (61.5%) | 26/34 (76.5%) | 0.149 | 31/57 (54.4%) | 22/32 (68.8%) | 0.185 | 0.436 |
| Lymphocytes (×109/L) | 0.9 (0.7–1.4) | 0.7 (0.5–1.3) | 0.298 | 0.9 (0.6–1.3) | 0.8 (0.4–1.0) | 0.090 | 0.9 (0.6–1.4) | 0.6 (0.4–0.8) | 0.002 | 0.668 |
| Decreased lymphocytes | 10/27 (37.0%) | 8/12 (66.7%) | 0.087 | 20/52 (38.5%) | 18/34 (52.9%) | 0.186 | 23/57 (40.4%) | 24/32 (75.0%) | 0.002 | 0.502 |
| Neutrophils (×109/L) | 9.5 (6.7–12.6) | 12.3 (9.3–16.6) | 0.104 | 9.3 (6.2–12.3) | 12.2 (9.0–16.1) | 0.003 | 8.7 (7.1–11.0) | 12.2 (8.3–17.2) | 0.010 | 0.694 |
| Increased neutrophils | 19/27 (70.4%) | 11/12 (91.7%) | 0.228 | 35/52 (67.3%) | 31/34 (91.2%) | 0.010 | 44/57 (77.2%) | 26/32 (81.3%) | 0.654 | 0.950 |
| Eosinophils (×109/L) | 0.09 (0.01–0.15) | 0.01 (0.00–0.11) | 0.118 | 0.06 (0.01–0.13) | 0.01 (0.00–0.05) | 0.004 | 0.08 (0.02–0.19) | 0.05 (0.01–0.13) | 0.229 | 0.207 |
| Decreased eosinophils | 7/27 (25.9%) | 7/12 (58.3%) | 0.075 | 16/52 (30.8%) | 20/34 (58.8%) | 0.010 | 15/57 (26.3%) | 13/32 (40.6%) | 0.163 | 0.359 |
| PLR | 353.0 (190.9–500.0) | 374.3 (200.2–508.8) | 0.869 | 346.7 (201.2–527.8) | 386.3 (248.9–526.3) | 0.318 | 341.7 (200.0–512.9) | 424.0 (304.2–590.4) | 0.207 | 0.610 |
| Increased PLR | 20/27 (74.1%) | 9/12 (75.0%) | 1.000 | 40/52 (76.9%) | 30/34 (88.2%) | 0.187 | 46/57 (80.7%) | 28/32 (87.5%) | 0.411 | 0.501 |
| NLR | 8.8 (5.8–16.0) | 17.8 (12.8–18.8) | 0.056 | 9.3 (4.9–18.4) | 14.1 (8.4–32.2) | 0.002 | 9.6 (6.1–15.7) | 15.4 (10.1–32.4) | <0.001 | 0.964 |
| Increased NLR | 26/27 (96.3%) | 11/12 (91.7%) | 0.526 | 49/52 (94.2%) | 34/34 (100.0%) | 0.274 | 56/57 (98.2%) | 32/32 (100.0%) | 1.000 | NR |
| First Wave | Second Wave | Third Wave | ||||
|---|---|---|---|---|---|---|
| 2nd Day | 7th Day | 2nd Day | 7th Day | 2nd Day | 7th Day | |
| Cardiac biomarkers | ns | In risk of CS LDH (U/L) | hs-cTn I (pg/mL) ⬆ hs-cTn I LDH (U/L) | hs-cTn I (pg/mL) | hs-cTn I (pg/mL) ⬆ hs-cTn I LDH (U/L) | hs-cTn I (pg/mL) LDH (U/L) CK ⬆ CK |
| Coagulation biomarkers | ns | D-dimers (µg/L) | ns | ns | Platelets (×109/L) ⬇ Platelets D-dimers (µg/L) | Platelets (×109/L) D-dimers (µg/L) |
| Inflammation Biomarkers | ns | ns | Lymphocytes (×109/L) Eosinophils (×109/L) PLR NLR | ⬆ Procalcitonin CRP (mg/L) WBCs (×109/L) Neutrophils (×109/L) ⬆ Neutrophils Eosinophils (×109/L) ⬇ Eosinophils NLR | Procalcitonin (ng/mL) CRP (mg/L) Lymphocytes (×109/L) Eosinophils (×109/L) ⬇ Eosinophils NLR | Procalcitonin (ng/mL) CRP (mg/L) WBCs (×109/L) Lymphocytes (×109/L) ⬇ Lymphocytes Neutrophils (×109/L) NLR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Von Rekowski, C.P.; Fonseca, T.A.H.; Araújo, R.; Brás-Geraldes, C.; Calado, C.R.C.; Bento, L.; Pinto, I. The Characteristics and Laboratory Findings of SARS-CoV-2 Infected Patients during the First Three COVID-19 Waves in Portugal—A Retrospective Single-Center Study. Medicina 2024, 60, 59. https://doi.org/10.3390/medicina60010059
Von Rekowski CP, Fonseca TAH, Araújo R, Brás-Geraldes C, Calado CRC, Bento L, Pinto I. The Characteristics and Laboratory Findings of SARS-CoV-2 Infected Patients during the First Three COVID-19 Waves in Portugal—A Retrospective Single-Center Study. Medicina. 2024; 60(1):59. https://doi.org/10.3390/medicina60010059
Chicago/Turabian StyleVon Rekowski, Cristiana P., Tiago A. H. Fonseca, Rúben Araújo, Carlos Brás-Geraldes, Cecília R. C. Calado, Luís Bento, and Iola Pinto. 2024. "The Characteristics and Laboratory Findings of SARS-CoV-2 Infected Patients during the First Three COVID-19 Waves in Portugal—A Retrospective Single-Center Study" Medicina 60, no. 1: 59. https://doi.org/10.3390/medicina60010059







