Immediate Loading Full-Arch 3D-Printed Implant-Supported Fixed Rehabilitation: A Case Report with 24-Month Follow-Up
Abstract
1. Introduction
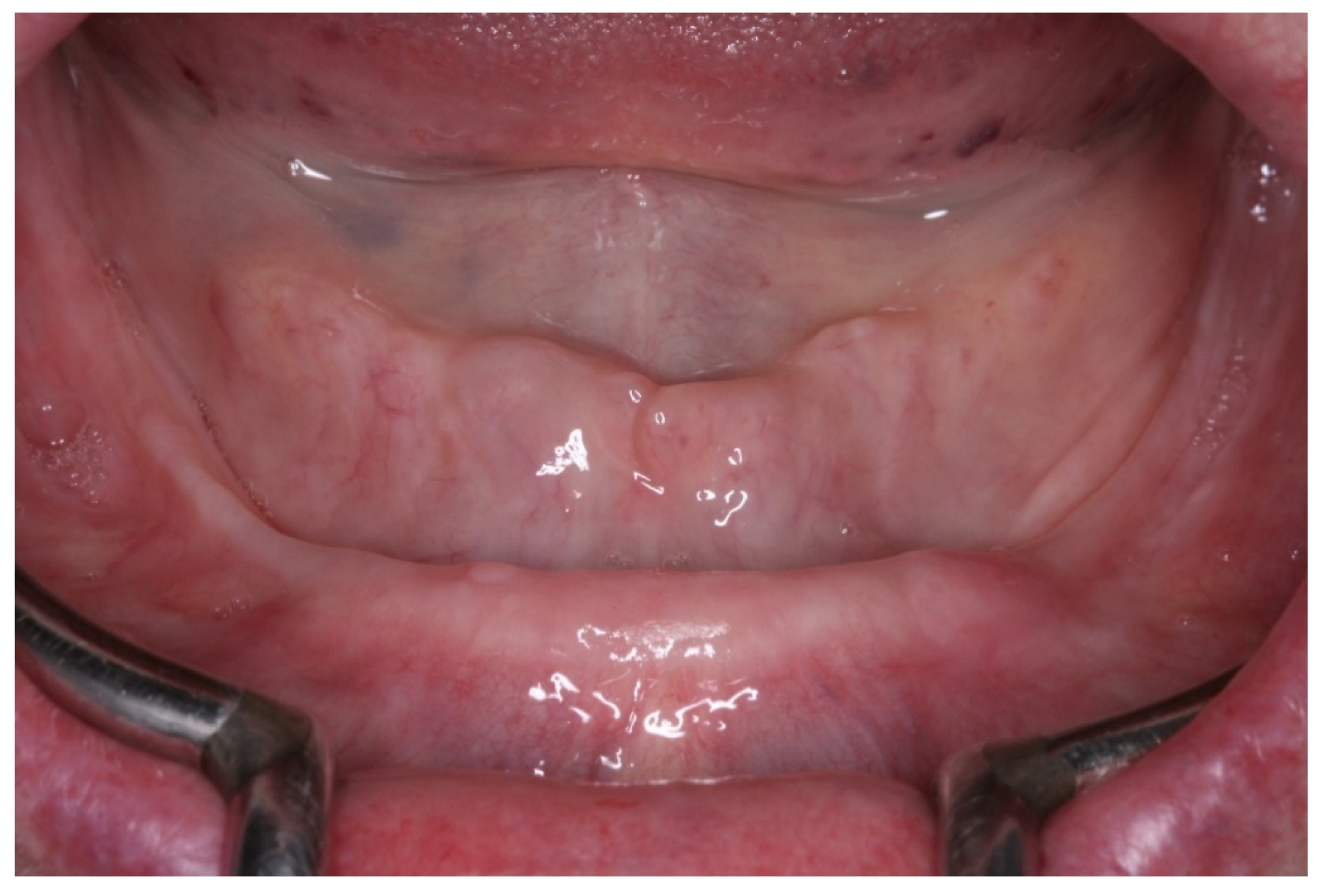
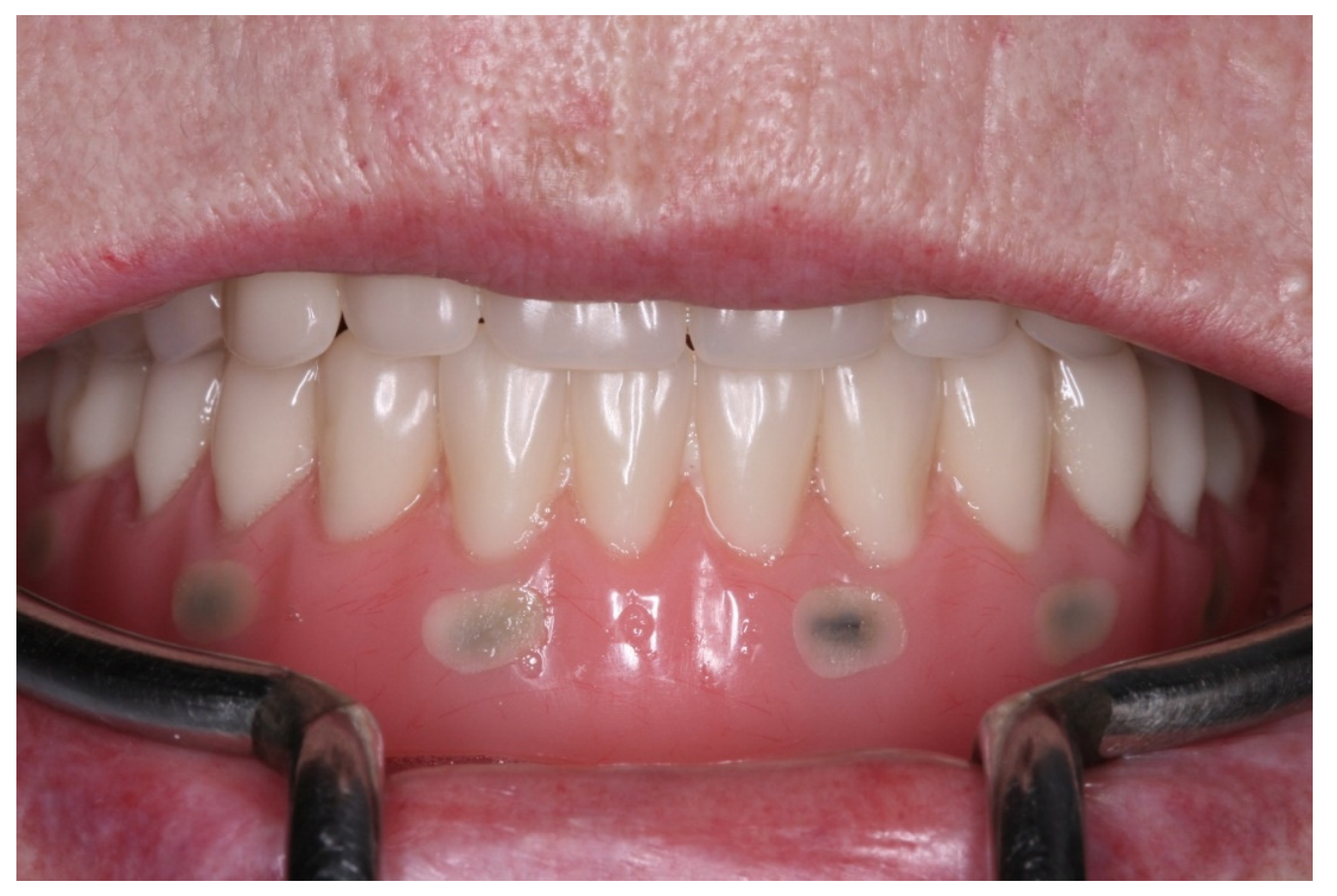
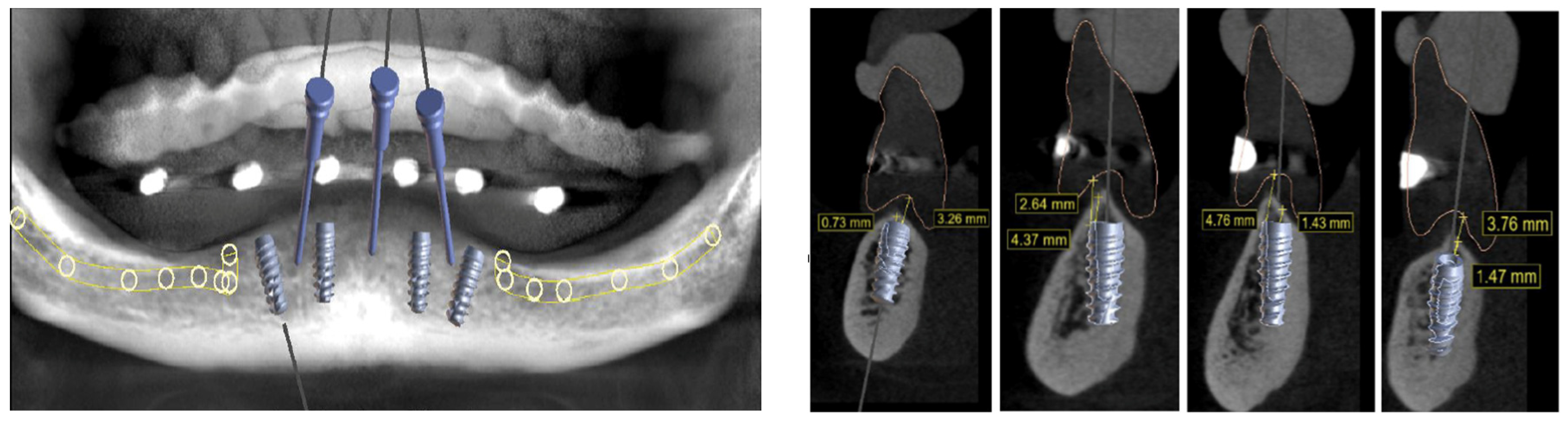
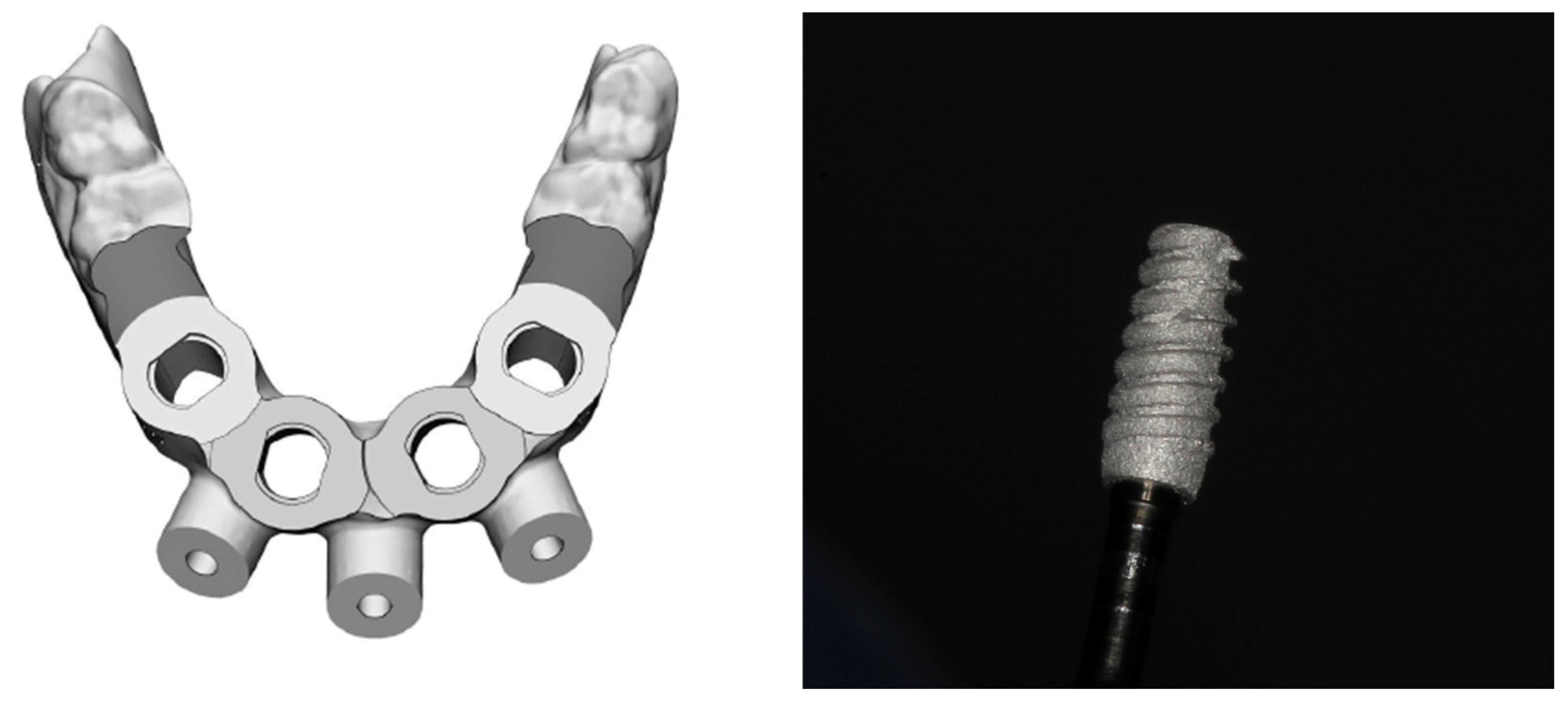
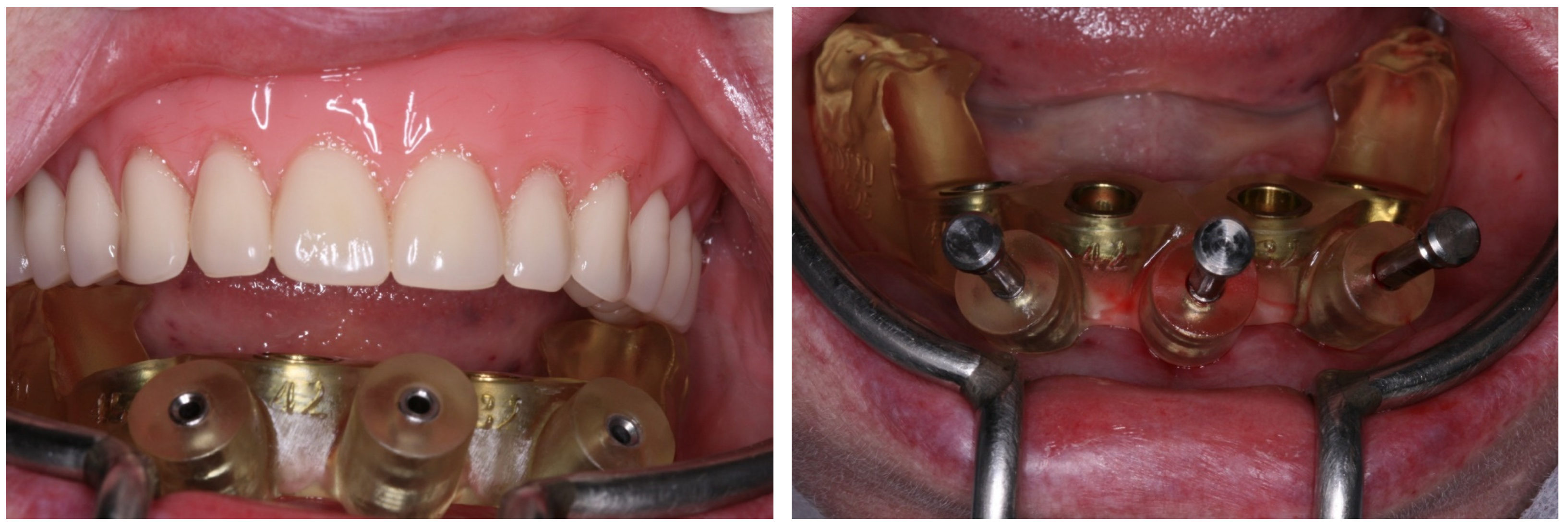
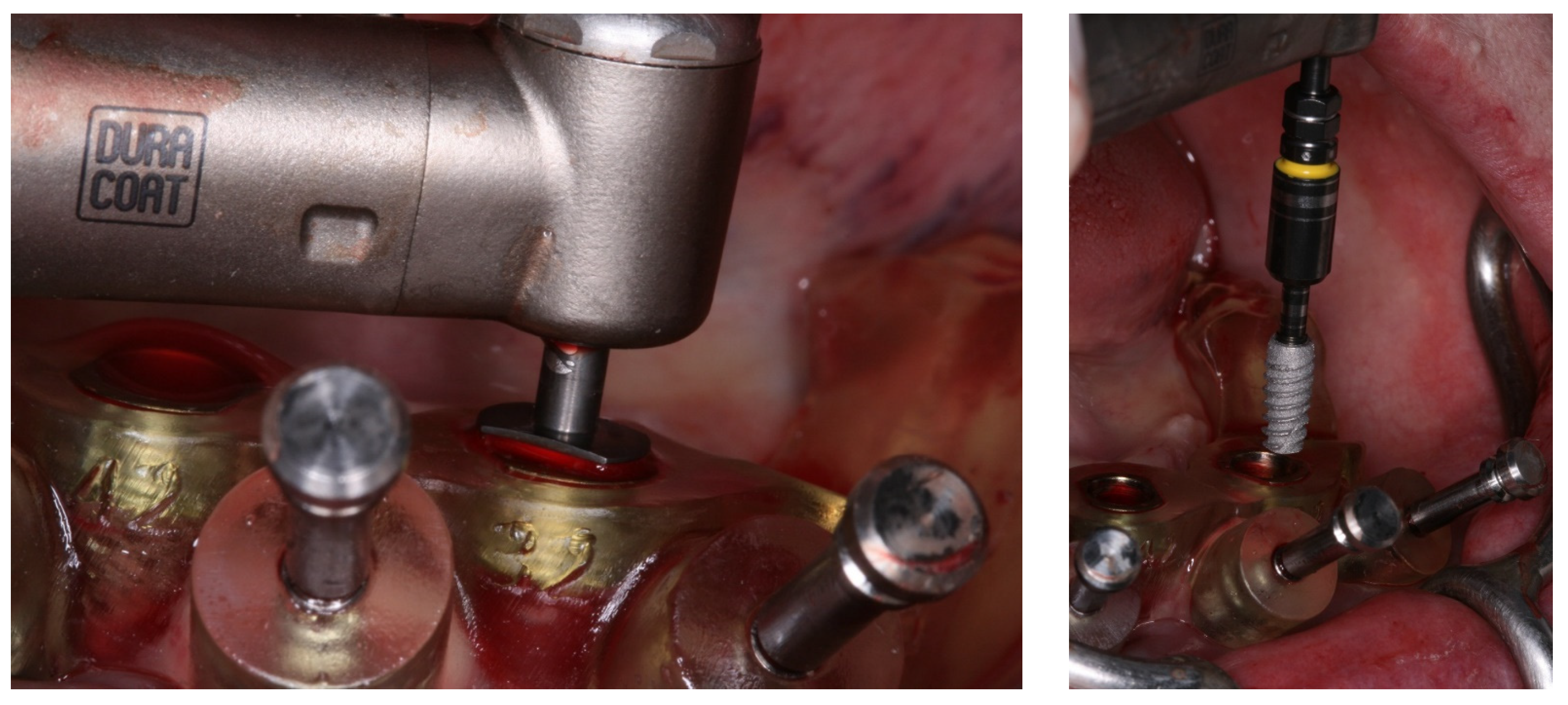
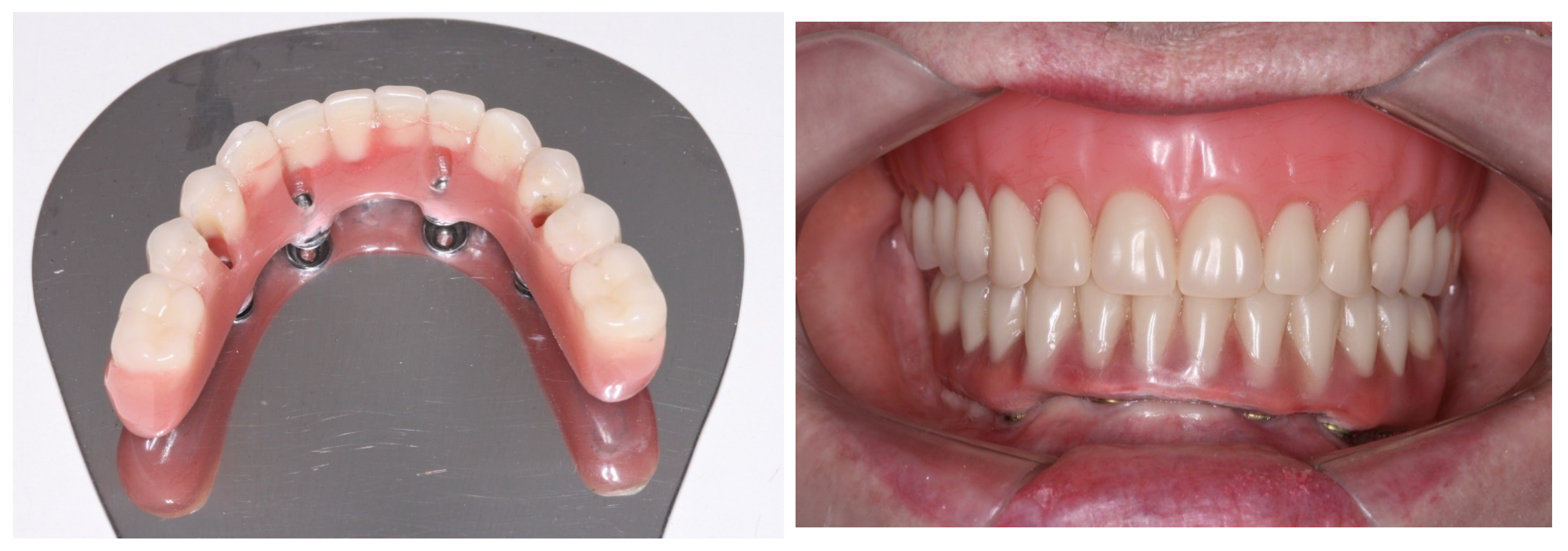
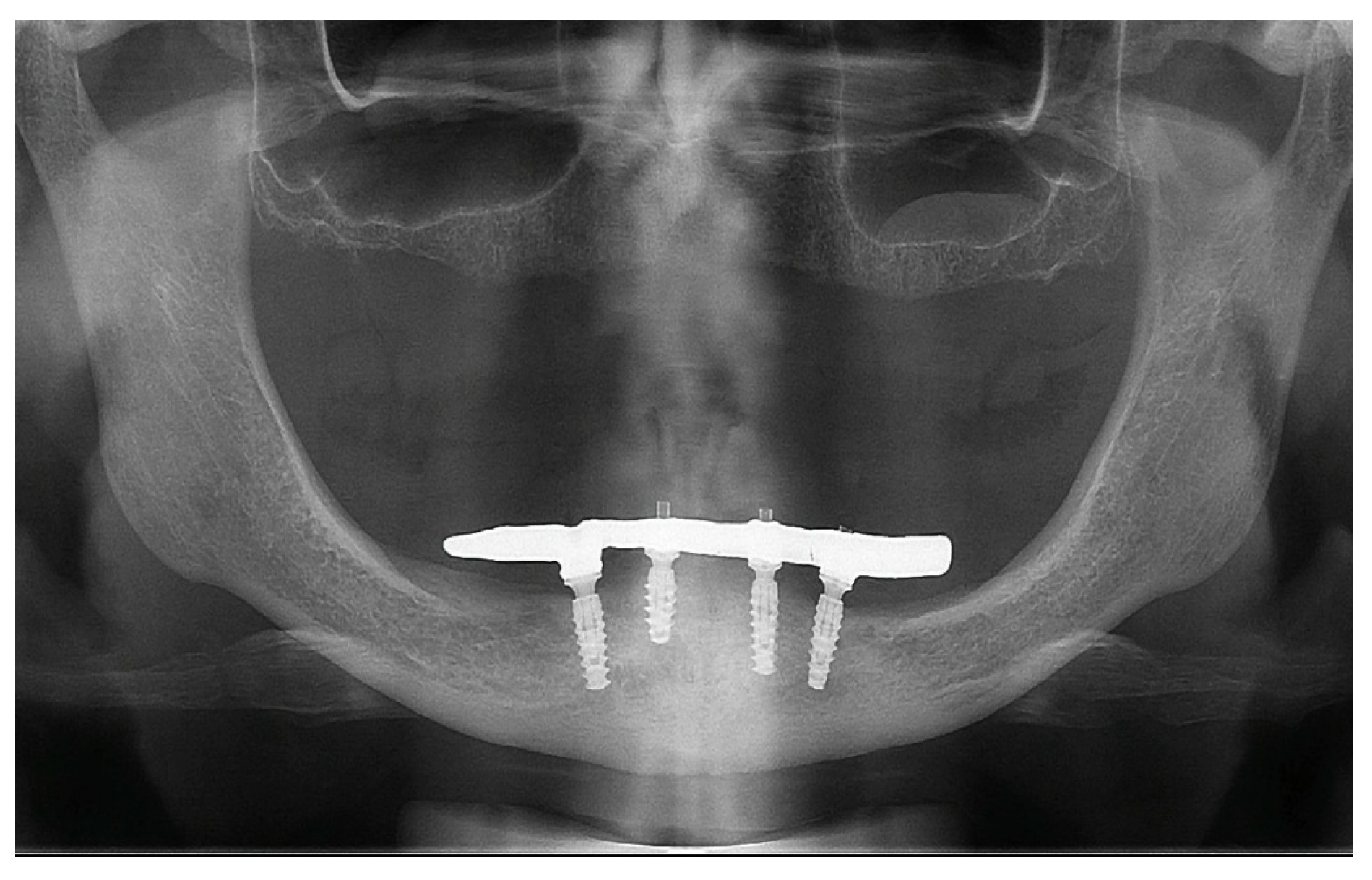
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Fabbro, M.; Testori, T.; Kekovic, V.; Goker, F.; Tumedei, M.; Wang, H.L. A systematic review of survival rates of osseointegrated implants in fully and partially edentulous patients following immediate loading. J. Clin. Med. 2019, 8, 2142. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.S.Y.; de Magalhães, K.M.F.; Rocha, E.P.; Dos Santos, P.H.; Assunção, W.G. Oral health-related quality of life and satisfaction in edentulous patients rehabilitated with implant-supported full dentures all-on-four concept: A systematic review. Clin. Oral Investig. 2022, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Agliardi, E.L.; Pozzi, A.; Romeo, D.; Del Fabbro, M. Clinical outcomes of full-arch immediate fixed prostheses supported by two axial and two tilted implants: A retrospective cohort study with 12-15 years of follow-up. Clin. Oral Implants Res. 2023, 34, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Malò, P.; De Araujo Norbre, M.; Lopes, A.; Ferro, A.; Botto, J. The All-on-4 treatment concept for the rehabilitation of the completely edentulous mandible: A longitudinal study with 10 to 18 years of follow-up. Clin. Implant. Dent. Relat. Res. 2019, 21, 565–577. [Google Scholar] [CrossRef]
- Badr, S.; Elawady, D.; Ibrahim, W.; Eldin, A. Axial versus tilted distal implants in All-on-4 mandibular screw-retained prosthesis. A randomized controlled trial. MSA Dent. J. 2023, 2, 24–33. [Google Scholar] [CrossRef]
- Mehta, S.P.; Sutariya, P.V.; Pathan, M.R.; Upadhyay, H.H.; Patel, S.R.; Kantharia, N.D.G. Clinical success between tilted and axial implants in edentulous maxilla: A systematic review and meta-analysis. J. Indian. Prosthodont. Soc. 2021, 21, 217–228. [Google Scholar] [CrossRef]
- Tironi, F.; Orlando, F.; Azzola, F.; Corbella, S.; Francetti, L.A. A retrospective analysis on marginal bone loss around tilted and axial implants in immediate-loaded All-On-4 with a long-term follow-up evaluation. Prosthesis 2022, 4, 15–23. [Google Scholar] [CrossRef]
- Cattoni, F.; Chirico, L.; Merlone, A.; Manacorda, M.; Vinci, R.; Gherlone, E.F. Digital smile designed computer-aided surgery versus traditional workflow in “All on Four” rehabilitations: A randomized clinical trial with 4-years follow up. Int. J. Environ. Res. Public Health 2021, 18, 3449. [Google Scholar] [CrossRef]
- Manacorda, M.; Poletti de Chaurand, B.; Merlone, A.; Tetè, G.; Mottola, F.; Vinci, R. Virtual implant rehabilitation of the severely atrophic maxilla: A radiographic study. Dent. J. 2020, 8, 14. [Google Scholar] [CrossRef]
- Miljanovic, D.; Seyedmahmoudian, M.; Horan, B.; Stojcevski, A. Novel and accurate 3D-printed surgical guide for mandibular reconstruction with integrated dental implants. Comput. Biol. Med. 2022, 151, 106327. [Google Scholar] [CrossRef]
- D’Haese, J.; Ackhurst, J.; Wismeijer, D.; De Bruyn, H.; Tahmaseb, A. Current state of the art of computer-guided implant surgery. Periodontol. 2000 2017, 73, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Naeini, E.N.; Atashkadeh, M.; De Bruyn, H.; D’Haese, J. Narrative review regarding the applicability, accuracy, and clinical outcome of flapless implant surgery with or without computer guidance. Clin. Implant. Dent. Relat. Res. 2020, 22, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Unsal, G.S.; Turkyilmaz, I.; Lakhia, S. Advantages and limitations of implant surgery with CAD/CAM surgical guides: A literature review. J. Clin. Exp. Dent. 2020, 12, e409–e417. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.M.; Souza, F.A.; Hadad, H.; Poli, P.P.; Maiorana, C.; Carvalho, P.S.P. Accuracy evaluation of computer-guided implant surgery associated with prototyped surgical guides. J. Prosthet. Dent. 2021, 125, 266–272. [Google Scholar] [CrossRef] [PubMed]
- La Monaca, G.; Pranno, N.; Annibali, S.; Di Carlo, S.; Pompa, G.; Cristalli, M.P. Immediate flapless full-arch rehabilitation of edentulous jaws on 4 or 6 implants according to the prosthetic-driven planning and guided implant surgery: A retrospective study on clinical and radiographic outcomes up to 10 years of follow-up. Clin. Implant. Dent. Relat. Res. 2022, 24, 831–844. [Google Scholar] [CrossRef]
- Atieh, M.A.; Baqain, Z.H.; Tawse-Smith, A.; Ma, S.; Almoselli, M.; Lin, L.; Alsabeeha, N.H.M. The influence of insertion torque values on the failure and complication rates of dental implants: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2021, 23, 341–360. [Google Scholar] [CrossRef]
- Darriba, I.; Seidel, A.; Moreno, F.; Botelho, J.; Machado, V.; Mendes, J.J.; Leira, Y.; Blanco, J. Influence of low insertion torque values on survival rate of immediately loaded dental implants: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 158–169. [Google Scholar] [CrossRef]
- Rossi, R.; Capri, D.; Risciotti, E.; Zeman, P. Randomized Clinical Investigation of Titanium Implants with and without Platform Switching: Six Months’ Radiographic and Clinical Outcome. Dent. J. 2015, 3, 55–66. [Google Scholar] [CrossRef]
- Spinato, S.; Bernardello, F.; Lombardi, T.; Soardi, C.M.; Messina, M.; Zaffe, D.; Stacchi, C. Influence of apico-coronal positioning of tissue-level implants on marginal bone stability during supracrestal tissue height establishment: A multi-center prospective study. Clin. Implant. Dent. Relat. Res. 2022, 24, 611–620. [Google Scholar] [CrossRef]
- Bambini, F.; Orilisi, G.; Quaranta, A.; Memè, L. Biological oriented immediate loading: A new mathematical implant vertical insertion protocol, Five-year follow-up study. Materials 2021, 14, 387. [Google Scholar] [CrossRef]
- Degidi, M.; Perrotti, V.; Shibli, J.A.; Novaes, A.B.; Piattelli, A.; Iezzi, G. Equicrestal and subcrestal dental implants: A histologic and histomorphometric evaluation of nine retrieved human implants. J. Periodontol. 2011, 82, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Aghaloo, T.; Jung, R.E.; Bertl, K.; Buser, D.; Chappuis, V.; de Stavola, L.; Monje, A.; Pispero, A.; Roccuzzo, A.; et al. Group 1 ITI Consensus Report: The role of bone dimensions and soft tissue augmentation procedures on the stability of clinical, radiographic, and patient-reported outcomes of implant treatment. Clin. Oral Implant. Res. 2023, 34, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Veis, A.; Parissis, N.; Tsirlis, A.; Papadeli, C.; Marinis, G.; Zogakis, A. Evaluation of peri-implant marginal bone loss using modified abutment connections at various crestal level placements. Int. J. Periodont. Rest. Dent. 2010, 30, 609–617. [Google Scholar]
- Alonso-González, R.; Aloy-Prósper, A.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M.A.; Peñarrocha-Diago, M. Marginal bone loss in relation to platform switching implant insertion depth: An update. J. Clin. Exp. Dent. 2012, 4, e173–e179. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Nardi, D.; Piattelli, A. One abutment at one time: Non-removal of an immediate abutment and its effect on bone healing around subcrestal tapered implants. Clin. Oral. Implant. Res. 2011, 22, 1303–1307. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Dai, R.; Cao, C.Y.; Fang, H.; Han, M.; Li, Q.L. One-time versus repeated abutment connection for platform-switched implant: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186385. [Google Scholar] [CrossRef]
- Tomar, S.; Saxena, D.; Kaur, N. Marginal bone loss around implants with platform switching and platform matched connection: A systematic review. J. Prosthet. Dent. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A narrative review of the histological and histomorphometrical evaluation of the peri-implant bone in loaded and unloaded dental implants. A 30-year experience (1988–2018). Int. J. Environ. Res. Public Health 2020, 17, 2088. [Google Scholar] [CrossRef]
- Bergamo, E.T.P.; Zahoui, A.; Barrera, R.B.; Huwais, S.; Coelho, P.G.; Karateew, E.D.; Bonfante, E.A. Osseodensification effect on implants primary and secondary stability: Multicenter controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 317–328. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, Y.C.; Ding, S.J. Primary stability of implant placement and loading related to dental implant materials and designs: A literature review. J. Dent. Sci. 2023, 18, 1467–1476. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Piattelli, A.; Raspanti, M.; Mangano, F.; Cassoni, A.; Iezzi, G.; Shibli, J.A. Scanning electron microscopy (SEM) and X-ray dispersive spectrometry evaluation of direct laser metal sintering surface and human bone interface: A case series. Lasers Med. Sci. 2011, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Raspanti, M.; Traini, T.; Piattelli, A.; Sammons, R. Stereo imaging and cytocompatibility of a model dental implant surface formed by direct laser fabrication. J. Biomed. Mater. Res. A 2009, 88, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Lee, D.; Lee, J.; Seol, Y.J.; Lee, Y.M.; Koo, K.T. Comparative evaluation of 3D-printed and conventional implants in vivo: A quantitative microcomputed tomographic and histomorphometric analysis. Sci. Rep. 2023, 13, 21041. [Google Scholar] [CrossRef]
- Iezzi, G.; Zavan, B.; Petrini, M.; Ferroni, L.; Pierfelice, T.V.; D’Amora, U.; Ronca, A.; D’Amico, E.; Mangano, C. 3D printed dental implants with a porous structure: The in vitro response of osteoblasts, fibroblasts, mesenchymal stem cells, and monocytes. J. Dent. 2024, 140, 104778. [Google Scholar] [CrossRef]
- Traini, T.; Mangano, C.; Sammons, R.L.; Mangano, F.; Macchi, A.; Piattelli, A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef]
- Huang, S.; Wei, H.; Li, D. Additive manufacturing technologies in the oral implant clinic: A review of current applications and progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef]
- Oliveira, T.T.; Reis, A.C. Fabrication of dental implants by the additive manufacturing method: A systematic review. J. Prosthet. Dent. 2019, 122, 270–274. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob. Adv. Health Med. 2013, 2, 38–43. [Google Scholar] [CrossRef]
- Louro, R.S.; Moraschini, V.; Melhem-Elias, F.; Sturzinger, G.P.S.; Amad, R.A.; Shibli, J.A. Digital implant-supported restoration planning placed in autologous graft using titanium implants produced by additive manufacturing. Dent. J. 2024, 12, 192. [Google Scholar] [CrossRef]
- Mafra, I.J.; Bordin, D.; Siroma, R.S.; Moraschini, V.; Faverani, L.P.; Souza, J.G.; Mourão, C.F.; Shibli, J.A. Additive manufacturing titanium dental implants placed in sinuses grafted with 70HA:30-TCP: A one-year retrospective study for evaluation of survival rate. Dent. J. 2024, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Soyfer, V. Review article on the All-On-Four treatment concept in dental implants. Arch. Surg. Clin. Res. 2023, 7, 19–23. [Google Scholar] [CrossRef]
- Chan, M.H.; Nudell, Y.A. All-on-4 concept update. Dent. Clin. N. Am. 2021, 65, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; de Araujo Nobre, M.; Lopes, A.; Ferro, A.; Nunes, M. The All-on-4 concept for full-arch rehabilitation of the edentulous maxillae: A longitudinal study with 5-13 years of follow-up. Clin. Implant. Dent. Relat. Res. 2019, 21, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Agliardi, E.; Basso, M.; Romeo, D.; Testori, T.; Francetti, L. Management of dental patients taking anticoagulant drugs. Dent. Cadmos 2008, 76, 13–23. [Google Scholar]
- Kelly, N.; Beaton, L.; Knights, J.; Stirling, D.; West, M.; Young, L. The practices and beliefs of dental professionals regarding the management of patients taking anticoagulant and antiplatelet drugs. BDJ Open 2023, 9, 1. [Google Scholar] [CrossRef]
- Tetè, G.; Polizzi, E.; D’orto, B.; Carinci, G.; Capparè, P. How to consider implant-prosthetic rehabilitation in elderly patients: A narrative review. J. Biol. Regul. Homeost. Agents 2021, 35, 119–126. [Google Scholar] [CrossRef]
- Subramani, K. Is computer-guided implant placement with a flapless approach more accurate than with a flapped surgical approach? Evid. Based Dent. 2022, 23, 110–111. [Google Scholar] [CrossRef]
- Tallarico, M. Computerization and digital workflow in medicine: Focus on digital dentistry. Materials 2020, 13, 2172. [Google Scholar] [CrossRef]
- Todaro, C.; Cerri, M.; Isola, G.; Manazza, A.; Storelli, S.; Rodriguez y Baena, R.; Lupi, S.M. Computer-guided osteotomy with simultaneous implant placement and immediately loaded full-arch fixed restoration: A case report. Prosthesis 2023, 5, 221–233. [Google Scholar] [CrossRef]
- Todaro, C.; Cerri, M.; Rodriguez, Y.; Baena, R.; Lupi, S.M. Full-arch guided restoration and bone regeneration: A complete digital workflow case report. Healthcare 2023, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Inchingolo, A.D.; Mancini, A.; Annicchiarico, C.; Inchingolo, F.; Dipalma, G.; et al. Surface Coatings of Dental Implants: A Review. J. Funct. Biomater. 2023, 22, 287. [Google Scholar] [CrossRef] [PubMed]
- Sonaye, S.Y.; Bokam, V.K.; Saini, A.; Nayak, V.V.; Witek, L.; Coelho, P.G.; Bhaduri, S.B.; Bottino, M.C.; Sikder, P. Patient-specific 3D printed Poly-ether-ether-ketone (PEEK) dental implant system. J. Mech. Behav. Biomed. Mater. 2022, 136, 105510. [Google Scholar] [CrossRef] [PubMed]
- Safi, I.N.; Hussein, B.M.A.; Aljudy, H.J.; Tukmachi, M.S. Effects of long durations of RF–magnetron sputtering deposition of hydroxyapatite on titanium dental implants. Eur. J. Dent. 2021, 15, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Plenum Bioengeharia. Available online: https://plenum.bio/en/products/implants (accessed on 19 September 2024).
- Goguta, L.; Lungeanu, D.; Negru, R.; Birdeanu, M.; Jivanescu, A.; Sinescu, C. Selective laser sintering versus Selective laser melting and Computer aided design—Computer aided manufacturing in double crowns retention. J. Prosthodont. Res. 2021, 65, 371–378. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.B.; Yun, J.; Rhyu, Y.C.; Lee, Y.M.; Lee, S.M.; Lee, M.K.; Kim, B.; Kim, P.; Koo, K.T. The impact of surface treatment in 3-dimensional printed implants for early osseointegration: A comparison study of three different surfaces. Sci. Rep. 2021, 11, 10453. [Google Scholar] [CrossRef]
- Chambrone, L.; Rincón-Castro, M.V.; Poveda-Marín, A.E.; Diazgranados-Lozano, M.P.; Fajardo-Escolar, C.E.; Bocanegra-Puerta, M.C.; Palma, L.F. Histological healing outcomes at the bone-titanium interface of loaded and unloaded dental implants placed in humans: A systematic review of controlled clinical trials. Int. J. Oral Implantol. 2020, 13, 321–342. [Google Scholar]
- Lang, N.P.; Imber, J.C.; Lang, K.N.; Schmid, B.; Muñoz, F.; Bosshardt, D.D.; Saulacic, N. Sequential osseointegration of a novel implant system based on 3D printing in comparison with conventional titanium implants. Clin. Oral Implant. Res. 2023, 34, 627–638. [Google Scholar] [CrossRef]
- Mangano, F.; Mangano, C.; Piattelli, A.; Iezzi, G. Histological evidence of the osseointegration of fractured direct metal laser sintering implants retrieved after 5 years of function. Biomed Res. Int. 2017, 2017, 9732136. [Google Scholar] [CrossRef]
- Łoginoff, J.; Majos, A.; Elgalal, M. The evolution of custom subperiosteal implants for treatment of partial or complete edentulism in patients with severe alveolar ridge atrophy. J. Clin. Med. 2024, 13, 3582. [Google Scholar] [CrossRef]
- Dantas, T.; Vaz, P.; Samuel, F. Subperiosteal dental implants: Past or future? A critical review on clinical trials/case reports and future directions. J. Dent. Implant. 2023, 13, 35–48. [Google Scholar] [CrossRef]
- Mangano, C.; Bianchi, A.; Mangano, F.G.; Dana, J.; Colombo, M.; Solop, I.; Admakin, O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: A case series. 3D Print. Med. 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Onică, N.; Budală, D.G.; Baciu, E.-R.; Onică, C.A.; Gelețu, G.L.; Murariu, A.; Balan, M.; Pertea, M.; Stelea, C. Long-term clinical outcomes of 3D-printed subperiosteal titanium implants: A 6-year follow-up. J. Pers. Med. 2024, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Pingueiro, J.; Piattelli, A.; Paiva, J.; Figueiredo, L.C.; Feres, M.; Shibli, J.; Bueno-Silva, B. Additive manufacturing of titanium alloy could modify the pathogenic microbial profile: An in vitro study. Braz. Oral Res. 2019, 30, e065. [Google Scholar] [CrossRef]
- Andrade, C.X.; Quirynen, M.; Rosenberg, D.R.; Pinto, N.R. Interaction between different implant surfaces and liquid fibrinogen: A pilot in vitro experiment. Biomed Res. Int. 2021, 2021, 9996071. [Google Scholar] [CrossRef]
- da Rocha Scalzer Lopes, G.; de Matos, J.D.M.; Oliveira, D.; Condé Oliveira Prado, P.H.; Garcia Rocha, M.; Coelho Sinhoreti, M.A.; Bottino, M.A.; de Carvalho Ramos, N. Posterior rehabilitation using 3D-printed dental implants and synthetic regenerative biomaterials. Braz. Dent. Sci. 2022, 25, e3572. [Google Scholar] [CrossRef]
- Heydari, M.; Ataei, A.; Riahi, S.M. Long-term effect of keratinized tissue width on peri-implant health status indices: An updated systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2021, 36, 1065–1075. [Google Scholar] [CrossRef]
- Oh, S.L.; Shahami, S.; Bernal-Cepeda, L.J.; Fu, Y.; Chung, M.K. Therapeutic effectiveness of keratinized mucosa augmentation for functioning dental implants: A systematic review and meta-analysis. J. Prosthodont. Res. 2024. Epub ahead of print. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho Formiga, M.; Fuller, R.; Ardelean, L.C.; Shibli, J.A. Immediate Loading Full-Arch 3D-Printed Implant-Supported Fixed Rehabilitation: A Case Report with 24-Month Follow-Up. Medicina 2024, 60, 1614. https://doi.org/10.3390/medicina60101614
de Carvalho Formiga M, Fuller R, Ardelean LC, Shibli JA. Immediate Loading Full-Arch 3D-Printed Implant-Supported Fixed Rehabilitation: A Case Report with 24-Month Follow-Up. Medicina. 2024; 60(10):1614. https://doi.org/10.3390/medicina60101614
Chicago/Turabian Stylede Carvalho Formiga, Márcio, Renato Fuller, Lavinia Cosmina Ardelean, and Jamil Awad Shibli. 2024. "Immediate Loading Full-Arch 3D-Printed Implant-Supported Fixed Rehabilitation: A Case Report with 24-Month Follow-Up" Medicina 60, no. 10: 1614. https://doi.org/10.3390/medicina60101614
APA Stylede Carvalho Formiga, M., Fuller, R., Ardelean, L. C., & Shibli, J. A. (2024). Immediate Loading Full-Arch 3D-Printed Implant-Supported Fixed Rehabilitation: A Case Report with 24-Month Follow-Up. Medicina, 60(10), 1614. https://doi.org/10.3390/medicina60101614








