Cardiac Magnetic Resonance Speckle Tracking Analysis of Right Ventricle Function in Myocarditis with Preserved Right Ventricular Ejection Fraction
Abstract
1. Introduction
2. Methods
2.1. Patient Selection and Exclusions
2.2. Diagnostic Criteria for Myocarditis
2.3. Statistics
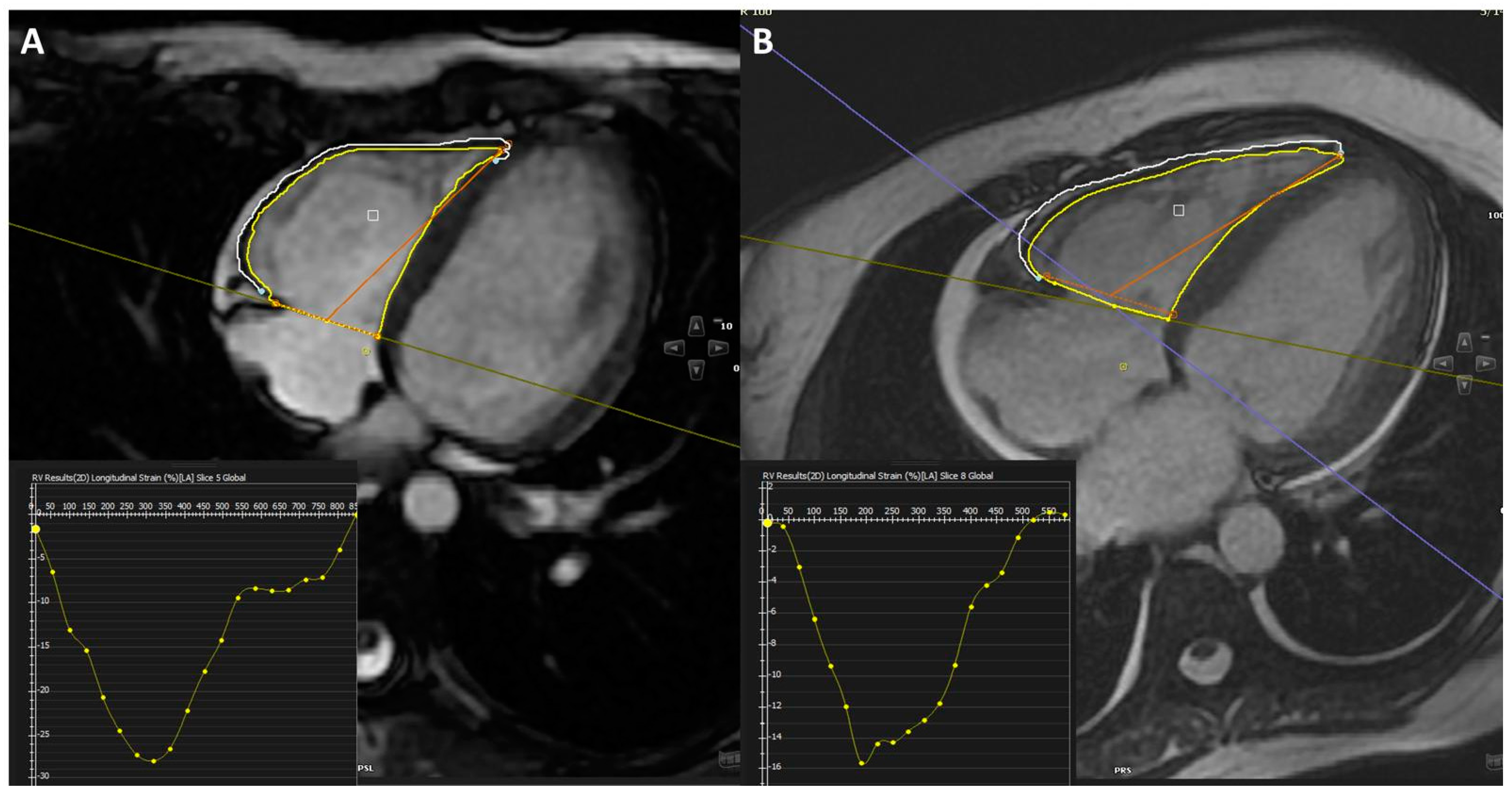
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Aretz, H.T.; Billingham, M.E.; Edwards, W.D.; Factor, S.M.; Fallon, J.T.; Fenoglio, J.J.J.; Olsen, E.G.; Schoen, F.J. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1987, 1, 3–14. [Google Scholar] [PubMed]
- Drory, Y.; Turetz, Y.; Hiss, Y.; Lev, B.; Fisman, E.Z.; Pines, A.; Kramer, M.R. Sudden unexpected death in persons <40 years of age. Am. J. Cardiol. 1991, 68, 1388–1392. [Google Scholar] [PubMed]
- Gaizauskiene, K.; Leketaite, K.; Glaveckaite, S.; Valeviciene, N. Diagnostic Value of Cardiovascular Magnetic Resonance T1 and T2 Mapping in Acute Myocarditis: A Systematic Literature Review. Medicina 2024, 60, 1162. [Google Scholar] [CrossRef]
- Neagu, O.; Chirică, V.; Luca, L.; Bosa, M.; Tița, A.; Ceaușu, M.C. Novel Immunohistochemical and Morphological Approaches in a Retrospective Study of Post-Mortem Myocarditis. Medicina 2024, 60, 1312. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Negri, F.; Luca ADe Todiere, G.; Bianco, F.; Barison, A.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lanzillo, C.; et al. Role of right ventricular involvement in acute myocarditis, assessed by cardiac magnetic resonance. Int. J. Cardiol. 2018, 271, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef]
- Pankuweit, S.; Maisch, B. Ätiologie, Diagnose, Management und Therapie der Myokarditis. Positionspapier der ESC Working Group on Myocardial and Pericardial Diseases [Etiology, diagnosis, management, and treatment of myocarditis. Position paper from the ESC Working Group on Myocardial and Pericardial Diseases]. Herz 2013, 38, 855–861. [Google Scholar]
- Di Bella, G.; Siciliano, V.; Aquaro, G.D.; De Marchi, D.; Rovai, D.; Carerj, S.; Molinaro, S.; Lombardi, M.; Pingitore, A. Right ventricular dysfunction: An independent and incremental predictor of cardiac deaths late after acute myocardial infarction. Int. J. Cardiovasc. Imaging 2015, 31, 379–387. [Google Scholar] [CrossRef]
- Meindl, C.; Paulus, M.; Poschenrieder, F.; Zeman, F.; Maier, L.S.; Debl, K. Patients with acute myocarditis and preserved systolic left ventricular function: Comparison of global and regional longitudinal strain imaging by echocardiography with quantification of late gadolinium enhancement by CMR. Clin. Res. Cardiol. 2021, 110, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- German Houard, L.; Militaru, S.; Tanaka, K.; Pasquet, A.; Vancraeynest, D.; Vanoverschelde, J.-L.; Pouleur, A.-C.; Gerber, B.L. Test–retest reliability of left and right ventricular systolic function by new and conventional echocardiographic and cardiac magnetic resonance parameters. Eur. Heart J. -Cardiovasc. Imaging 2020, 22, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Obrist, S.J.; Erne, S.A.; Stark, A.W.; Marggraf, M.; Kaneko, K.; Guensch, D.P.; Huber, A.T.; Greulich, S.; Aghayev, A.; et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc. Imaging 2020, 13, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, H.; Pan, J.; Shu, J.; Hu, Y.; Yu, R. Performance of cardiovascular magnetic resonance strain in patients with acute myocarditis. Cardiovasc. Diagn. Ther. 2020, 10, 725–737. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeong, Y.J.; Lee, G.; Lee, N.K.; Lee, H.W.; Kim, J.Y.; Choi, B.-S.; Choo, K.S. Predictive Value of Cardiac Magnetic Resonance Imaging-Derived Myocardial Strain for Poor Outcomes in Patients with Acute Myocarditis. Korean J. Radiol. 2017, 18, 643–654. [Google Scholar] [CrossRef]
- Sanders, J.L.; Koestenberger, M.; Rosenkranz, S.; Maron, B.A. Right ventricular dysfunction and long-term risk of death. Cardiovasc. Diagn. Ther. 2020, 10, 1646–1658. [Google Scholar] [CrossRef]
- Mattei, A.; Strumia, A.; Benedetto, M.; Nenna, A.; Schiavoni, L.; Barbato, R.; Mastroianni, C.; Giacinto, O.; Lusini, M.; Chello, M.; et al. Perioperative Right Ventricular Dysfunction and Abnormalities of the Tricuspid Valve Apparatus in Patients Undergoing Cardiac Surgery. J. Clin. Med. 2023, 12, 7152. [Google Scholar] [CrossRef]
- Khanna, S.; Gan, G.; Gupta, K.; Khan, W.; Bhat, A.; Chen, H.; Tan, T. Characterisation of Right Ventricular Size and Systolic Function in a Cohort of Myocarditis Patients with Normal LVEF. Heart Lung Circ. 2019, 28, S240–S241. [Google Scholar] [CrossRef]
- Luetkens, J.; Petry, P.; Kuetting, D.; Dabir, D.; Schmeel, F.; Homsi, R.; Schild, H.; Thomas, D. Left and right ventricular strain in the course of acute myocarditis: A cardiovascular magnetic resonance study. RöFo-Fortschritte Auf Dem Geb. Der Röntgenstrahlen Und Der Bild. Verfahr. 2018, 190, 722–732. [Google Scholar] [CrossRef]
- Di Bella, G.; Carerj, S.; Recupero, A.; Donato, R.; Pugliatti, P.; Falanga, G.; Pedri, S.; Vizzari, G.; Campisi, M.; Zito, C.; et al. Left ventricular endocardial longitudinal dysfunction persists after acute myocarditis with preserved ejection fraction. Echocardiography 2018, 35, 1966–1973. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Zhou, Z.; Jiang, H.; Yan, Z.; Tao, X.; Li, H.; Xu, L. Cardiac involvement in COVID-19 patients: Mid-term follow up by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Toia, P.; Maffei, E.; Mantini, C.; Runza, G.; La Grutta, L.; Grassedonio, E.; Guaricci, A.; Punzo, B.; Cavaliere, C.; Cademartiri, F. Cardiac Magnetic Resonance with Delayed Enhancement of the Right Ventricle in patients with Left Ventricle primary involvement: Diagnosis and evaluation of functional parameters. Acta Biomed. 2022, 93, e2022023. [Google Scholar]
- Manautou, L.; Jerjes-Sanchez, C.; Meraz, M.; Perez-Garcia, L.F.; Diaz-Cid, A.; de la Peña-Almaguer, E.; Avila, C.; Sanchez, L. Myopericarditis with predominantly right ventricular involvement with normal B-type natriuretic peptide and cardiac tamponade as the initial manifestation of systemic lupus erythematosus. Lupus 2014, 23, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Manca, P.; Cannatà, A.; Nuzzi, V.; Bromage, D.I.; Varrà, G.G.; Rossi, M.; Ferro, M.D.; Paldino, A.; Gigli, M.; Barbati, G.; et al. Prevalence and Evolution of Right Ventricular Dysfunction Among Different Genetic Backgrounds in Dilated Cardiomyopathy. Can. J. Cardiol. 2021, 37, 1743–1750. [Google Scholar] [CrossRef]
| Parameters | Myocarditis Group (n = 28) | Control Group (n = 27) | p |
|---|---|---|---|
| Age (y) | 35.1 ± 12.2 | 34.2 ± 9.3 | 0.787 |
| Gender (f) (n, %) | 9 (32.2) | 13 (48.1) | 0.277 |
| Conventional coronary angiography (n, %) | 23 (82.1) | - | - |
| Coronary angiography with computed tomography (n, %) | 5 (17.9) | - | - |
| Pericardial effusion (n, %) | 18 (64.3) | - | - |
| İndication for CMR | |||
| Chest pain (n, %) | 20 (71.4) | 2 (7.4) | <0.001 |
| Dyspnea (n, %) | 4 (14.3) | - | - |
| Palpitation (n, %) | 4 (14.3) | 16 (59.3) | <0.001 |
| COVID-19 vaccination | 3 (10.7) | - | - |
| Suspected cardiac pathologies (n, %) | - | 4 (14.8) | - |
| Suspected cardiac involvement in arrhythmic disorders (n, %) | - | 3 (11.1) | - |
| Family history of dilated cardiomyopathy (n, %) | - | 2 (7.4) | - |
| Parameters | Myocarditis Group (n = 28) | Control Group (n = 27) | p |
|---|---|---|---|
| Left ventricle | |||
| LV EF (%) | 56.6 ± 10.6 | 62.1 ± 2.6 | 0.035 |
| Septal thickness (mm) | 8 ± 2.6 | 8.7 ± 2.1 | 0.299 |
| LV posterior wall thickness (mm) | 7.3 ± 1.2 | 7.1 ± 1.6 | 0.537 |
| LV EDD (mm) | 50.9 ± 7.6 | 47.9 ± 3.9 | 0.106 |
| LV ESD (mm) | 36.8 ± 8.1 | 33.1 ± 3.8 | 0.069 |
| MAPSE (mm) | 12.2 ± 4.8 | 14.2 ± 2.2 | 0.096 |
| RA area (cm2) | 20.2 ± 3.8 | 20.8 ± 3.6 | 0.570 |
| LA area (cm2) | 19.8 ± 5.7 | 18.5 ± 5.5 | 0.446 |
| LV EDVi (mL/m2) | 85.9 ± 27 | 75.2 ± 12.2 | 0.088 |
| LV ESVi (mL/m2) | 39.3 ± 25.7 | 29 ± 6.3 | 0.096 |
| Right ventricle | |||
| RV EF (%) | 56 ± 4.3 | 57.7 ± 3.2 | 0.129 |
| TAPSE (mm) | 21.2 ± 5 | 20.8 ± 4.2 | 0.775 |
| RV EDV (mL) | 77.9 ± 15.4 | 75.3 ± 15.1 | 0.568 |
| RV ESV (mL) | 35 ± 8.1 | 31.3 ± 7.8 | 0.140 |
| RV strain (%) | 18.4 ± 5.4 | 21.8 ± 2.8 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özden, Ö.; Ünlü, S.; Şahin, A.A.; Barutçu, A.; Gövdeli, E.A.; Sherif, S.A.; Papadopoulos, K.; Bingöl, G.; Kılıç, I.D.; Özmen, E.; et al. Cardiac Magnetic Resonance Speckle Tracking Analysis of Right Ventricle Function in Myocarditis with Preserved Right Ventricular Ejection Fraction. Medicina 2024, 60, 1569. https://doi.org/10.3390/medicina60101569
Özden Ö, Ünlü S, Şahin AA, Barutçu A, Gövdeli EA, Sherif SA, Papadopoulos K, Bingöl G, Kılıç ID, Özmen E, et al. Cardiac Magnetic Resonance Speckle Tracking Analysis of Right Ventricle Function in Myocarditis with Preserved Right Ventricular Ejection Fraction. Medicina. 2024; 60(10):1569. https://doi.org/10.3390/medicina60101569
Chicago/Turabian StyleÖzden, Özge, Serkan Ünlü, Ahmet Anıl Şahin, Ahmet Barutçu, Elif Ayduk Gövdeli, Sara Abou Sherif, Konstantinos Papadopoulos, Gülsüm Bingöl, Ismail Doğu Kılıç, Emre Özmen, and et al. 2024. "Cardiac Magnetic Resonance Speckle Tracking Analysis of Right Ventricle Function in Myocarditis with Preserved Right Ventricular Ejection Fraction" Medicina 60, no. 10: 1569. https://doi.org/10.3390/medicina60101569
APA StyleÖzden, Ö., Ünlü, S., Şahin, A. A., Barutçu, A., Gövdeli, E. A., Sherif, S. A., Papadopoulos, K., Bingöl, G., Kılıç, I. D., Özmen, E., Seçkin Göbüt, Ö., Landra, F., Cameli, M., & Göktekin, Ö. (2024). Cardiac Magnetic Resonance Speckle Tracking Analysis of Right Ventricle Function in Myocarditis with Preserved Right Ventricular Ejection Fraction. Medicina, 60(10), 1569. https://doi.org/10.3390/medicina60101569










