Abstract
Anterior myocardial infarction is a critical condition with significant implications for cardiac function and patient prognosis. Despite advancements in reperfusion therapies, optimizing recovery during the early phases of myocardial infarction remains challenging. Anterior myocardial infarction can lead to substantial long-term effects on a patient’s health due to extensive damage to the heart muscle, particularly the left ventricle, impacting both quality of life and overall prognosis. Vericiguat, a soluble guanylate cyclase stimulator, has shown promise in heart failure, but its role in early anterior myocardial infarction has not yet been fully explored. By enhancing soluble guanylate cyclase activity, vericiguat may increase cyclic guanosine monophosphate production, leading to vasodilation, inhibition of platelet aggregation, and potential cardioprotective effects. Currently, treatment options for anterior myocardial infarction primarily focus on reperfusion strategies and managing complications. However, there is a critical need for adjunctive therapies that specifically target the pathophysiological changes occurring in the early phases of myocardial infarction. Vericiguat’s mechanism of action offers a novel approach to improving vascular function and myocardial health, potentially contributing to innovative treatment strategies that could transform the care and prognosis of patients with anterior myocardial infarction.
1. Introduction
Anterior myocardial infarction (MI) is a serious, life-threatening event characterized by the blockage of blood flow to the anterior wall of the heart, leading to significant damage to cardiac muscle. Anterior MIs are among the most severe types of heart attacks, associated with worse outcomes compared to other infarct locations, such as inferior or lateral MIs. This type of MI accounts for approximately 33% of all cases [1]. Globally, coronary artery disease (CAD), the primary cause of MI, remains the leading cause of death, with around 17.9 million deaths annually, representing approximately 31% of all global deaths [2]. A substantial fraction of these deaths is attributed to anterior MI due to its higher morbidity and mortality rates, particularly in older adults [3].
In the short term, complications such as acute heart failure, arrhythmias, and cardiogenic shock can arise from substantial damage to the left ventricle, compromising its pumping ability [4]. Despite advances in acute MI management, including timely reperfusion via primary percutaneous coronary intervention (PCI) [5,6] and improved pharmacotherapy, anterior MI continues to carry a higher risk of complications and mortality compared to other MI types. Recent data indicate in-hospital mortality rates of 8–12% for anterior MI, compared to 4–6% for other types, with 30-day mortality ranging from 8 to 14% for anterior MI versus 5–8% for others [7]. This highlights the disproportionate burden of anterior MI, particularly due to the larger area of myocardial damage associated with left anterior descending (LAD) artery occlusion (Figure 1). Short- and long-term complications are significantly influenced by factors such as heart failure development (seen in up to 25% of anterior MI patients), as well as left ventricular (LV) dysfunction, remodeling, fibrosis, and an increased risk of recurrent MI (Table 1) [8]. Additionally, fibrosis and electrical instability in the damaged myocardium can lead to persistent arrhythmias, further exacerbating heart failure. While timely reperfusion therapy has effectively reduced mortality [9], additional therapeutic strategies are necessary to optimize cardiac recovery, particularly in the early phases of MI.
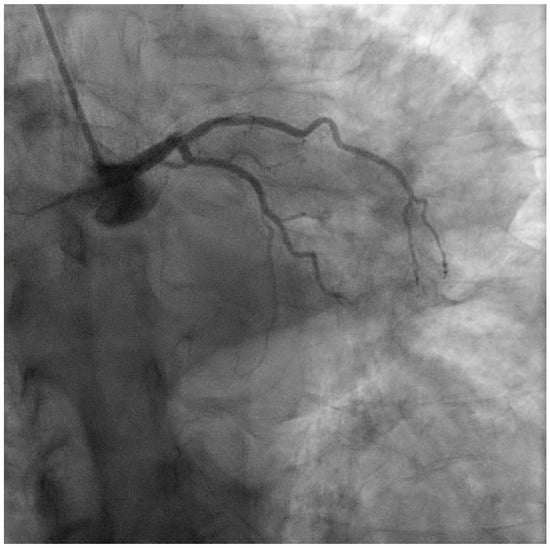
Figure 1.
Coronary angiography performed during anterior STEMI, showing thrombotic occlusion of the ostial LAD.

Table 1.
Early and late complications of anterior myocardial infarction (MI) along with the potential beneficial effects of vericiguat.
One potential therapeutic candidate is vericiguat, a soluble guanylate cyclase (sGC) stimulator that has shown promise in treating heart failure with reduced ejection fraction (HFrEF). The VICTORIA trial highlighted vericiguat’s role in improving outcomes for patients with HFrEF [10], making it a compelling candidate for use in acute anterior MI. However, its role in the specific setting of early anterior MI has yet to be fully explored, despite its potential to address critical pathophysiological changes and optimize recovery, thereby preventing long-term cardiac dysfunction [11].
The pathophysiology of myocardial infarction involves complex biochemical pathways, including the nitric oxide (NO)-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) signaling cascade [12,13]. Under healthy conditions, NO stimulates sGC, leading to increased cGMP production, a molecule that promotes vasodilation and helps regulate blood flow [14]. However, during MI, NO availability is often significantly reduced due to ischemia and endothelial dysfunction, impairing the sGC-cGMP pathway and leading to decreased cGMP production, vasoconstriction, and exacerbation of ischemic injury [15].
The findings from the VICTORIA trial suggest that vericiguat’s ability to enhance myocardial function by increasing cGMP production [16] may be particularly beneficial in anterior MI, where left ventricular function is often compromised. The cardioprotective effects observed in HFrEF, such as improved vascular tone, reduced myocardial remodeling, and decreased fibrosis, may also translate to better outcomes in anterior MI patients. By directly stimulating sGC independent of NO, vericiguat can bypass the impaired NO-sGC interaction [17]. This unique mechanism can lead to increased cGMP levels, even under conditions of reduced NO availability, which are typical during MI. Vericiguat’s potential to enhance vasodilation, reduce myocardial oxygen demand, and inhibit platelet aggregation presents a compelling case for its use in the early stages of MI, where swift intervention is crucial for limiting myocardial damage [18].
Further supporting this, a recent systematic review and network meta-analysis on the efficacy of modern therapies for HFrEF highlights vericiguat as a promising option across various population subgroups [19]. This study showed that vericiguat’s benefits extend beyond general heart failure management and are consistent across different patient demographics. These findings raise the possibility that vericiguat could offer similar cardioprotective benefits in the acute phase of MI, potentially reducing the progression to heart failure and other long-term complications.
2. Rationale
2.1. The Role of Nitric Oxide Deficiency in Post-Myocardial Infarction and the Therapeutic Potential of Vericiguat
Nitric oxide (NO) plays a key role in cardiovascular physiology by maintaining vascular tone, reducing inflammation, and protecting against ischemic injury [20]. However, in post-MI patients, NO bioavailability is significantly reduced due to endothelial dysfunction, oxidative stress, and the upregulation of pathways that degrade NO [21]. This deficiency contributes to increased myocardial damage, impaired vascular function, and the progression of heart failure (HF), a common and deadly complication in post-MI survivors.
NO is synthesized primarily in endothelial cells by endothelial nitric oxide synthase (eNOS). Upon release, NO diffuses into vascular smooth muscle cells, where it binds to soluble guanylate cyclase (sGC), triggering the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). Elevated cGMP levels induce vasodilation, reduce vascular resistance, and inhibit smooth muscle proliferation and platelet aggregation. This pathway is crucial for maintaining cardiac function and vascular homeostasis, especially in ischemic injury settings like MI [22].
In post-MI patients, the NO-sGC-cGMP pathway becomes dysregulated due to endothelial dysfunction, oxidative stress, and sGC inactivation. Reduced NO bioavailability following MI profoundly affects both the acute and chronic phases of myocardial injury. With lower NO levels, vasoconstriction becomes more prominent, worsening myocardial ischemia by reducing blood flow to already oxygen-deprived areas. Furthermore, a deficient NO system leads to endothelial dysfunction, contributing to atherosclerosis progression, poor angiogenesis, and impaired coronary blood flow regulation [23]. NO deficiency also promotes adverse ventricular remodeling, including myocardial fibrosis, ventricular dilatation, and reduced cardiac output, all worsening heart failure. A lack of NO exacerbates HF by increasing afterload, impairing myocardial contractility, and promoting the development of pulmonary hypertension [24]. Finally, NO stabilizes myocardial cell electrical activity, and its deficiency increases the risk of arrhythmias [25], a major cause of sudden death in post-MI patients.
These adverse effects highlight the critical need for therapeutic strategies that can restore or enhance NO-cGMP signaling to improve outcomes in post-MI patients. Vericiguat is an oral soluble guanylate cyclase stimulator designed to enhance cGMP production independently of NO availability. It addresses the limitations of current heart failure therapies, particularly in post-MI patients with NO deficiency, by directly stimulating sGC even in the presence of oxidative stress, which can impair NO-mediated signaling [26].
Vericiguat directly binds to sGC and stimulates it to produce cGMP without requiring NO. This is particularly advantageous in post-MI patients, where oxidative stress often reduces NO availability and sGC becomes less responsive to endogenous NO. Moreover, in tissues where NO is still partially available, vericiguat enhances the sensitivity of sGC to the remaining NO, amplifying its effects on cGMP production [27]. This combination of NO-independent stimulation and NO-sensitization gives vericiguat a distinct advantage over traditional vasodilators or earlier-generation sGC modulators, which rely more heavily on the presence of endogenous NO for therapeutic effects.
By stimulating cGMP production in vascular smooth muscle cells, vericiguat promotes vasodilation, reducing afterload and improving coronary blood flow. This helps mitigate ischemic injury and supports better oxygen delivery to the myocardium. Increased cGMP levels help prevent pathological myocardial remodeling by reducing fibrosis, preserving ventricular geometry, and improving myocardial contractility. Furthermore, clinical trials such as VICTORIA have demonstrated that vericiguat significantly reduces the risk of hospitalization due to heart failure [28]. This is particularly relevant for post-MI patients, who are at high risk of recurrent decompensations.
2.2. The Promise of Vericiguat in Early Myocardial Infarction
In the context of early anterior MI, the application of vericiguat could represent a significant therapeutic advancement. By enhancing sGC activity and increasing cGMP production, vericiguat could address critical challenges associated with MI (Table 2). Increased cGMP levels can facilitate the relaxation of vascular smooth muscle, promoting vasodilation and improving blood flow to the ischemic myocardium. This effect could reduce the extent of myocardial damage and enhance overall cardiac function. Moreover, the anti-platelet effects of cGMP could help prevent further coronary artery occlusion, thereby reducing the risk of subsequent cardiac events.

Table 2.
Potential beneficial effects of vericiguat in the early phases of anterior myocardial infarction.
The cardioprotective effects associated with elevated cGMP levels, such as reduced cardiac remodeling and fibrosis, are particularly relevant in the setting of MI [29]. Specifically, the guanylyl cyclase B (GC-B)/cGMP signaling pathway has consistently been reported as a potent inhibitor of organ fibrosis in various experimental models of cardiovascular diseases [30]. By mitigating fibrosis and stabilizing cardiac electrophysiology, vericiguat could aid in managing heart failure, prevent adverse cardiac remodeling, reduce the incidence of arrhythmias, and lower the risk of recurrent cardiovascular events in patients following an anterior MI. These effects could counteract adverse cardiac remodeling, a common consequence of extensive myocardial injury and a precursor to heart failure.
2.3. Potential Challenges and Considerations
The theoretical benefits of vericiguat in early phases of MI are promising; however, several challenges and considerations must be addressed. First, the safety profile of vericiguat in the acute MI setting requires thorough evaluation. While the drug has shown safety in chronic heart failure populations, the acute context presents unique risks. Its integration with existing therapies must be carefully considered to ensure both efficacy and safety.
One primary treatment for MI is the use of angiotensin-converting enzyme (ACE) inhibitors, which reduce the production of angiotensin II, a potent vasoconstrictor. By promoting vasodilation and decreasing afterload, ACE inhibitors play a crucial role in improving long-term survival and preventing adverse cardiac remodeling [31]. Vericiguat’s mechanism, enhancing cGMP production via the sGC pathway, also promotes vasodilation but through a different biochemical process. This complementary action could theoretically improve myocardial oxygen supply and reduce cardiac workload post-MI. However, since both ACE inhibitors and vericiguat can lower blood pressure, there is concern for additive hypotension, particularly in patients with borderline or low baseline blood pressure. This underscores the need for the careful monitoring of blood pressure and renal function when these drugs are used together.
Another critical therapy following MI is the administration of beta-blockers, which lower myocardial oxygen demand by reducing heart rate, contractility, and blood pressure. Beta-blockers also possess anti-arrhythmic properties, helping prevent life-threatening arrhythmias during the post-MI period [32,33]. While beta-blockers focus on decreasing oxygen consumption, vericiguat aims to enhance oxygen supply by promoting vasodilation and improving coronary and systemic perfusion. This combination could potentially optimize myocardial oxygen balance, offering a more comprehensive approach to post-MI care. However, similar to ACE inhibitors, beta-blockers can amplify hypotensive effects when combined with vericiguat, necessitating careful titration to avoid symptomatic hypotension. Additionally, since beta-blockers can slow heart rate, their interaction with vericiguat may pose a risk of bradycardia or excessive heart rate reduction, requiring close monitoring in clinical practice.
Vericiguat could also interact with aldosterone antagonists, often prescribed after MI to reduce heart failure progression [34]. These agents block aldosterone’s effects, reducing sodium retention and preventing adverse cardiac remodeling [35]. Vericiguat’s ability to enhance vasodilation and improve myocardial perfusion could complement aldosterone antagonists, potentially offering a dual approach to minimizing heart failure progression. However, both vericiguat and aldosterone antagonists may contribute to hyperkalemia, making potassium level monitoring crucial for patients receiving both therapies.
In addition to heart failure-focused treatments, antiplatelet therapy, often in the form of dual antiplatelet therapy (DAPT) with aspirin and P2Y12 inhibitors, is essential following MI to prevent thrombosis [36,37]. Vericiguat’s ability to increase cGMP levels may also confer anti-platelet effects by inhibiting platelet aggregation, providing an additional protective mechanism against clot formation. While this synergy might enhance thrombo-protection, there is a risk of increased bleeding when combining vericiguat with potent antiplatelet agents. Clinicians must carefully assess the bleeding risk in individual patients before combining these therapies.
Vericiguat may also play a beneficial role in patients undergoing reperfusion therapy, such as percutaneous coronary intervention (PCI) or thrombolytic therapy. By promoting vasodilation and improving microvascular flow, vericiguat could enhance the effects of reperfusion, helping to preserve myocardial tissue after large coronary arteries are reopened. Additionally, its ability to reduce platelet aggregation and vasoconstriction may help prevent no-reflow phenomena, where microvascular blockages persist despite successful reperfusion. While no significant direct interactions between vericiguat and reperfusion therapies are anticipated, there is potential for cumulative vasodilatory effects, which could lead to hypotension in some patients.
Moreover, diuretics are often used after MI, especially in patients with heart failure, to relieve fluid overload and reduce pulmonary congestion [38]. Vericiguat’s vasodilatory effects could complement diuretics by reducing afterload and venous congestion, further alleviating symptoms of heart failure. However, combining these therapies could increase the risk of volume depletion and hypotension, necessitating close monitoring of electrolytes and kidney function.
Ultimately, while vericiguat offers potential benefits in enhancing vasodilation, improving myocardial oxygen supply, and reducing adverse remodeling after MI, its use in combination with standard MI treatments requires careful consideration. The risks of hypotension, electrolyte imbalances, and drug interactions must be closely managed.
To effectively assess the impact of vericiguat in this patient population, it is crucial to carefully select high-risk individuals, such as those experiencing cardiogenic shock [39]. Including these patients will provide valuable insights into the potential benefits or harms of this therapy in critically ill populations, particularly those with higher TIMI, GRACE, and Intermountain Risk Scores (IMRSs) [40].
Future studies are needed to explore these potential interactions in greater depth to determine how vericiguat can be safely integrated into current MI treatment protocols, optimizing outcomes while minimizing risks for patients.
3. Conclusions
The application of vericiguat in the early phases of anterior myocardial infarction (MI) could represent a novel approach with the potential to transform the management of this critical condition. By targeting the nitric oxide (NO)-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway, vericiguat may improve outcomes by promoting vasodilation, reducing myocardial injury, and preventing adverse cardiac remodeling (Figure 2). While the promise of vericiguat is evident, further research is necessary to fully understand its benefits, optimize its use, and ensure safety in the acute MI setting.
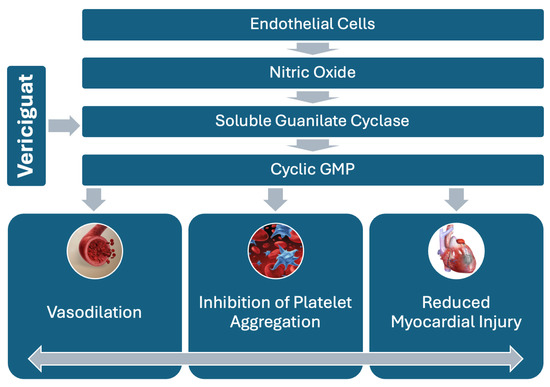
Figure 2.
NO-sGC-cGMP pathway, highlighting vericiguat’s role in stimulating sGC to increase cGMP production. This leads to vasodilation and reduced platelet aggregation, offering therapeutic benefits in myocardial infarction.
Should vericiguat demonstrate efficacy and safety in this context, it could pave the way for larger-scale trials and ultimately establish a new standard of care in the management of anterior MI. In the ongoing battle against myocardial infarction, vericiguat could emerge as a valuable addition to the therapeutic arsenal, offering hope for improved outcomes in a high-risk patient population.
Author Contributions
Conceptualization, F.C. (Federico Cacciapuoti) and F.C. (Fulvio Cacciapuoti); methodology, F.C. (Fulvio Cacciapuoti), F.C. (Federico Cacciapuoti), and C.M.; validation, F.C. (Fulvio Cacciapuoti), F.C. (Federico Cacciapuoti), C.M., S.C. (Salvatore Crispo), and R.G.; formal analysis, F.C. (Fulvio Cacciapuoti), F.C. (Federico Cacciapuoti), and F.M.; resources, V.C. and S.C. (Salvatore Chianese); data curation, L.G.T.; writing—original draft preparation, F.C. (Fulvio Cacciapuoti) and F.C. (Federico Cacciapuoti); writing—review and editing, F.C. (Fulvio Cacciapuoti) and F.C. (Federico Cacciapuoti); supervision, F.M.; project administration, F.C. (Fulvio Cacciapuoti), R.G. and F.C. (Federico Cacciapuoti). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Valentina Capone and Salvatore Chianese have been supported by the CardioPath PhD program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Newman, J.D.; Shimbo, D.; Baggett, C.; Liu, X.; Crow, R.; Abraham, J.M.; Loehr, L.R.; Wruck, L.M.; Folsom, A.R.; Rosamond, W.D. Trends in myocardial infarction rates and case fatality by anatomical location in four United States communities, 1987 to 2008 (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2013, 112, 1714–1719. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association [published correction appears in Circulation. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Yu, B.; Akushevich, I.; Yashkin, A.P.; Kravchenko, J. Epidemiology of Geographic Disparities of Myocardial Infarction Among Older Adults in the United States: Analysis of 2000–2017 Medicare Data. Front. Cardiovasc. Med. 2021, 8, 7102. [Google Scholar] [CrossRef] [PubMed]
- Hayıroğlu, M.; Keskin, M.; Uzun, A.O.; Yıldırım, D.I.; Kaya, A.; Çinier, G.; Bozbeyoğlu, E.; Yıldırımtürk, Ö.; Kozan, Ö.; Pehlivanoğlu, S. Predictors of In-Hospital Mortality in Patients With ST-Segment Elevation Myocardial Infarction Complicated with Cardiogenic Shock. Heart Lung Circ. 2019, 28, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Karkabi, B.; Meir, G.; Zafrir, B.; Jaffe, R.; Adawi, S.; Lavi, I.; Flugelman, M.Y.; Shiran, A. Door-to-balloon time and mortality in patients with ST-elevation myocardial infarction undergoing primary angioplasty. Eur. Heart J. Quality Care Clinic Outcomes 2017, 7, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients with ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 1235–1250, Erratum in J. Am. Coll. Cardiol. 2016, 67, 1506. [Google Scholar] [CrossRef]
- Kwok, C.S.; Qureshi, A.I.; Lip, G.Y. The impact of the site of myocardial infarction on in-hospital outcomes for patients with STEMI. Coron. Artery Dis. 2024, 35, 286–291. [Google Scholar] [CrossRef]
- Shabbir, M.; Kayani, A.M.; Qureshi, O.; Mughal, M.M. Predictors of fatal outcome in acute myocardial infarction. J. Ayub Med. Coll. Abbottabad 2009, 20, 14–16. [Google Scholar]
- Keeley, E.C.; Boura, J.A.; Grines, C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003, 361, 13–20. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Hui, S.K.; Sharma, A.; Docherty, K.; McMurray, J.J.V.; Pitt, B.; Dickstein, K.; A Pfeffer, M.; Girerd, N.; Rossignol, P.; Ferreira, J.P.; et al. Non-fatal cardiovascular events preceding sudden cardiac death in patients with an acute myocardial infarction complicated by heart failure: Insights from the high-risk myocardial infarction database. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Ayalasomayajula, S.; Blaustein, R.O.; Gheyas, F. Vericiguat, a novel sGC stimulator: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2023, 16, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Marti, C.N.; Sabbah, H.N.; Roessig, L.; Greene, S.J.; Böhm, M.; Burnett, J.C.; Campia, U.; Cleland, J.G.F.; Collins, S.P.; et al. Soluble guanylate cyclase: A potential therapeutic target for heart failure. Heart Fail. Rev. 2013, 18, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. et Biophys. Acta 1999, 1411, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.R.; Marletta, M.A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Anstrom, K.J.; Armstrong, P.W. Comparing the Benefit of Novel Therapies Across Clinical Trials: Insights From the VICTORIA Trial. Circulation 2020, 142, 717–719. [Google Scholar] [CrossRef]
- Zhu, W.; Ben, Y.; Shen, Y.; Liu, W. Vericiguat protects against cardiac damage in a pig model of ischemia/reperfusion. PLoS ONE 2023, 18, e0295566. [Google Scholar] [CrossRef]
- Chen, T.; Kong, B.; Shuai, W.; Gong, Y.; Zhang, J.; Huang, H. Vericiguat alleviates ventricular remodeling and arrhythmias in mouse models of myocardial infarction via CaMKII signaling. Life Sci. 2023, 334, 122184. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, C.; Mariani, M.V.; Severino, P.; Palombi, M.; Trivigno, S.; D’Amato, A.; Silvetti, G.; Pierucci, N.; Di Lullo, L.; Chimenti, C.; et al. Efficacy of modern therapies for heart failure with reduced ejection fraction in specific population subgroups: A systematic review and network meta-analysis. Cardiorenal Med. 2024, 1–23. [Google Scholar] [CrossRef]
- Ziolo, M.T.; Kohr, M.J.; Wang, H. Nitric oxide signaling and the regulation of myocardial function. J. Mol. Cell. Cardiol. 2008, 45, 625–632. [Google Scholar] [CrossRef]
- Bice, J.S.; Jones, B.R.; Chamberlain, G.R.; Baxter, G.F. Nitric oxide treatments as adjuncts to reperfusion in acute myocardial infarction: A systematic review of experimental and clinical studies. Basic Res. Cardiol. 2016, 111, 23. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, J.; Stark, K.; Esslinger, U.B.; Rumpf, P.M.; Koesling, D.; de Wit, C.; Kaiser, F.J.; Braunholz, D.; Medack, A.; Fischer, M.; et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 2013, 504, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; van der Laarse, A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol. Cell. Biochem. 2009, 333, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Roman-Campos, D.; Sales-Junior, P.; Santos-Miranda, A.; Joviano-Santos, J.V.; Ropert, C.; Cruz, J.S. Deletion of inducible nitric oxide synthase delays the onset of cardiomyocyte electrical remodeling in experimental Chagas disease. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165949. [Google Scholar] [CrossRef]
- Sahana, U.; Wehland, M.; Simonsen, U.; Schulz, H.; Grimm, D. A Systematic Review of the Effect of Vericiguat on Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 11826. [Google Scholar] [CrossRef]
- Vericiguat. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2023.
- Lam, C.S.; Giczewska, A.; Sliwa, K.; Edelmann, F.; Refsgaard, J.; Bocchi, E.; Ezekowitz, J.A.; Hernandez, A.F.; O’Connor, C.M.; Roessig, L.; et al. Clinical Outcomes and Response to Vericiguat According to Index Heart Failure Event: Insights from the VICTORIA Trial. JAMA Cardiol. 2021, 6, 706–712, Erratum in JAMA Cardiol. 2021, 6, e214194. [Google Scholar] [CrossRef]
- Peddibhotla, S.; Zheng, Y.; Pan, S.; Mehta, A.; Moroni, D.G.; Chen, Q.-Y.; Ma, X.; Burnett, J.C.; Malany, S.; Sangaralingham, S.J. Discovery of small molecule guanylyl cyclase B receptor positive allosteric modulators. PNAS Nexus 2024, 3, 225. [Google Scholar] [CrossRef]
- Park, M.; Sandner, P.; Krieg, T. cGMP at the centre of attention: Emerging strategies for activating the cardioprotective PKG pathway. Basic Res. Cardiol. 2018, 113, 24. [Google Scholar] [CrossRef]
- Borghi, C.; Omboni, S.; Reggiardo, G.; Bacchelli, S.; Degli Esposti, D.; Ambrosioni, E. Effects of the concomitant administration of xanthine oxidase inhibitors with zofenopril or other ACE-inhibitors in post-myocardial infarction patients: A meta-analysis of individual data of four randomized, double-blind, prospective studies. BMC Cardiovasc. Disord. 2018, 18, 112. [Google Scholar] [CrossRef]
- Bangalore, S.; Makani, H.; Radford, M.; Thakur, K.; Toklu, B.; Katz, S.D.; DiNicolantonio, J.J.; Devereaux, P.; Alexander, K.P.; Wetterslev, J.; et al. Clinical outcomes with β-blockers for myocardial infarction: A meta-analysis of randomized trials. Am. J. Med. 2014, 127, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Desta, L.; Raposeiras-Roubin, S.; Ibanez, B. The art of prescribing β-blockers after myocardial infarction. Circ. Cardiovasc. Interv. 2021, 14, 399–401. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. New Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Buffolo, F.; Tetti, M.; Mulatero, P.; Monticone, S. Aldosterone as a Mediator of Cardiovascular Damage. Hypertension 2022, 79, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155, Correction in Circulation 2016, 134, e192–e194. [Google Scholar]
- Gelbenegger, G.; Jilma, B. Clinical pharmacology of antiplatelet drugs. Expert Rev. Clin. Pharmacol. 2022, 15, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Trêpa, M.; Oliveira, M.; Frias, A.; Campinas, A.; Luz, A.; Santos, M.; Torres, S. Heart Failure Incidence Following ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2022, 164, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Van Spall, H.G.; Mamas, M.A. Cardiogenic Shock in the Setting of Acute Myocardial Infarction History Repeating Itself? Circ. Cardiovasc. Interv. 2020, 13, e009034. [Google Scholar] [CrossRef]
- Çınar, T.; Şaylık, F.; Akbulut, T.; Korkmaz, Y.; Çiçek, V.; Asal, S.; Erdem, A.; Selçuk, M.; Hayıroğlu, M. Evaluation of Intermountain Risk Score for Short- and Long-Term Mortality in ST Elevation Myocardial Infarction Patients. Angiology 2023, 74, 357–364. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).