The Neutrophil-to-Lymphocyte Ratio in Patients with Spinal Cord Injury: A Narrative Review Study
Abstract
1. Introduction
Importance and Novelty
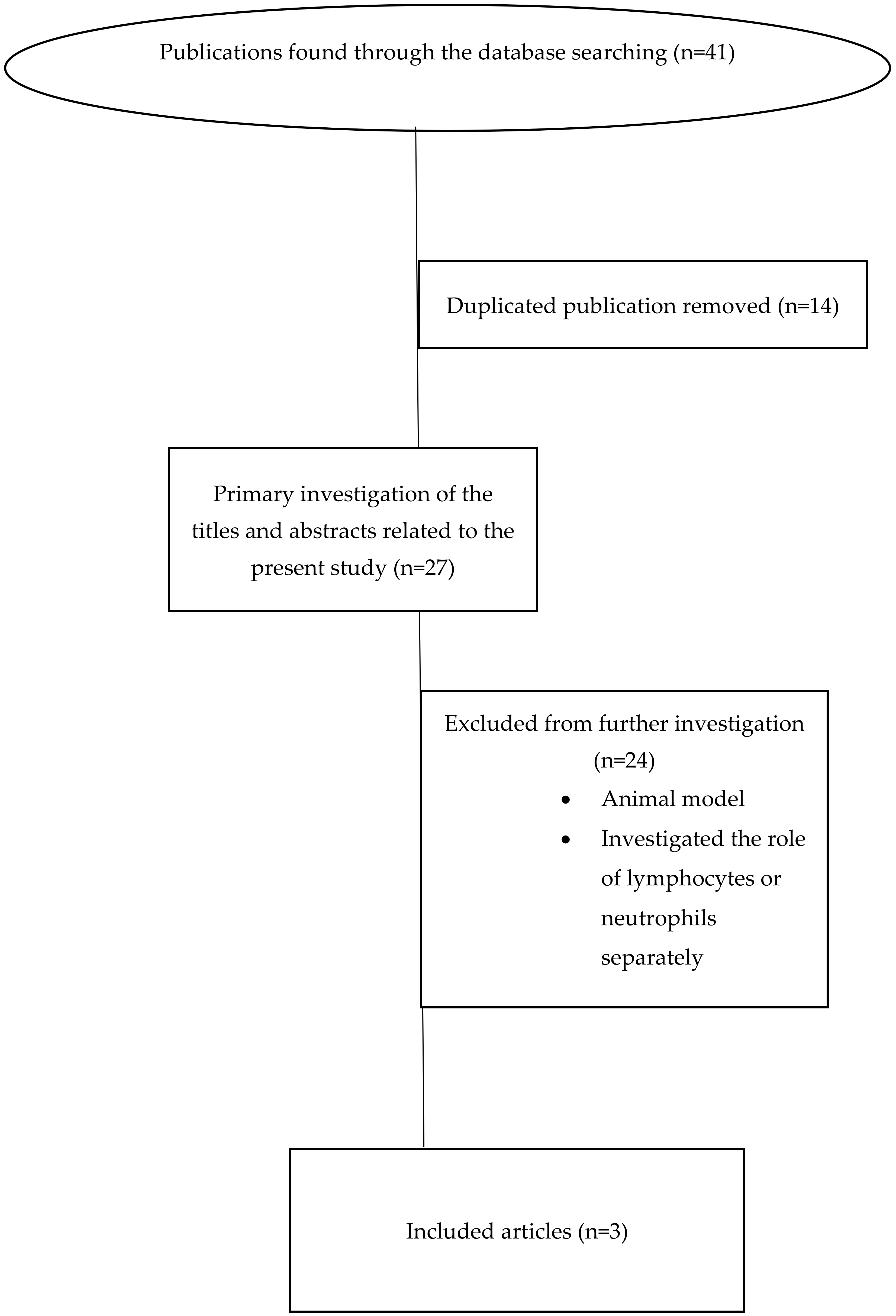
2. Materials and Methods
2.1. Object
2.2. Databases and Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Outcomes
2.5. Spinal Cord Injury
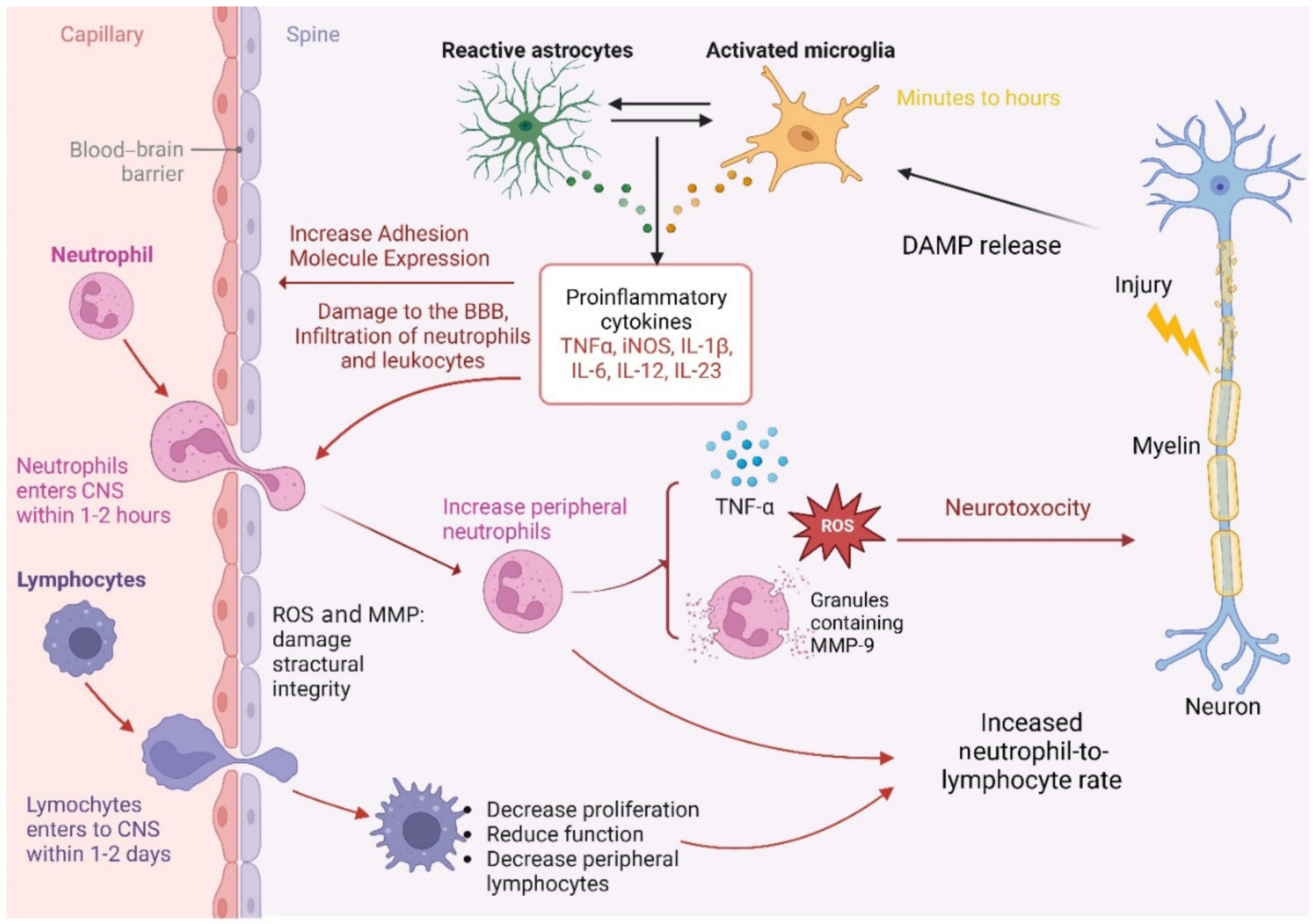
2.6. The Neutrophils’ Role after SCI
2.7. The Lymphocytes’ Role after SCI
2.8. The Role of the Neutrophil-to-Lymphocyte Ratio in SCI
2.9. Pathophysiology of NLR Role Following SCI
3. Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chhabra, H.S.; Sharawat, R.; Vishwakarma, G. In-hospital mortality in people with complete acute traumatic spinal cord injury at a tertiary care center in India—A retrospective analysis. Spinal Cord. 2022, 60, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Kowalski, R.G.; Sciubba, D.M.; Geocadin, R.G. Critical care of traumatic spinal cord injury. J. Intensive Care Med. 2013, 28, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Jogia, T.; Lübstorf, T.; Jacobson, E.; Scriven, E.; Atresh, S.; Nguyen, Q.H.; Liebscher, T.; Schwab, J.M.; Kopp, M.A.; Walsham, J.; et al. Prognostic value of early leukocyte fluctuations for recovery from traumatic spinal cord injury. Clin. Transl. Med. 2021, 11, e272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dilektasli, E.; Inaba, K.; Haltmeier, T.; Wong, M.D.; Clark, D.; Benjamin, E.R.; Lam, L.; Demetriades, D. The prognostic value of neutrophil-to-lymphocyte ratio on mortality in critically ill trauma patients. J. Trauma Acute Care Surg. 2016, 81, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, E.; Majdi, A.; Jangjui, P.; Rahigh Aghsan, S.; Naseri Alavi, S.A. Neutrophil-to-Lymphocyte Ratio and Traumatic Brain Injury: A Review Study. World Neurosurg. 2020, 140, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Lai, S.T.; Du, Z.Y.; Xu, J.; Sun, Y.-R.; Yuan, Q.; Wu, X.; Li, Z.-Q.; Hu, J.; Xie, R. Circulating neutrophil-to-lymphocyte ratio at admission predicts the long-term outcome in acute traumatic cervical spinal cord injury patients. BMC Musculoskelet. Disord. 2020, 21, 548. [Google Scholar] [CrossRef]
- Xue, J.; Huang, W.; Chen, X.; Li, Q.; Cai, Z.; Yu, T.; Shao, B. Neutrophil-to-Lymphocyte Ratio Is a Prognostic Marker in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, J.; Zileli, M.; Sharif, S.Y. Outcomes of Spinal Cord Injury: WFNS Spine Committee Recommendations. Neurospine 2020, 17, 809–819. [Google Scholar] [CrossRef]
- Santos-Lima, B.; Pietronigro, E.C.; Terrabuio, E.; Zenaro, E.; Constantin, G. The role of neutrophils in the dysfunction of central nervous system barriers. Front. Aging Neurosci. 2022, 14, 965169. [Google Scholar] [CrossRef]
- McColl, S.R.; Staykova, M.A.; Wozniak, A.; Fordham, S.; Bruce, J.; Willenborg, D.O. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 1998, 161, 6421–6426. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Li, Z.; Wang, X.Y.; Yi, H. Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front. Immunol. 2018, 9, 2456. [Google Scholar] [CrossRef]
- Fahmi, R.M.; Ramadan, B.M.; Salah, H.; Elsaid, A.F.; Shehta, N. Neutrophil-lymphocyte ratio as a marker for disability and activity in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 51, 102921. [Google Scholar] [CrossRef]
- Akıl, E.; Bulut, A.; Kaplan, İ.; Özdemir, H.H.; Arslan, D.; Aluçlu, M.U. The increase of carcinoembryonic antigen (CEA), high-sensitivity C-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s disease. Neurol. Sci. 2015, 36, 423–428. [Google Scholar] [CrossRef]
- Cordeiro, M.D.; Ilario, E.N.; Abe, D.K.; Carvalho, P.A.; Muniz, D.Q.B.; Sarkis, A.S.; Coelho, R.F.; Guimarães, R.M.; Haddad, M.V.; Nahas, W.C. Neutrophil-to-Lymphocyte Ratio Predicts Cancer Outcome in Locally Advanced Clear Renal Cell Carcinoma. Clin. Genitourin. Cancer 2022, 20, 102–106. [Google Scholar] [CrossRef]
- Lattanzi, S.; Cagnetti, C.; Provinciali, L.; Silvestrini, M. Neutrophil-to-Lymphocyte Ratio Predicts the Outcome of Acute Intracerebral Hemorrhage. Stroke 2016, 47, 1654–1657. [Google Scholar] [CrossRef]
- Afari, M.E.; Bhat, T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: An update. Expert Rev. Cardiovasc. Ther. 2016, 14, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Mputu Mputu, P.; Beauséjour, M.; Richard-Denis, A.; Mac-Thiong, J.M. Early Predictors of Neurological Outcomes After Traumatic Spinal Cord Injury: A Systematic Review and Proposal of a Conceptual Framework. Am. J. Phys. Med. Rehabil. 2021, 100, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Phan, P.; Budhram, B.; Zhang, Q.; Rivers, C.S.; Noonan, V.K.; Plashkes, T.; Wai, E.K.; Paquet, J.; Roffey, D.M.; Tsai, E.; et al. Highlighting discrepancies in walking prediction accuracy for patients with traumatic spinal cord injury: An evaluation of validated prediction models using a Canadian Multicenter Spinal Cord Injury Registry. Spine J. 2019, 19, 703–710. [Google Scholar] [CrossRef]
- Schnell, L.; Fearn, S.; Klassen, H.; Schwab, M.E.; Perry, V.H. Acute inflammatory responses to mechanical lesions in the CNS: Differences between brain and spinal cord. Eur. J. Neurosci. 1999, 11, 3648–3658. [Google Scholar] [CrossRef]
- Schwab, M.E.; Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996, 76, 319–370. [Google Scholar] [CrossRef]
- Hayashi, M.; Ueyama, T.; Nemoto, K.; Tamaki, T.; Senba, E. Sequential mRNA expression for immediate early genes, cytokines, and neutrophils in spinal cord injury. J. Neurotrauma 2000, 17, 203–218. [Google Scholar] [CrossRef]
- Acarin, L.; González, B.; Castellano, B. Neuronal, astroglial, and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur. J. Neurosci. 2000, 12, 3505–3520. [Google Scholar] [CrossRef]
- Hausmann, O. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003, 41, 369–378. [Google Scholar] [CrossRef]
- Carlson, S.L.; Parrish, M.E.; Springer, J.E.; Doty, K.; Dossett, L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998, 151, 77–88. [Google Scholar] [CrossRef]
- Chatzipanteli, K.; Yanagawa, Y.; Marcillo, A.E.; Kraydieh, S.; Yezierski, R.P.; Dietrich, W.D. Post-traumatic hypothermia reduces polymorphnuclear leucocyte accumulation following spinal cord injury in rats. J. Neurotrauma 2000, 17, 321–332. [Google Scholar] [CrossRef]
- Neirinckx, V.; Coste, C.; Franzen, R.; Gothot, A.; Rogister, B.; Wislet, S. Neutrophil contribution to spinal cord injury and repair. J. Neuroinflamm. 2014, 11, 150. [Google Scholar] [CrossRef]
- Taoka, Y.; Okajima, K.; Uchiba, M.; Murakami, K.; Kushimoto, S.; Johno, M.; Naruo, M.; Okabe, H.; Takatsuki, K. Role of neutrophils in spinal cord injury in the rat. Neuroscience 1997, 79, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.P.; Liu, S.; Kubes, P.; Yong, V.W. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J. Neurosci. 2009, 29, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.K.; von der Ohe, M.; Kolling, U.; Altstaedt, J.; Uciechowski, P.; Fleischer, D.; Dalhoff, K.; Ju, X.; Zenke, M.; Heussen, N.; et al. Polymorphonuclear leucocytes selectively produce anti-inflammatory interleukin-1 receptor antagonist and chemokines, but fail to produce pro-inflammatory mediators. Immunology 2006, 119, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Lymphocytes and autoimmunity after spinal cord injury. Exp. Neurol. 2014, 258, 78–90. [Google Scholar] [CrossRef]
- Jones, T.B.; Basso, D.M.; Sodhi, A.; Pan, J.Z.; Hart, R.P.; MacCallum, R.C.; Lee, S.; Whitacre, C.C.; Popovich, P.G. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: Implications for autoimmune vaccine therapy. J. Neurosci. 2002, 22, 2690–2700. [Google Scholar] [CrossRef]
- Hauben, E.; Nevo, U.; Yoles, E.; Moalem, G.; Agranov, E.; Mor, F.; Akselrod, S.; Neeman, M.; Cohen, I.R.; Schwartz, M. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet 2000, 355, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, D.P.; Popovich, P.G. B cells and autoantibodies: Complex roles in CNS injury. Trends Immunol. 2010, 31, 332–338. [Google Scholar] [CrossRef]
- Ren, X.; Akiyoshi, K.; Dziennis, S.; Vandenbark, A.A.; Herson, P.S.; Hurn, P.D.; Offner, H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J. Neurosci. 2011, 31, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
- Taoka, Y.; Okajima, K. Role of leukocytes in spinal cord injury in rats. J. Neurotrauma 2000, 17, 219–229. [Google Scholar] [CrossRef]
- Farooque, M.; Isaksson, J.; Olsson, Y. Improved recovery after spinal cord trauma in ICAM-1 and P-selectin knockout mice. Neuroreport 1999, 10, 131–134. [Google Scholar] [CrossRef]
- Gris, D.; Marsh, D.R.; Oatway, M.A.; Chen, Y.; Hamilton, E.F.; Dekaban, G.A.; Weaver, L.C. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. 2004, 24, 4043–4051. [Google Scholar] [CrossRef]
- van den Berg, M.E.; Castellote, J.M.; Mahillo-Fernandez, I.; de Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology 2010, 34, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Du, Z.Y.; Yuan, Q.; Yu, J.; Sun, Y.R.; Wu, X.; Li, Z.-Q.; Wu, X.-H.; Hu, J. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Predicting the 6-Month Outcome of Patients with Traumatic Brain Injury: A Retrospective Study. World Neurosurg. 2019, 124, e411–e416. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.C.; Krassioukov, A.V.; Fehlings, M.G. Hematologic abnormalities within the first week after acute isolated traumatic cervical spinal cord injury: A case-control cohort study. Spine 2006, 31, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Riegger, T.; Conrad, S.; Liu, K.; Schluesener, H.J.; Adibzahdeh, M.; Schwab, J.M. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur. J. Neurosci. 2007, 25, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Prüss, H.; Tedeschi, A.; Thiriot, A.; Lynch, L.; Loughhead, S.M.; Stutte, S.; Mazo, I.B.; Kopp, M.A.; Brommer, B.; Blex, C.; et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 2017, 20, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Salehpour, F.; Aghazadeh, J.; Mirzaei, F.; Naseri Alavi, S.A. Riluzole Can Improve Sensory and Motor Function in Patients with Acute Spinal Cord Injury. Asian J. Neurosurg. 2018, 13, 656–659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salehpour, F.; Bazzazi, A.M.; Aghazadeh, J.; Hasanloei, A.V.; Pasban, K.; Mirzaei, F.; Naseri Alavi, S.A. What do You Expect from Patients with Severe Head Trauma? Asian J. Neurosurg. 2018, 13, 660–663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aghazadeh, J.; Samadi Motlagh, P.; Salehpour, F.; Meshkini, A.; Fatehi, M.; Mirzaei, F.; Naseri Alavi, S.A. Effects of Atorvastatin in Patients with Acute Spinal Cord Injury. Asian Spine J. 2017, 11, 903–907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, W.; Mao, Z.; Wang, Z.; Zhu, H.; Zhao, Y.; Zhang, Z.; Zeng, Y.; Li, M. Diagnostic and Predictive Value of Novel Inflammatory Markers of the Severity of Acute Traumatic Spinal Cord Injury: A Retrospective Study. World Neurosurg. 2023, 171, e349–e354. [Google Scholar] [CrossRef] [PubMed]
- Naseri Alavi, S.A.; Kobets, A.J.; Rezakhah, A.; Habibi, M.A.; Rezvani, K.; Sigaroudi, F.E. Can Neutrophil to Lymphocyte Ratio predict early outcome in patients with Spinal Cord Injury? World Neurosurg. 2023, 180, e243–e249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Q.; Luo, X.; Hong, J.; Pan, K.; Lin, X.; Liu, X.; Zhou, L.; Wang, H.; Xu, Y.; et al. Neutrophil-to-Lymphocyte Ratio Positively Correlates to Age in Healthy Population. J. Clin. Lab. Anal. 2015, 29, 437–443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trivedi, A.; Olivas, A.D.; Noble-Haeusslein, L.J. Inflammation and Spinal Cord Injury: Infiltrating Leukocytes as Determinants of Injury and Repair Processes. Clin. Neurosci. Res. 2006, 6, 283–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Reference (Year) | Type of Study | Type of Trauma | Number of Subjects | Mean Age _ SD (y) or Range | Sex (M/F) | NL Ratio | Outcome | Additional Data Based on ROC-AUC |
|---|---|---|---|---|---|---|---|---|
| Zhao et al. (2020) [9] | Retrospective | Mild | 377 (human) | 46.05 ± 17.93 | 212/156 | 13.28 ± 11.46 | The NLR is an independent predictor of outcomes in the 6-month follow-up period in patients with acute cervical SCI | N/A |
| Zhou et al. (2023) [49] | Retrospective | Mild, Moderate, Severe | 526 (human) | 52.2 ± 12.5 | 439/87 | N/A | The NLR showed higher diagnostic performance than the platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune-inflammatory index | Sensitivity = 0.79, specificity = 0.57 |
| Naseri Alavi et al. (2023) [50] | Retrospective | Mild, Moderate, Severe | 536 (human) | 40.93 ± 12.77 (min = 18, max = 64) | 399/137 | 8.43 ± 8.34 (min = 0.68, max = 70.69) | There was no significant relationship between neutrophil counts at admission and outcome following SCI; however, these counts decreased significantly over time. | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseri Alavi, S.A.; Habibi, M.A.; Naseri Alavi, S.H.; Zamani, M.; Kobets, A.J. The Neutrophil-to-Lymphocyte Ratio in Patients with Spinal Cord Injury: A Narrative Review Study. Medicina 2024, 60, 1567. https://doi.org/10.3390/medicina60101567
Naseri Alavi SA, Habibi MA, Naseri Alavi SH, Zamani M, Kobets AJ. The Neutrophil-to-Lymphocyte Ratio in Patients with Spinal Cord Injury: A Narrative Review Study. Medicina. 2024; 60(10):1567. https://doi.org/10.3390/medicina60101567
Chicago/Turabian StyleNaseri Alavi, Seyed Ahmad, Mohammad Amin Habibi, Seyed Hamed Naseri Alavi, Mahsa Zamani, and Andrew J. Kobets. 2024. "The Neutrophil-to-Lymphocyte Ratio in Patients with Spinal Cord Injury: A Narrative Review Study" Medicina 60, no. 10: 1567. https://doi.org/10.3390/medicina60101567
APA StyleNaseri Alavi, S. A., Habibi, M. A., Naseri Alavi, S. H., Zamani, M., & Kobets, A. J. (2024). The Neutrophil-to-Lymphocyte Ratio in Patients with Spinal Cord Injury: A Narrative Review Study. Medicina, 60(10), 1567. https://doi.org/10.3390/medicina60101567






