Muscle-Derived Stem/Progenitor Cells Ameliorate Acute Kidney Injury in Rats through the Anti-Apoptotic Pathway and Demonstrate Comparable Effects to Bone Marrow Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Isolation and Characterization of MDSPCs and BM-MSCs
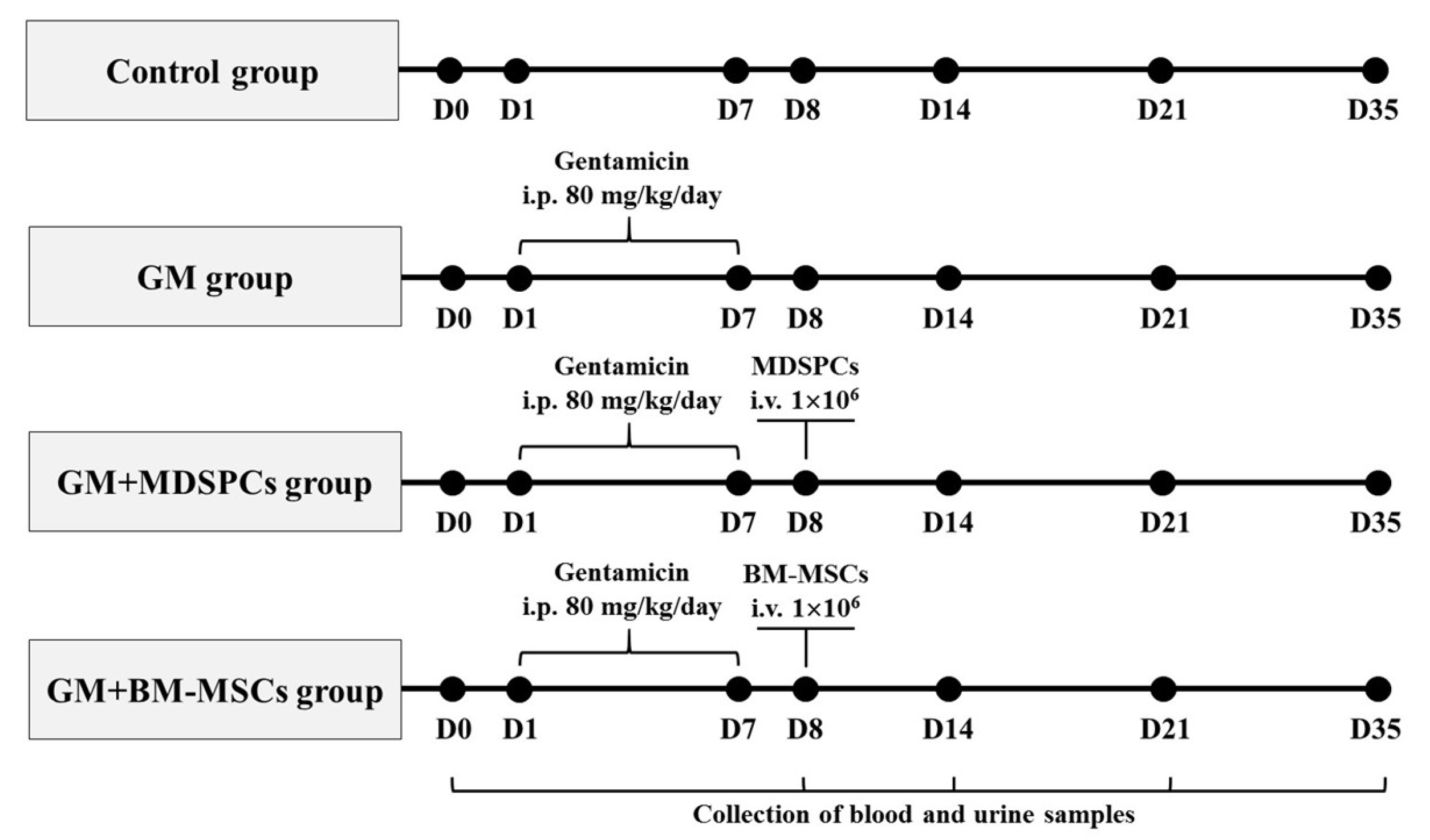
2.3. Nephrotoxicity Model
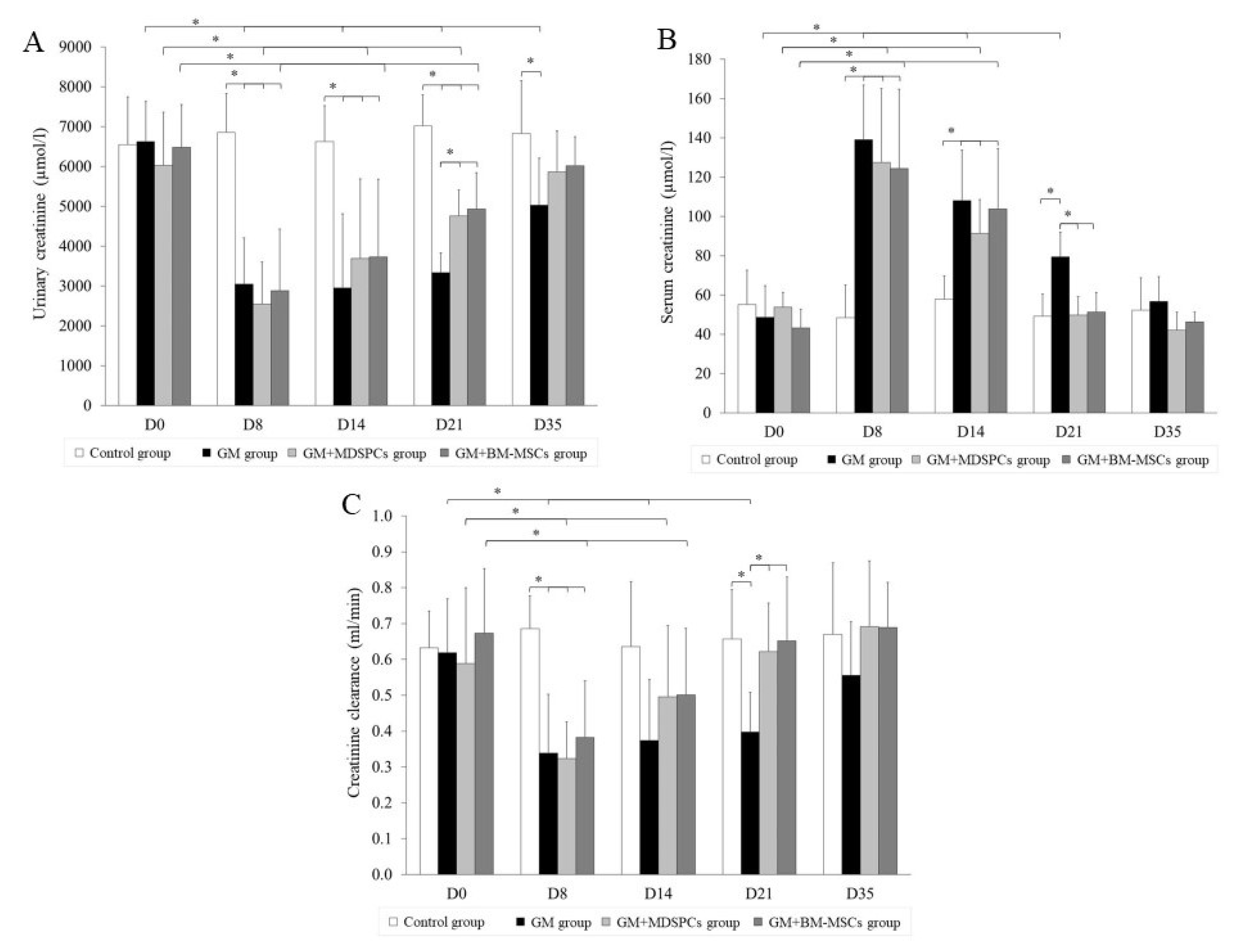
2.4. Biochemical Functional Analysis
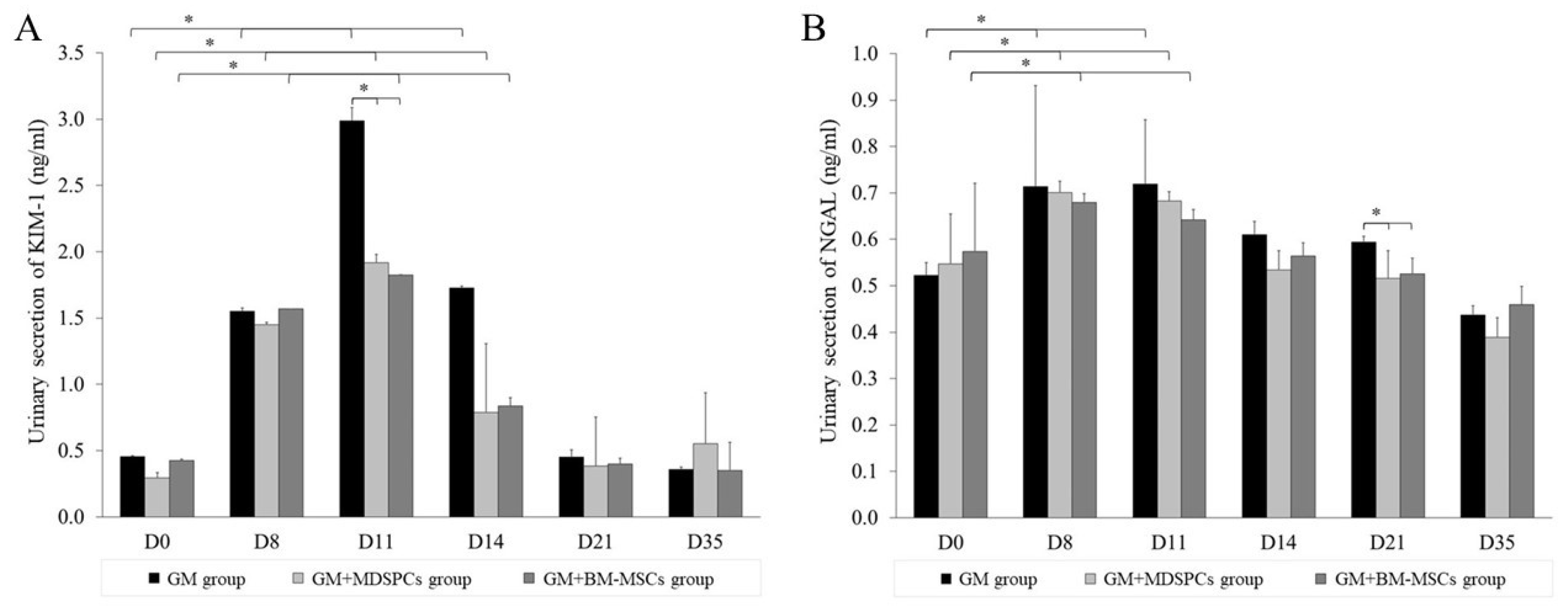
2.5. Biochemical Injury Markers KIM-1 and NGAL Secretion Analysis
2.6. Renal Histology
2.7. Apoptosis Assay
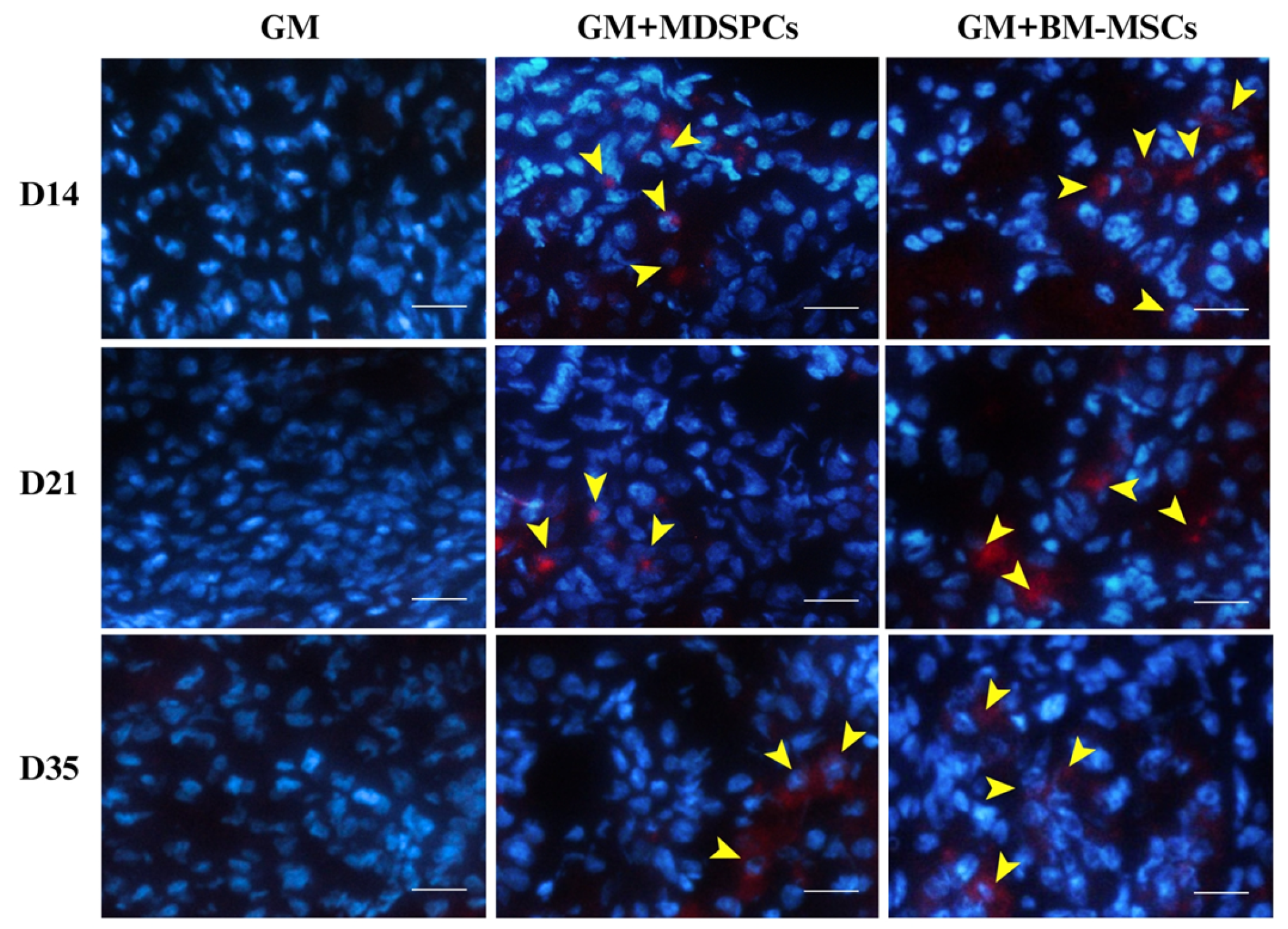
2.8. In Vivo Tracking of MDSPCs and BM-MSCs
2.9. Statistical Analysis
3. Results
3.1. Characteristics of MDSPCs and BM-MSCs
3.2. Effects of MDSPCs and BM-MSCs on Kidney Function after AKI
3.3. Effects of MDSPCs and BM-MSCs on KIM-1 and NGAL Secretion
3.4. Effects of MDSPCs and BM-MSCs on Kidney Histology after AKI
3.5. Anti-Apoptotic Effects of MDSPCs and BM-MSCs
3.6. Tracking of MDSPCs and BM-MSCs In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part J. Int. Soc. Anal. Cytol. 2018, 93, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Thanh, V.V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, M.; Lu, A.; Thompson, S.D.; Robbins, P.D.; Huard, J.; Niedernhofer, L.J. Isolation of muscle-derived stem/progenitor cells based on adhesion characteristics to collagen-coated surfaces. Methods Mol. Biol. 2013, 976, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Jing, X.; Guo, W.; Hao, C.; Zhang, Y.; Gao, S.; Chen, M.; Zhang, Z.; Zhang, X.; et al. Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stem Cells: Characterization, Differentiation, and Applications in Cartilage Tissue Engineering. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Snyder, L.; Knights, K.; He, H.; Springer, N.L.; Lillich, J.; Weiss, M.L. A Protocol for the Isolation, Culture, and Cryopreservation of Umbilical Cord-Derived Canine Mesenchymal Stromal Cells: Role of Cell Attachment in Long-Term Maintenance. Stem Cells Dev. 2020, 29, 695–713. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Mao, Q.; Xia, H. A simple, efficient and economical method for isolating and culturing human umbilical cord blood-derived mesenchymal stromal cells. Mol. Med. Rep. 2019, 20, 5257–5264. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Lee, A.R.; Moon, D.K.; Siregar, A.; Moon, S.Y.; Jeon, R.-H.; Son, Y.-B.; Kim, B.G.; Hah, J.H.; Hwang, S.C.; Byun, J.H.; et al. Involvement of mitochondrial biogenesis during the differentiation of human periosteum-derived mesenchymal stem cells into adipocytes, chondrocytes and osteocytes. Arch. Pharm. Res. 2019, 42, 1052–1062. [Google Scholar] [CrossRef]
- Hodgson, B.; Mafi, R.; Mafi, P.; Khan, W.S. The Regulation of Differentiation of Mesenchymal Stem-cells into Skeletal Muscle: A Look at Signalling Molecules Involved in Myogenesis. Curr. Stem Cell Res. Ther. 2018, 13, 384–407. [Google Scholar] [CrossRef]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.J.; Ji, H.; Guan, W.; Zhao, Y. Isolation, culture, and characterization of chicken lung-derived mesenchymal stem cells. Can. J. Vet. Res. 2018, 82, 225–235. [Google Scholar] [PubMed]
- De Chiara, L.; Famulari, E.S.; Fagoonee, S.; van Daalen, S.K.M.; Buttiglieri, S.; Revelli, A.; Tolosano, E.; Silengo, L.; Pelt, A.M.M.; Altruda, F. Characterization of Human Mesenchymal Stem Cells Isolated from the Testis. Stem Cells Int. 2018, 2018, 4910304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Zheng, Y.; Wen, H.; Ji, H.; Zhao, Y.; Guan, W. Isolation and biological characterization of mesenchymal stem cells from goose dermis. Poult. Sci. 2018, 97, 3236–3247. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-Y.; Cheong, S.-K.; Mok, P.-L.; Leong, C.-F. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology 2008, 40, 52–57. [Google Scholar] [CrossRef]
- Qian, H.; Yang, H.; Xu, W.; Yan, Y.; Chen, Q.; Zhu, W.; Cao, H.; Yin, Q.; Zhou, H.; Mao, F.; et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int. J. Mol. Med. 2008, 22, 325–332. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, X.; Li, P.; Zhou, Y.; Xue, T.; Liu, J.; Yu, C. Bone Marrow Derived Mesenchymal Stromal Cells Ameliorate Ischemia/Reperfusion Injury-Induced Acute Kidney Injury in Rats via Secreting Tumor Necrosis Factor-Inducible Gene 6 Protein. BioMed Res. Int. 2019, 2019, 9845709. [Google Scholar] [CrossRef]
- Xiu, G.-H.; Zhou, X.; Li, X.-L.; Chen, X.-Z.; Li, B.-Q.; Chen, X.-L.; Jin, H.; Pan, X.H.; Sun, J.; Ling, B. Role of Bone Marrow Mesenchymal Stromal Cells in Attenuating Inflammatory Reaction in Lipopolysaccaride-induced Acute Kidney Injury of Rats Associated with TLR4-NF-κB Signaling Pathway Inhibition. Ann. Clin. Lab. Sci. 2018, 48, 743–750. [Google Scholar]
- He, W.; Qin, D.; Li, B.; Zhang, H.; Cheng, X.; Sun, J.; Hua, J.; Peng, S. Immortalized canine adipose-derived mesenchymal stem cells alleviate gentamicin-induced acute kidney injury by inhibiting endoplasmic reticulum stress in mice and dogs. Res. Vet. Sci. 2021, 136, 39–50. [Google Scholar] [CrossRef]
- Selim, R.E.; Ahmed, H.H.; Abd-Allah, S.H.; Sabry, G.M.; Hassan, R.E.; Khalil, W.K.B.; Abouhashem, N.S. Mesenchymal Stem Cells: A Promising Therapeutic Tool for Acute Kidney Injury. Appl. Biochem. Biotechnol. 2019, 189, 284–304. [Google Scholar] [CrossRef]
- Lee, K.-H.; Tseng, W.-C.; Yang, C.-Y.; Tarng, D.-C. The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3529. [Google Scholar] [CrossRef] [PubMed]
- Sadek, E.M.; Afifi, N.M.; Elfattah, L.I.A.; Mohsen, M.A.A.-E. Histological study on effect of mesenchymal stem cell therapy on experimental renal injury induced by ischemia/reperfusion in male albino rat. Int. J. Stem Cells 2013, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Alfarano, C.; Roubeix, C.; Chaaya, R.; Ceccaldi, C.; Calise, D.; Mias, C.; Cussac, D.; Bascands, J.L.; Parini, A. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant. 2012, 21, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Zilberman-Itskovich, S.; Abu-Hamad, R.; Zarura, R.; Sova, M.; Hachmo, Y.; Stark, M.; Neuman, S.; Slavin, S.; Efrati, S. Human mesenchymal stromal cells ameliorate complement induced inflammatory cascade and improve renal functions in a rat model of ischemia-reperfusion induced acute kidney injury. PLoS ONE 2019, 14, e0222354. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Polymeri, A.; Giannobile, W.V.; Kaigler, D. Bone Marrow Stromal Stem Cells in Tissue Engineering and Regenerative Medicine. Horm. Metab. Res. 2016, 48, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J. Am. Soc. Nephrol. JASN 2018, 29, 260–267. [Google Scholar] [CrossRef]
- Swaminathan, M.; Kopyt, N.; Atta, M.G.; Radhakrishnan, J.; Umanath, K.; Nguyen, S.; O’Rourke, B.; Allen, A.; Vaninov, N.; Tilles, A.; et al. Pharmacological effects of ex vivo mesenchymal stem cell immunotherapy in patients with acute kidney injury and underlying systemic inflammation. Stem Cells Transl. Med. 2021, 10, 1588–1601. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, Z.; Han, F.; Zhao, M.; Cao, R.; Zhao, D.; Hong, L.; Na, N.; Li, H.; Miao, B.; et al. Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: Pilot results of a multicenter randomized controlled trial. J. Transl. Med. 2018, 16, 52. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Deasy, B.M.; Gharaibeh, B.M.; Pollett, J.B.; Jones, M.M.; Lucas, M.A.; Kanda, Y.; Huard, J. Long-term self-renewal of postnatal muscle-derived stem cells. Mol. Biol. Cell 2005, 16, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Testa, S.; Riera, C.S.; Fornetti, E.; Riccio, F.; Fuoco, C.; Bernardini, S.; Baldi, J.; Costantini, M.; Foddai, M.L.; Cannata, S.; et al. Skeletal Muscle-Derived Human Mesenchymal Stem Cells: Influence of Different Culture Conditions on Proliferative and Myogenic Capabilities. Front. Physiol. 2020, 11, 553198. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Counsell, J.R.; Reza, M.; Laval, S.H.; Danos, O.; Thrasher, A.; Lochmüller, H.; Muntoni, H.; Morgan, J.E. Autologous skeletal muscle derived cells expressing a novel functional dystrophin provide a potential therapy for Duchenne Muscular Dystrophy. Sci. Rep. 2016, 6, 19750. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, M.; Thompson, S.D.; Pollett, J.B.; Usas, A.; Lu, A.; Stolz, D.B.; Clark, K.A.; Sun, B.; Péault, B.; Huard, J. Human muscle-derived stem/progenitor cells promote functional murine peripheral nerve regeneration. J. Clin. Investig. 2014, 124, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.-C.; Chiu, C.-Y.; Lan, K.-C.; Weng, T.-I.; Yang, R.-S.; Liu, S.-H. Transplantation of human skeletal muscle-derived progenitor cells ameliorates knee osteoarthritis in streptozotocin-induced diabetic mice. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Haring, G.; Herman, S.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res. Ther. 2020, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-X.; Lu, H.-B.; Jin, X.-L.; Feng, W.-F.; Yang, X.-N.; Qi, Z.-L. Skeletal muscle-derived cells repair peripheral nerve defects in mice. Neural Regen. Res. 2020, 15, 152–161. [Google Scholar] [CrossRef]
- Elashry, M.I.; Gaertner, K.; Klymiuk, M.C.; Eldaey, A.; Wenisch, S.; Arnhold, S. Characterisation of stemness and multipotency of ovine muscle-derived stem cells from various muscle sources. J. Anat. 2021, 239, 336–350. [Google Scholar] [CrossRef]
- Tey, S.-R.; Robertson, S.; Lynch, E.; Suzuki, M. Coding Cell Identity of Human Skeletal Muscle Progenitor Cells Using Cell Surface Markers: Current Status and Remaining Challenges for Characterization and Isolation. Front. Cell Dev. Biol. 2019, 7, 284. [Google Scholar] [CrossRef]
- Lavasani, M.; Robinson, A.R.; Lu, A.; Song, M.; Feduska, J.M.; Ahani, B.; Tilstra, J.S.; Feldman, C.H.; Robbins, P.D.; Niedernhofer, L.J.; et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat. Commun. 2012, 3, 608. [Google Scholar] [CrossRef]
- Pavyde, E.; Maciulaitis, R.; Mauricas, M.; Sudzius, G.; Ivanauskaite Didziokiene, E.; Laurinavicius, A.; Sutkeviciene, N.; Stankevicius, E.; Maciulaitis, J.; Usas, A. Skeletal Muscle-Derived Stem/Progenitor Cells: A Potential Strategy for the Treatment of Acute Kidney Injury, Skeletal Muscle-Derived Stem/Progenitor Cells: A Potential Strategy for the Treatment of Acute Kidney Injury. Stem Cells Int. 2016, 2016, e9618480. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.A.; Borges, F.T.; Simões, M.J.; Borges, A.A.; Sinigaglia-Coimbra, R.; Schor, N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS ONE 2012, 7, e44092. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. JASN 2004, 15, 1794–1804. [Google Scholar] [CrossRef]
- Žymantienė, J.; Želvytė, R.; Oberauskas, V.; Daunoras, G.; Pockevičius, A.; Jokimas, J.; Milius, J.; Maciulaitis, R. Rat—An animal’s model for correction of nephrotoxicity using gentamicin. Vet. Med. Zoot. 2010, 51, 83–88. [Google Scholar]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Sun, C.-K.; Lin, Y.-C.; Chang, L.-T.; Chen, Y.-L.; Tsai, T.-H.; Chung, S.-Y.; Chua, S.; Kao, Y.-H.; Yen, C.-H.; et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J. Transl. Med. 2011, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Vanderbrink, B.A.; Campbell, M.T.; Hile, K.L.; Zhang, H.; Meldrum, D.R.; Meldrum, K.K. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J. Surg. Res. 2011, 168, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- La Manna, G.; Bianchi, F.; Cappuccilli, M.; Cenacchi, G.; Tarantino, L.; Pasquinelli, G.; Valente, S.; Bella, E.D.; Cantoni, S.; Claudia, C.; et al. Mesenchymal stem cells in renal function recovery after acute kidney injury: Use of a differentiating agent in a rat model. Cell Transplant. 2011, 20, 1193–1208. [Google Scholar] [CrossRef]
- Sinha, V.; Vence, L.M.; Salahudeen, A.K. Urinary tubular protein-based biomarkers in the rodent model of cisplatin nephrotoxicity: A comparative analysis of serum creatinine, renal histology, and urinary KIM-1, NGAL, and NAG in the initiation, maintenance, and recovery phases of acute kidney injury. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2013, 61, 564–568. [Google Scholar] [CrossRef]
- Sohotnik, R.; Nativ, O.; Abbasi, A.; Awad, H.; Frajewicki, V.; Bishara, B.; Sukhotnik, I.; Armaly, Z.; Aronson, D.; Heyman, S.N.; et al. Phosphodiesterase-5 inhibition attenuates early renal ischemia-reperfusion-induced acute kidney injury: Assessment by quantitative measurement of urinary NGAL and KIM-1. Am. J. Physiol. Renal Physiol. 2013, 304, F1099–F1104. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, P.Y.; Lee, J.S.; Hwang, S.; Sohn, J.H.; Yoon, Y.; Bae, K.S.; Eom, Y.W. Cultured human skeletal muscle satellite cells exhibit characteristics of mesenchymal stem cells and play anti-inflammatory roles through prostaglandin E2 and hepatocyte growth factors. Cell Biol. Int. 2021, 45, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Uehara, K.; Nozaki, M.; Kobayashi, T.; Terada, S.; Tobita, K.; Fu, F.H.; Huard, J. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am. J. Sports Med. 2011, 39, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Hwang, S.; Hong, J.E.; Jo, M.; Rhee, K.-J.; Kim, S.; Jung, P.Y.; Yoon, Y.; Kang, S.H.; Ryu, H.; et al. Skeletal muscle satellite cell-derived mesenchymal stem cells ameliorate acute alcohol-induced liver injury. Int. J. Med. Sci. 2022, 19, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Osman, Y.; Hamed, S.M.; Barakat, N.M.; Khater, S.; Gabr, M.; Mosbah, A.; Gaballah, M.A.; Shaaban, A. Prophylaxis against renal ischemia-reperfusion injury in canine model: Stem cell approach. Indian J. Urol. IJU 2020, 36, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.; Gkioka, V.; Michalopoulos, E.; Katsimpoulas, M.; Noutsias, M.; Sarri, E.F.; Stavropoulos, C.; Kostakis, A. Advanced Therapy Medicinal Products Challenges and Perspectives in Regenerative Medicine. J. Clin. Med. Res. 2020, 12, 780–786. [Google Scholar] [CrossRef]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wie, Q.; Shen, A.; Fu, Y.; et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef]
- Zhang, J.; He, W.; Zheng, D.; He, Q.; Tan, M.; Jin, J. Exosomal-miR-1184 derived from mesenchymal stem cells alleviates cisplatin-associated acute kidney injury. Mol. Med. Rep. 2021, 24, 795. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, D.H.; Go, H.-K.; Youn, J.; Kim, H.-K.; Jin, R.C.; Miller, R.B.; Kim, D.-H.; Cho, B.S.; Yi, Y.W. Reproducible Large-Scale Isolation of Exosomes from Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Application in Acute Kidney Injury. Int. J. Mol. Sci. 2020, 21, 4774. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavyde, E.; Usas, A.; Pockevicius, A.; Maciulaitis, R. Muscle-Derived Stem/Progenitor Cells Ameliorate Acute Kidney Injury in Rats through the Anti-Apoptotic Pathway and Demonstrate Comparable Effects to Bone Marrow Mesenchymal Stem Cells. Medicina 2024, 60, 63. https://doi.org/10.3390/medicina60010063
Pavyde E, Usas A, Pockevicius A, Maciulaitis R. Muscle-Derived Stem/Progenitor Cells Ameliorate Acute Kidney Injury in Rats through the Anti-Apoptotic Pathway and Demonstrate Comparable Effects to Bone Marrow Mesenchymal Stem Cells. Medicina. 2024; 60(1):63. https://doi.org/10.3390/medicina60010063
Chicago/Turabian StylePavyde, Egle, Arvydas Usas, Alius Pockevicius, and Romaldas Maciulaitis. 2024. "Muscle-Derived Stem/Progenitor Cells Ameliorate Acute Kidney Injury in Rats through the Anti-Apoptotic Pathway and Demonstrate Comparable Effects to Bone Marrow Mesenchymal Stem Cells" Medicina 60, no. 1: 63. https://doi.org/10.3390/medicina60010063
APA StylePavyde, E., Usas, A., Pockevicius, A., & Maciulaitis, R. (2024). Muscle-Derived Stem/Progenitor Cells Ameliorate Acute Kidney Injury in Rats through the Anti-Apoptotic Pathway and Demonstrate Comparable Effects to Bone Marrow Mesenchymal Stem Cells. Medicina, 60(1), 63. https://doi.org/10.3390/medicina60010063





