A Potential Radiomics–Clinical Model for Predicting Failure of Lymph Node Control after Definite Radiotherapy in Locally Advanced Head and Neck Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. CT Imaging and Radiotherapy
2.3. Radiomics Feature Extraction and Clinical Features
2.4. Radiomics Feature Selection
2.5. Statistical Test
3. Results
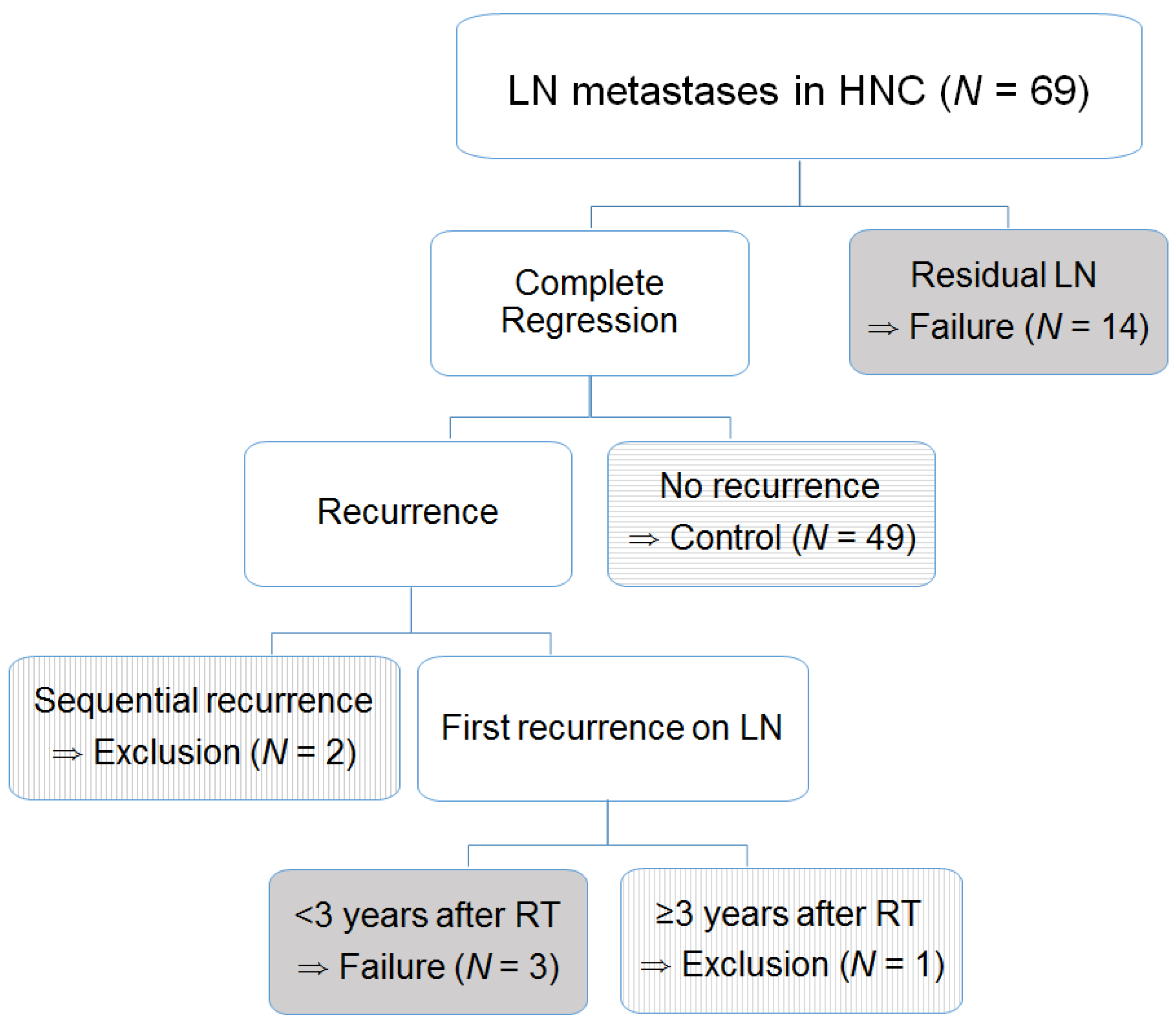
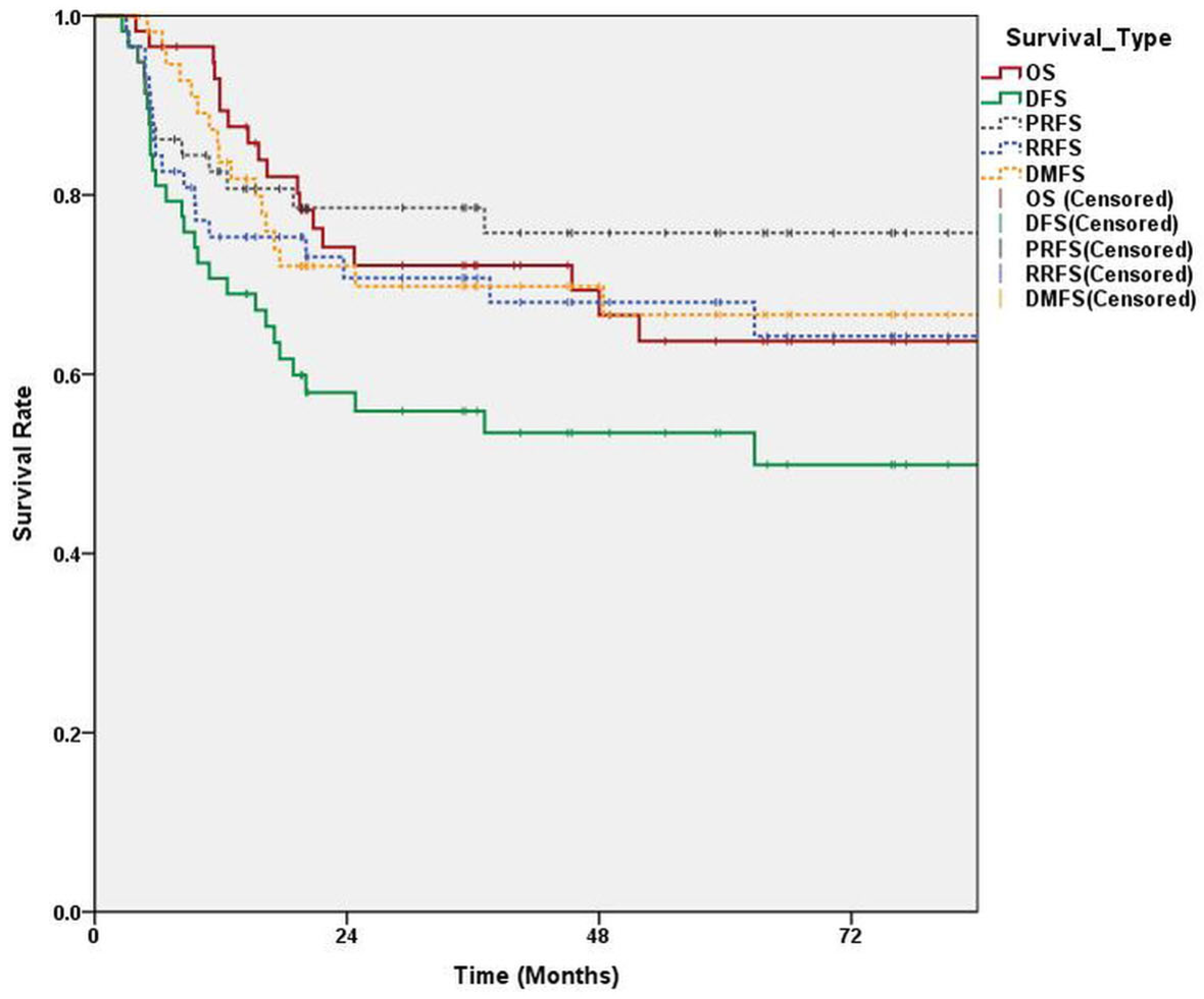
3.1. Survival and Failure of LN Control
3.2. Feature Selection
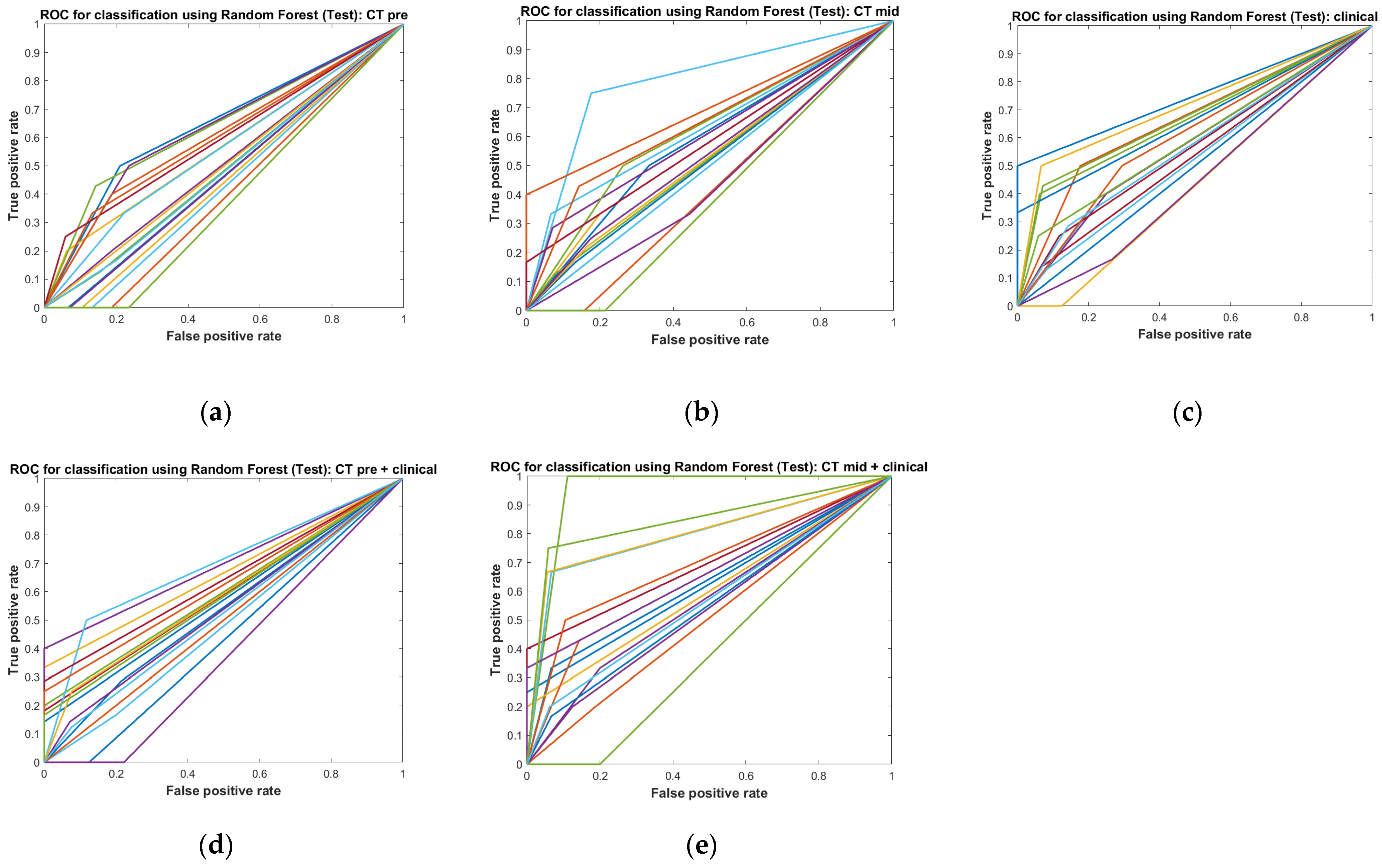
3.3. Performance Test
3.4. Feature Importance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Li, W.F.; Chen, N.Y.; Zhang, N.; Hu, G.Q.; Xie, F.Y.; Sun, Y.; Chen, X.Z.; Li, J.G.; Zhu, X.D.; et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016, 17, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.C.J.; Kelly, J.R.; Park, H.S.; An, Y.; Judson, B.L.; Burtness, B.A.; Husain, Z.A. Patterns of failure in high-metastatic node number human papillomavirus-positive oropharyngeal carcinoma. Oral. Oncol. 2018, 85, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Pagh, A.; Grau, C.; Overgaard, J. Failure pattern and salvage treatment after radical treatment of head and neck cancer. Acta Oncol. 2016, 55, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, J.; Chatterjee, A.; Dora, T.K.; Bose, S.; Goel, A.; Kakade, A.; Saini, A.; Pahwa, S.; Singh, A.; Laskar, S.G.; et al. Recurrence pattern with respect to two different dose fractionations in patients with locally advanced head and neck cancer treated with chemoradiation using image-guided volumetric arc therapy. Head Neck 2022, 44, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Ferreira, S.; Caetano, M. PET/CT in the Evaluation of Hypoxia for Radiotherapy Planning in Head and Neck Tumors: Systematic Literature Review. J. Nucl. Med. Technol. 2021, 49, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Soni, T.P.; Patni, N.; Singh, D.K.; Jakhotia, N.; Gupta, A.K.; Sharma, L.M.; Sharma, S.; Gothwal, R.S. A randomized prospective study comparing acute toxicity, compliance and objective response rate between simultaneous integrated boost and sequential intensity-modulated radiotherapy for locally advanced head and neck cancer. Radiat. Oncol. J. 2021, 39, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maitre, A.; Bourhis, J.; Group, M.-N.C. Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): An update. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, S112–S114. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, L.; Franzese, C.; Fogliata, A.; Franceschini, D.; Navarria, P.; Tomatis, S.; Scorsetti, M. Predicting survival and local control after radiochemotherapy in locally advanced head and neck cancer by means of computed tomography based radiomics. Strahlenther. Onkol. 2019, 195, 805–818. [Google Scholar] [CrossRef]
- Zhai, T.T.; Langendijk, J.A.; van Dijk, L.V.; Halmos, G.B.; Witjes, M.J.H.; Oosting, S.F.; Noordzij, W.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. The prognostic value of CT-based image-biomarkers for head and neck cancer patients treated with definitive (chemo-)radiation. Oral. Oncol. 2019, 95, 178–186. [Google Scholar] [CrossRef]
- Van den Bossche, V.; Zaryouh, H.; Vara-Messler, M.; Vignau, J.; Machiels, J.P.; Wouters, A.; Schmitz, S.; Corbet, C. Microenvironment-driven intratumoral heterogeneity in head and neck cancers: Clinical challenges and opportunities for precision medicine. Drug Resist. Updat. 2022, 60, 100806. [Google Scholar] [CrossRef]
- Zhai, T.T.; Wesseling, F.; Langendijk, J.A.; Shi, Z.; Kalendralis, P.; van Dijk, L.V.; Hoebers, F.; Steenbakkers, R.J.; Dekker, A.; Wee, L.; et al. External validation of nodal failure prediction models including radiomics in head and neck cancer. Oral. Oncol. 2021, 112, 105083. [Google Scholar] [CrossRef]
- Alfieri, S.; Romano, R.; Bologna, M.; Calareso, G.; Corino, V.; Mirabile, A.; Ferri, A.; Bellanti, L.; Poli, T.; Marcantoni, A.; et al. Prognostic role of pre-treatment magnetic resonance imaging (MRI)-based radiomic analysis in effectively cured head and neck squamous cell carcinoma (HNSCC) patients. Acta Oncol. 2021, 60, 1192–1200. [Google Scholar] [CrossRef]
- Rabasco Meneghetti, A.; Zwanenburg, A.; Linge, A.; Lohaus, F.; Grosser, M.; Baretton, G.B.; Kalinauskaite, G.; Tinhofer, I.; Guberina, M.; Stuschke, M.; et al. Integrated radiogenomics analyses allow for subtype classification and improved outcome prognosis of patients with locally advanced HNSCC. Sci. Rep. 2022, 12, 16755. [Google Scholar] [CrossRef]
- Luo, L.M.; Huang, B.T.; Chen, C.Z.; Wang, Y.; Su, C.H.; Peng, G.B.; Zeng, C.B.; Wu, Y.X.; Wang, R.H.; Huang, K.; et al. A Combined Model to Improve the Prediction of Local Control for Lung Cancer Patients Undergoing Stereotactic Body Radiotherapy Based on Radiomic Signature Plus Clinical and Dosimetric Parameters. Front. Oncol. 2021, 11, 819047. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Yu, Y.; Apte, A.P.; Leeman, J.E.; Katabi, N.; Morris, L.; Deasy, J.O.; Chan, T.A.; Lee, N.Y.; Riaz, N.; et al. Radiomic analysis identifies tumor subtypes associated with distinct molecular and microenvironmental factors in head and neck squamous cell carcinoma. Oral. Oncol. 2020, 110, 104877. [Google Scholar] [CrossRef]
- Bagher-Ebadian, H.; Lu, M.; Siddiqui, F.; Ghanem, A.I.; Wen, N.; Wu, Q.; Liu, C.; Movsas, B.; Chetty, I.J. Application of radiomics for the prediction of HPV status for patients with head and neck cancers. Med. Phys. 2020, 47, 563–575. [Google Scholar] [CrossRef]
- Lafata, K.J.; Chang, Y.; Wang, C.; Mowery, Y.M.; Vergalasova, I.; Niedzwiecki, D.; Yoo, D.S.; Liu, J.; Brizel, D.M.; Yin, F. Intrinsic radiomic expression patterns after 20 Gy demonstrate early metabolic response of oropharyngeal cancers. Med. Phys. 2021, 48, 3767–3777. [Google Scholar] [CrossRef]
- Carles, M.; Fechter, T.; Grosu, A.L.; Sorensen, A.; Thomann, B.; Stoian, R.G.; Wiedenmann, N.; Rühle, A.; Zamboglou, C.; Ruf, J.; et al. 18F-FMISO-PET Hypoxia Monitoring for Head-and-Neck Cancer Patients: Radiomics Analyses Predict the Outcome of Chemo-Radiotherapy. Cancers 2021, 13, 3449. [Google Scholar] [CrossRef]
- Berger, T.; Noble, D.J.; Shelley, L.E.A.; McMullan, T.; Bates, A.; Thomas, S.; Carruthers, L.J.; Beckett, G.; Duffton, A.; Paterson, C.; et al. Predicting radiotherapy-induced xerostomia in head and neck cancer patients using day-to-day kinetics of radiomics features. Phys. Imaging Radiat. Oncol. 2022, 24, 95–101. [Google Scholar] [CrossRef]
- Xi, Y.; Ge, X.; Ji, H.; Wang, L.; Duan, S.; Chen, H.; Wang, M.; Hu, H.; Jiang, F.; Ding, Z. Prediction of Response to Induction Chemotherapy Plus Concurrent Chemoradiotherapy for Nasopharyngeal Carcinoma Based on MRI Radiomics and Delta Radiomics: A Two-Center Retrospective Study. Front. Oncol. 2022, 12, 824509. [Google Scholar] [CrossRef] [PubMed]
- Sellami, S.; Bourbonne, V.; Hatt, M.; Tixier, F.; Bouzid, D.; Lucia, F.; Pradier, O.; Goasduff, G.; Visvikis, D.; Schick, U. Predicting response to radiotherapy of head and neck squamous cell carcinoma using radiomics from cone-beam CT images. Acta Oncol. 2022, 61, 73–80. [Google Scholar] [CrossRef] [PubMed]


| Category | Sub-Category | |
|---|---|---|
| Sex | Male: female | 58:11 |
| Age (years) | Median (range) | 55 (26–84) |
| Primary site | Nasopharynx:oropharynx:larynx:oral cavity: hypopharynx | 25:31:3:1:9 |
| Virus status | EBV:HPV:negative:unknown | 21:17:11:20 |
| Smoking | Never, ex-smoker, current smoker | 24:17:28 |
| ≤10:>10 (pack years) | 30:39 | |
| Primary tumor | ||
| T-stage | T1–2: T3–4 | 39:30 |
| Size | ≤2:2.1–4:>4 (cm) | 15:35:19 |
| Lymph node (LN) | ||
| N-Stage | N1:N2–3 | 15: 54 |
| Size | ≤3:3.1–6:>6 | 27:38:4 |
| Multiplicity | ≤2:>2 (lymph node stations) | 29:40 |
| Laterality | Unilateral: bilateral | 33:36 |
| Extra capsular extension | No:yes | 29:40 |
| Central necrosis | No:yes | 27:42 |
| Total radiation dose | <70:≥70 (Gy) | 9:60 |
| Concurrent chemotherapy | No:yes | 2:67 |
| Regression of the largest LN size (long diameter CTmid/CTpre) | Median (range) | 0.762 (0.436–1.250) |
| CTpre | CTmid | Clinical Parameters | CTpre Plus Clinical Parameters | CTmid Plus Clinical Parameters | |
|---|---|---|---|---|---|
| ACC | 0.681 ± 0.069 | 0.698 ± 0.089 | 0.726 ± 0.089 | 0.731 ± 0.100 | 0.790 ± 0.095 |
| AUC | 0.521 ± 0.008 | 0.568 ± 0.093 | 0.593 ± 0.085 | 0.582 ± 0.088 | 0.662 ± 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, S.; Rim, C.H.; Lee, Y.H.; Kwon, S.Y.; Oh, K.H.; Yoon, W.S. A Potential Radiomics–Clinical Model for Predicting Failure of Lymph Node Control after Definite Radiotherapy in Locally Advanced Head and Neck Cancer. Medicina 2024, 60, 92. https://doi.org/10.3390/medicina60010092
Lee S, Park S, Rim CH, Lee YH, Kwon SY, Oh KH, Yoon WS. A Potential Radiomics–Clinical Model for Predicting Failure of Lymph Node Control after Definite Radiotherapy in Locally Advanced Head and Neck Cancer. Medicina. 2024; 60(1):92. https://doi.org/10.3390/medicina60010092
Chicago/Turabian StyleLee, Seunghak, Sunmin Park, Chai Hong Rim, Young Hen Lee, Soon Young Kwon, Kyung Ho Oh, and Won Sup Yoon. 2024. "A Potential Radiomics–Clinical Model for Predicting Failure of Lymph Node Control after Definite Radiotherapy in Locally Advanced Head and Neck Cancer" Medicina 60, no. 1: 92. https://doi.org/10.3390/medicina60010092
APA StyleLee, S., Park, S., Rim, C. H., Lee, Y. H., Kwon, S. Y., Oh, K. H., & Yoon, W. S. (2024). A Potential Radiomics–Clinical Model for Predicting Failure of Lymph Node Control after Definite Radiotherapy in Locally Advanced Head and Neck Cancer. Medicina, 60(1), 92. https://doi.org/10.3390/medicina60010092








