Magnetic Resonance Imaging of Temporomandibular Joint and Aortic Root Score in Fibrillinopathies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Methods
2.3.1. Systemic Score
| Sign | Points |
| Wrist and thumb sign | 3 |
| Wrist or thumb sign | 1 |
| Pectus carinatum deformity | 2 |
| Pectus excavatum or chest asymmetry | 1 |
| Hindfoot deformity | 2 |
| Plain pes planus | 1 |
| Pneumothorax | 2 |
| Dural ectasia | 2 |
| Protrusio acetabuli | 2 |
| Reduced upper/lower skeleton and increased arm/height and no severe scoliosis | 1 |
| Scoliosis or thoracolumbar kyphosis | 1 |
| Reduced elbow extension | 1 |
| Facial features (3/5): dolichocephaly, enophtalmos, downslanting palpebral fissures, malar hyoplasia, retrognathia | 1 |
| Skin striae | 1 |
| Myopia > 3 diopters | 1 |
| Mitral valve prolapse | 1 |
2.3.2. Transthoracic Echocardiography
2.3.3. Fibrillinopathy Diagnosis
2.3.4. TMDs Diagnosis
2.3.5. Treatment
2.3.6. Job Strain Score in Employees with Fibrillinopathies
2.3.7. Statistical Analysis
3. Results
3.1. The Clinical Characteristics of the Entire Sample
3.2. The Relationship between TMDs, and Z Score
3.3. The Predictive Value of TMDs for Z Score
- R = 0.894, a powerful correlation (0.7 ≤ R ≤ 1) between DD and Z score
- R-square = 0.799, and Adjusted R-square = 0.794. All three of these parameters: R, R-square, and Adjusted R-square, confirmed that the statistical model explained the clinical supposition very well.
3.4. Correlation between Echocardiographic Parameters
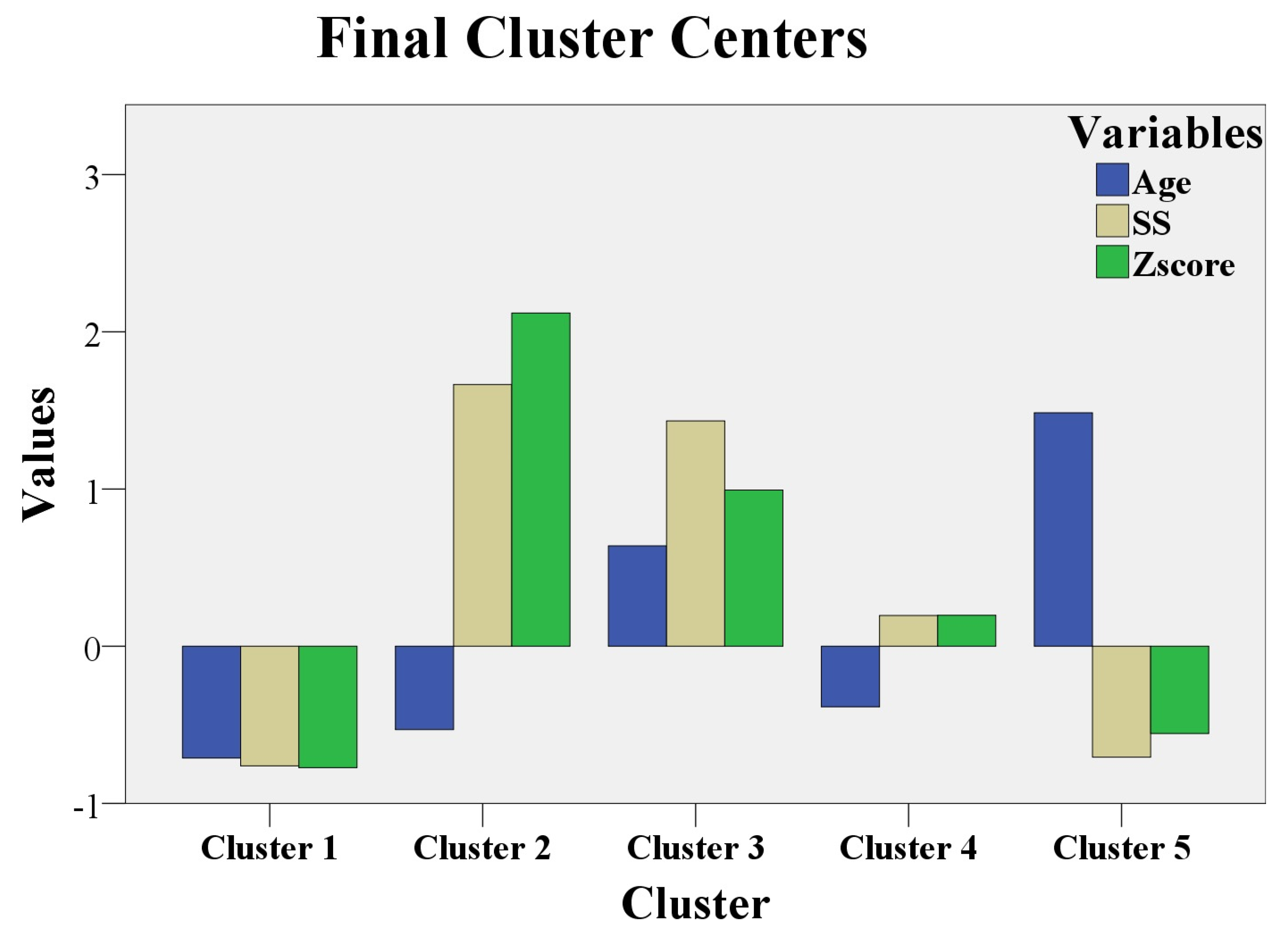
3.5. The Clustering Analysis
- Cluster 1 had 17 patients with MLSF and 14 patients with MVPS. Low values for age, Z score, and SS were noticed in this cluster.
- Cluster 2 had 8 patients with MS. Low values for age, but the maximum values for Z score and for SS, were registered in this cluster.
- Cluster 3 had 13 patients with MS. High values for age (above 21 years), high values for Z score, and for SS (but not as high as in cluster 2) were noticed in this cluster.
- Cluster 4 had 15 patients with MASS. Low values for age (below 21 years) and middle levels for Z score and for SS were revealed in this cluster.
- Cluster 5 had 1 patient with MASS, 7 patients with MLSF, and 9 patients with MVPS. They were the “eldest” patients (the highest values for age); low values for Z score and SS were registered in this cluster.
- MLSF and MVPS (cluster 1 and cluster 5) had the lowest Z scores and SS, independent of age values. Figure 2 shows that Z score and SS in cluster 1 and cluster 5 were represented below 0 (mean = 0); the meaning of these results is that Z- score and SS had the lowest values for MLSF and MVPS patients. Age was below 0 (below mean age = 21 years) in cluster 1, with the youngest patients from the study. In cluster 5, age exceeded 1 (standard deviation = 1), and the patients were “the eldest” from the study (above mean age = 21 years). MLSF and MVPS patients were the youngest (cluster 1) and ‘’the eldest” (cluster 5) patients of our study.
- The majority of MASS patient (15 of 16 MASS patients, 93%) had average levels of Z score and SS. MASS had a moderate expression in our study and the phenotypic findings were defined early, during childhood, or teenage; the majority of MASS, 93%, were aged below 21 years in cluster 4.
- MS patients with the maximum values for Z score, and maximum values for SS were children, or teenaged (cluster 2). These young MS patients (age is below 0, so patient age was below 21 years) had the most severe phenotypic expression of the fibrillinopathy. The other MS patients from cluster 3 had high values (but not as high as in cluster 2) for Z score and for SS; the 3thid cluster had “elderly” MS patients, aged above 21 years. This last category of MS patients (cluster 3) had a severe form of the disease, but not as severe as the first MS category (cluster 2). The youngest MS patients (cluster 2) had the worst prognosis in our study (the highest values for Z score, above 2, and the highest values for SS, above 1.5). The “eldest” MS patients had a severe prognosis, but not as severe as the youngest MS patients (high values for Z score and for SS in cluster 3, but not as high as in cluster 2).
3.6. SWWS Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietz, H.; Adam, M.P.; Feldman, J.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.H.; Gripp, K.W.; Amemiya, A. FBN1-Related Marfan Syndrome; GeneReviews®; University of Washington: Seattle, WA, USA, 2022. [Google Scholar] [PubMed]
- Delwarde, C.; Capoulade, R.; Mérot, J.; Le Scouarnec, S.; Bouatia-Naji, N.; Yu, M.; Huttin, O.; Selton-Suty, C.; Sellal, J.M.; Piriou, N.; et al. Genetics and pathophysiology of mitral valve prolapse. Front. Cardiovasc. Med. 2023, 10, 1077788. [Google Scholar] [CrossRef] [PubMed]
- Judge, D.P.; Markwald, R.R.; Hagège, A.A.; Levine, R.A. Translational research on the mitral valve: From developmental mechanisms to new therapies. J. Cardiovasc. Transl. Res. 2011, 4, 699–701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.Y.; Martin, N.; Hsia, E.C.; Pyeritz, R.E.; Albert, D.A. Management of Aortic Disease in Marfan Syndrome: A Decision Analysis. Arch. Intern. Med. 2005, 165, 749–755. [Google Scholar] [CrossRef]
- Singh, J.; Wanjari, A. Cardiac Complications in Marfan Syndrome: A Review. Cureus 2022, 14, e29800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Marelli, S.; Micaglio, E.; Taurino, J.; Salvi, P.; Rurali, E.; Perrucci, G.L.; Dolci, C.; Udugampolage, N.S.; Caruso, R.; Gentilini, D.; et al. Marfan Syndrome: Enhanced Diagnostic Tools and Follow-up Management Strategies. Diagnostics 2023, 13, 2284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coelho, S.G.; Almeida, A.G. Marfan syndrome revisited: From genetics to the clinic. Rev. Port. Cardiol. 2020, 39, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.C.; Tran, J.R.; Bektas, A. Marfan’s syndrome. Lancet 2005, 366, 1978–1981. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.G.; Pyeritz, R.E. Medical management of Marfan syndrome. Circ 2008, 117, 2802–2813. [Google Scholar] [CrossRef] [PubMed]
- Klemenzdottir, E.O.; Arnadottir, G.A.; Jensson, B.O. A population-based survey of FBN1 variants in Iceland reveals underdiagnosis of Marfan syndrome. Eur. J. Hum. Genet 2024, 32, 44–51. [Google Scholar] [CrossRef]
- Chan, Y.C.; Ting, C.W.; Ho, P.; Poon, J.T.; Cheung, G.C.; Cheng, S.W. Ten-year epidemiological review of in-hospital patients with Marfan syndrome. Ann. Vasc. Surg. 2008, 22, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Braverman, A.C.; De Backer, J.; Morris, S.A.; Boileau, C.; Maumenee, I.H.; Jondeau, G.; Evangelista, A.; Pyeritz, R.E. Marfan syndrome. Nat. Rev. Dis. Primers 2021, 7, 64, Erratum in Nat. Rev. Dis. Primers. 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stheneur, C.; Tubach, F.; Jouneaux, M.; Roy, C.; Benoist, G.; Chevallier, B.; Boileau, C.; Jondeau, G. Study of phenotype evolution during childhood in Marfan syndrome to improve clinical recognition. Genet Med. 2014, 16, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.L.; Walker, B.A.; Halpern, B.L.; Kuzma, J.W.; McKusick, V.A. Life expectancy and causes of death in the Marfan syndrome. N. Engl. J. Med. 1972, 286, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.F.; Mano, T.B.; Rito, T.; Branco, L.M.; Fragata, J.; Martins, J.D.; Ferreira, R.C.; Sousa, L. Long term follow-up of Marfan Syndrome—Experience of an adult congenital heart disease centre. Am. J. Cardiovasc. Dis. 2022, 12, 92–101. [Google Scholar] [PubMed] [PubMed Central]
- Sheikhzadeh, S.; De Backer, J.; Gorgan, N.R.; Rybczynski, M.; Hillebrand, M.; Schüler, H.; Bernhardt, A.M.; Koschyk, D.; Bannas, P.; Keyser, B.; et al. The main pulmonary artery in adults: A controlled multicenter study with assessment of echocardiographic reference values, and the frequency of dilatation and aneurysm in Marfan syndrome. Orphanet. J. Rare Dis. 2014, 9, 203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campens, L.; Renard, M.; Trachet, B. Intrinsic cardiomyopathy in Marfan syndrome: Results from in- vivo and ex-vivo studies of the Fbn1C1039G/+ model and longitudinal findings in humans. Pediatr. Res. 2015, 78, 256–263. [Google Scholar] [CrossRef]
- de Witte, P.; Aalberts, J.J.; Radonic, T.; Timmermans, J.; Scholte, A.J.; Zwinderman, A.H.; Mulder, B.J.; Groenink, M.; van den Berg, M.P. Intrinsic biventricular dysfunction in Marfan syndrome. Heart 2011, 97, 2063–2068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rybczynski, M.; Mir, T.S.; Sheikhzadeh, S.; Bernhardt, A.M.; Schad, C.; Treede, H.; Veldhoen, S.; Groene, E.F.; Kühne, K.; Koschyk, D.; et al. Frequency and age-related course of mitral valve dysfunction in the Marfan syndrome. Am. J. Cardiol. 2010, 106, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- von Kodolitsch, Y.; Demolder, A.; Girdauskas, E.; Kaemmerer, H.; Kornhuber, K.; Muino Mosquera, L.; Morris, S.; Neptune, E.; Pyeritz, R.; Rand-Hendriksen, S.; et al. Features of Marfan syndrome not listed in the Ghent nosology—The dark side of the disease. Expert Rev. Cardiovasc. Ther. 2019, 17, 883–915. [Google Scholar] [CrossRef] [PubMed]
- Stark, V.C.; Olfe, J.; Pesch, J.; Tahir, E.; Weinrich, J.M.; Wiegand, P.; Kozlik-Feldmann, R.; von Kodolitsch, Y.; Mir, T.S. Tricuspid valve prolapse as an early predictor for severe phenotype in children with Marfan syndrome. Acta Paediatr. 2022, 111, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.S.; Silber, E.N.; Bavishi, N.; Varga, P.; Burton, B.K.; Clark, W.A.; Denes, P. Effect of long-term beta- blockade on aortic root compliance in patients with Marfan syndrome. Am. Heart J. 1999, 137, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Yetman, A.T.; Bornemeier, R.A.; McCrindle, B.W. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am. J. Cardiol. 2005, 95, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Teixido-Tura, G.; Forteza, A.; Rodríguez-Palomares, J. Losartan versus atenolol for prevention of aortic dilation in patients with Marfan syndrome. JACC 2018, 72, 1613–1618. [Google Scholar] [CrossRef]
- Velandia-Sánchez, A.; Polanía-Sandoval, C.A.; Senosiain-González, J.; Álvarez-Martínez, J.V.; Gallo-Bernal, S.; Barrera-Carvajal, J.G.; Umana, J.P.; Camacho-Mackenzie, J. Challenges in prompt identification and surgical correction of Marfan Syndrome aortic disease in a middle-income country: A case series study. J. Cardiothorac. Surg. 2024, 19, 323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moisii, P.; Jari, I.; Naum, A.G.; Butcovan, D.; Tinica, G. Takayasu’s arteritis: A special case report and review of the literature. Medicina 2024, 60, 456. [Google Scholar] [CrossRef]
- David, T.E.; Park, J.; Steve Fan, C.P. Mitral valve surgery in patients with Marfan syndrome. J. Thorac. Cardiovasc. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef]
- Docimo, R.; Maturo, P.; D’Auria, F.; Grego, S.; Costacurta, M.; Perugia, C.; Chiariello, L. Association between Oro-Facial Defects and Systemic Alterations in Children Affected by Marfan Syndrome. J. Clin. Diagn. Res. 2013, 7, 700–703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bauss, O.; Sadat-Khonsari, R.; Fenske, C.; Engelke, W.; Schwestka-Polly, R. Temporomandibular joint dysfunction in Marfan syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J. Oral Facial. Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.A.; Boboc, A.M.; Horodynski, M.; Impellizzeri, A.; Serritella, E.; Galluccio, G. Severity of temporomandibular joint disc displacement and generalized joint hypermobility in growing patients: A cross-sectional magnetic resonance image study. Appl. Sci. 2023, 13, 12495. [Google Scholar] [CrossRef]
- Boboc, A.M.; De Stefano, A.; Impellizzeri, A.; Barbato, E.; Galluccio, G. Correlation between generalised joint hypermobility and temporomandibular joint disc displacement in adolescent patients: Magnetic Resonance Imaging study. Eur. J. Paediatr. Dent. 2022, 23, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; de Simone, G.; Arnett, D.K.; Best, L.G.; Boerwinkle, E.; Howard, B.V.; Kitzman, D.; Lee, E.T.; Mosley, T.H., Jr.; Weder, A.; et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am. J. Cardiol. 2012, 110, 1189–1194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumgartner, H.; Schima, H.; Kühn, P. Value and limitations of proximal jet dimensions for the quantitation of valvular regurgitation: An in vitro study using Doppler flow imaging. J. Am. Soc. Echocardiogr. 1991, 4, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L. European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Blais, M.R.; Lachance, L.; Forget, J.; Richer, S.; Dulude, D.M. L’eschelle de satisfaction globale au travail. In Proceedings of the Annual Congress of the Society Quebecoise for Research in Psychology, Trois Riviers, QC, Canada, 30 September 1991. [Google Scholar]
- Nundy, S.; Zulfqar, A.K.; Bhutta, A. How to Practice Academic Medicine and Publish from Developing Countries? A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Détaint, D.; Faivre, L.; Collod-Beroud, G.; Child, A.H.; Loeys, B.L.; Binquet, C.; Gautier, E.; Arbustini, E.; Mayer, K.; Arslan-Kirchner, M.; et al. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur. Heart J. 2010, 31, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Devereux, R.B.; Preiss, L.R.; Asch, F.M.; Eagle, K.A.; Holmes, K.W.; LeMaire, S.A.; Maslen, C.L.; Milewicz, D.M.; Morris, S.A.; et al. GenTAC Investigators*. Associations of Age and Sex With Marfan Phenotype: The National Heart, Lung, and Blood Institute GenTAC (Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) Registry. Circ. Cardiovasc. Genet. 2017, 10, e001647. [Google Scholar] [CrossRef] [PubMed]
- Nucera, M.; Heinisch, P.P.; Langhammer, B.; Jungi, S.; Mihalj, M.; Schober, P.; Luedi, M.M.; Yildiz, M.; Schoenhoff, F.S. The impact of sex and gender on aortic events in patients with Marfan syndrome. Eur. J. Cardiothorac. Surg. 2022, 62, ezac305. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495, quiz 576–577. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, M.D.; Du, W.; Skeens, M.E.; Humes, R.A. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. J. Am. Soc. Echocardiogr. 2008, 21, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Gautier, M.; Detaint, D.; Fermanian, C.; Aegerter, P.; Delorme, G.; Arnoult, F.; Milleron, O.; Raoux, F.; Stheneur, C.; Boileau, C.; et al. Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am. J. Cardiol. 2010, 105, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Rhee, D.; Solowiejczyk, D.; Altmann, K.; Prakash, A.; Gersony, W.M.; Stolar, C.; Kleinman, C.; Anyane-Yeboa, K.; Chung, W.K.; Hsu, D. Incidence of aortic root dilatation in pectus excavatum and its association with Marfan syndrome. Arch. Pediatr. Adolesc. Med. 2008, 162, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Lombardo, M.; Grasso, E.; Gensini, G.F.; Ambrosio, G. The influence of pectus excavatum on cardiac kinetics and function in otherwise healthy individuals: A systematic review. Int. J. Cardiol. 2023, 381, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Jenabzadeh, T.; Bohner, L.; Köppe, J.; Kleinheinz, J.; Hanisch, M.; Oelerich, O. Temporomandibular disorders in individuals with Marfan syndrome: An exploratory analysis. Head Face Med. 2024, 20, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, X.; He, Y.; Li, Z.; Han, J.; Chen, J.; Nixon, J.V. Echocardiographic versus histologic findings in Marfan syndrome. Tex Heart Inst. J. 2015, 42, 30–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moisii, P.; Jari, I.; Ursu, A.M.; Naum, A.G. The relationship between job strain and ischemic heart disease mediated by endothelial dysfunction markers and imaging. Medicina 2024, 60, 1048. [Google Scholar] [CrossRef]
- Fiabane, E.; Giorgi, I.; Candura, S.M.; Argentero, P. Return to work after coronary revascularization procedures and a patient’s job satisfaction: A prospective study. Int. J. Occup. Med. Environ. Health 2015, 28, 52–61. [Google Scholar] [CrossRef]

| Disease Type | Z Score | SS | Ectopia Lentis |
|---|---|---|---|
| MS | ≥3 (children)/≥2 (adults) | - | Yes |
| MS | ≥3 (children)/≥2 (adults) | ≥7 | No |
| MASS | <3 (children)/<2 (adults) | ≤5 | No |
| MVPS | <3 (children)/<2 (adults) | <5 | No |
| Characteristics | n | % | Mean ± SD |
|---|---|---|---|
| Sex - females | 43 | 51.8 | - |
| - males | 40 | 48.2 | - |
| Age | - | - | 20.9 ± 8.8 |
| Wrist ± thumb sign | 36 | 43.3 | - |
| Chest deformity | 68 | 81.9 | - |
| Foot deformity | 56 | 67.4 | - |
| Cutaneous striae | 58 | 69.8 | - |
| Cranio-facial aspects | 69 | 83.1 | - |
| Aortic Z-score | - | - | 1.88 ± 1.18 |
| Systemic score | - | - | 6.36 ± 3.61 |
| MVP | 59 | 71 | - |
| TMD | 26 | 31.2 | |
| Family medical history | 45 | 54.1 | - |
| Disease Type | DDwR: n (%) | DDwoR (n, %) | Rho † (Zscore-DD) | p † (Zscore-DD) |
|---|---|---|---|---|
| MVPS | 1 (4.5%) | - | 0.276 | 0.213 |
| MASS | 10 (19.2%) | - | 0.787 ** | <0.01 ** |
| MS | 6 (7.2%) | - | 0.143 | 0.536 |
| MS | - | 9 (10.8%) | 0.819 ** | <0.01 ** |
| ANOVA a | |||||
|---|---|---|---|---|---|
| Model | Sum of Squares | Df | Mean Square | F | p-Value |
| 1 | Regression | 92,494 | 2 | 46,247 159,041 | <0.05 b |
| Residual | 23,263 | 80 | 0.291 | ||
| Total | 115,757 | 82 | |||
| Coefficients a | ||||||
|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | T | p-Value | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | 1205 | 0.072 | 16,717 | <0.05 | |
| [DDwR] | 1661 | 0.140 | 0.601 | 11,824 | <0.05 | |
| [DDwoR] | 3281 | 0.216 | 0.772 | 15,177 | <0.05 | |
| Disease Type | Rho † | p-Value † |
|---|---|---|
| All the patients | 0.817 ** | p < 0.01 ** |
| MASS | 0.244 | p = 0.362 |
| MVPS | 0.094 | p = 0.676 |
| MS | 0.442 * | p = 0.045 * |
| C1 (n) | C2 (n) | C3 (n) | C4 (n) | C5 (n) | Total n | |
|---|---|---|---|---|---|---|
| Disease type | ||||||
| MASS | 0 | 0 | 0 | 15 | 1 | 16 |
| MLSF | 17 | 0 | 0 | 0 | 7 | 24 |
| MS | 0 | 8 | 13 | 0 | 0 | 21 |
| MVPS | 14 | 0 | 0 | 0 | 8 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisii, P.; Naum, A.G.; Ursu, A.M.; Vilcu, A.; Esanu, I.; Jari, I. Magnetic Resonance Imaging of Temporomandibular Joint and Aortic Root Score in Fibrillinopathies. Medicina 2024, 60, 1572. https://doi.org/10.3390/medicina60101572
Moisii P, Naum AG, Ursu AM, Vilcu A, Esanu I, Jari I. Magnetic Resonance Imaging of Temporomandibular Joint and Aortic Root Score in Fibrillinopathies. Medicina. 2024; 60(10):1572. https://doi.org/10.3390/medicina60101572
Chicago/Turabian StyleMoisii, Paloma, Alexandru Gratian Naum, Andra Mara Ursu, Adrian Vilcu, Irina Esanu, and Irina Jari. 2024. "Magnetic Resonance Imaging of Temporomandibular Joint and Aortic Root Score in Fibrillinopathies" Medicina 60, no. 10: 1572. https://doi.org/10.3390/medicina60101572
APA StyleMoisii, P., Naum, A. G., Ursu, A. M., Vilcu, A., Esanu, I., & Jari, I. (2024). Magnetic Resonance Imaging of Temporomandibular Joint and Aortic Root Score in Fibrillinopathies. Medicina, 60(10), 1572. https://doi.org/10.3390/medicina60101572






