Association between Polypharmacy and Adverse Events in Patients with Alzheimer’s Disease: An Analysis of the Japanese Adverse Drug Event Report Database (JADER)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
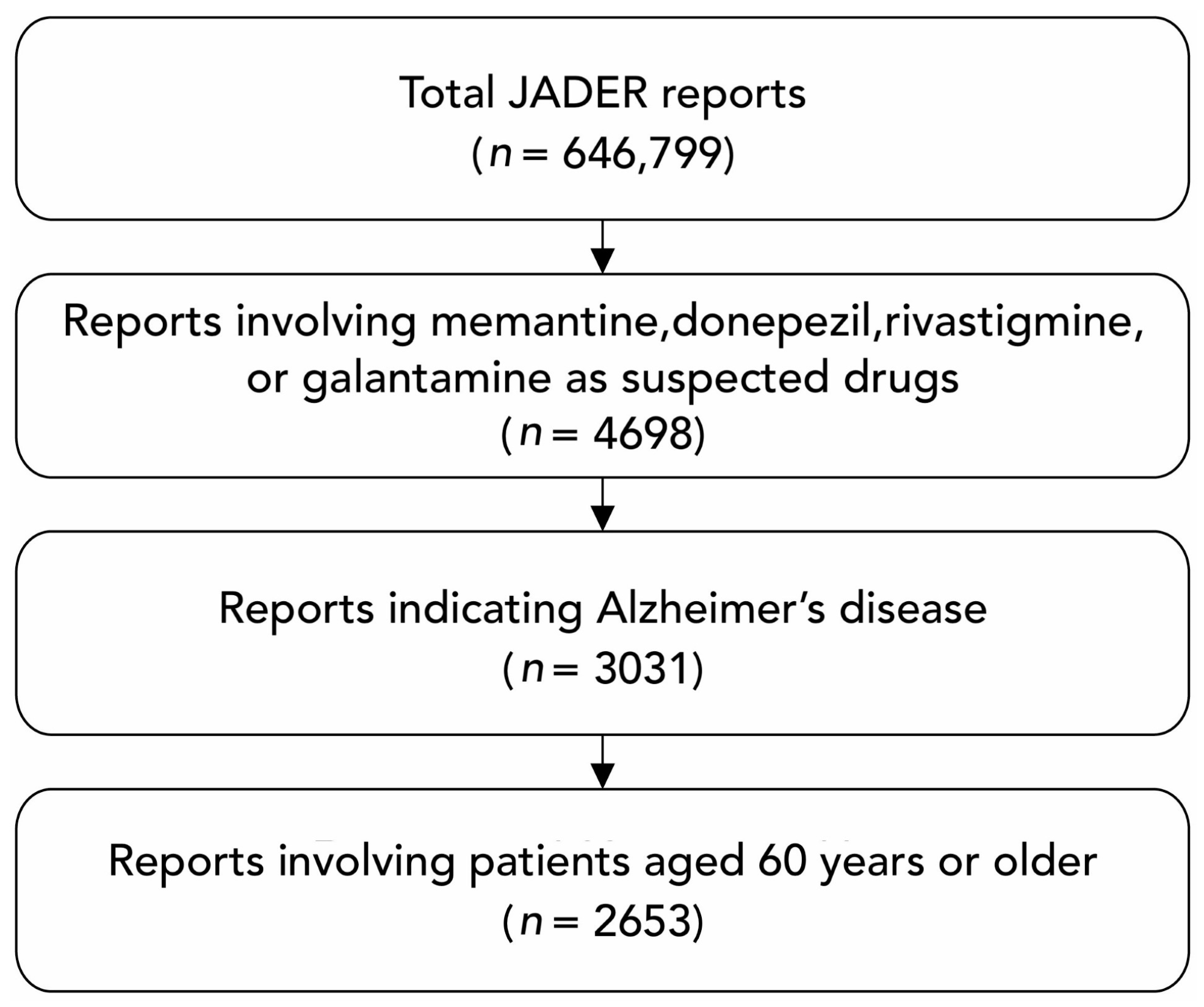
2.2. Data Processing
2.3. Adverse Events
2.4. Variables
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Characteristics of the Study Population
3.2. Association between Alzheimer’s Disease Treatment Combinations and Adverse Events
3.3. Association between the Number of Concomitant Medications and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Dementia: Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 4 August 2024).
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013.
- Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef]
- Japanese Society of Neurology: Clinical Practice Guideline for Dementia. 2017. Available online: https://www.neurology-jp.org/guidelinem/dementia/documents/guideline2017.pdf (accessed on 24 August 2024).
- Alzheimer’s Disease International; Wimo, A.; Gemma-Claire, A.; Guerchet, M.; Prince, M.; Prina, M.; Wu, Y.-T. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Available online: https://www.alzint.org/u/WorldAlzheimerReport2015.pdf (accessed on 3 August 2024).
- Ikejima, C.; Hisanaga, A.; Meguro, K.; Yamada, T.; Ouma, S.; Kawamuro, Y.; Hyouki, K.; Nakashima, K.; Wada, K.; Yamada, S.; et al. Multicentre population-based dementia prevalence survey in Japan: A preliminary report. Psychogeriatrics 2012, 12, 120–123. [Google Scholar] [CrossRef]
- The Ministry of Health Labour and Welfare, Health Labour Sciences Research Grant: Report on Future Projections of the Elderly Population with Dementia in Japan. Available online: https://mhlw-grants.niph.go.jp/project/23685 (accessed on 4 August 2024).
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Chandra Das, D.; Sunna, T.C.; Beyene, J.; Hossain, A. Global and regional prevalence of multimorbidity in the adult population in community settings: A systematic review and meta-analysis. EClinicalMedicine 2023, 57, 101860. [Google Scholar] [CrossRef]
- Bunn, F.; Burn, A.M.; Goodman, C.; Rait, G.; Norton, S.; Robinson, L.; Schoeman, J.; Brayne, C. Comorbidity and dementia: A scoping review of the literature. BMC Med. 2014, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Parsons, P. Polypharmacy and inappropriate medication use in patients with dementia: An underresearched problem. Ther. Adv. Drug. Saf. 2017, 8, 31–46. [Google Scholar] [CrossRef]
- Growdon, M.E.; Gan, S.; Yaffe, K.; Steinman, M.A. Polypharmacy among older adults with dementia compared with those without dementia in the United States. J. Am. Geriatr. Soc. 2021, 69, 2464–2475. [Google Scholar] [CrossRef]
- World Health Organization. Medication Safety in Polypharmacy. Available online: https://www.who.int/docs/default-source/patient-safety/who-uhc-sds-2019-11-eng.pdf (accessed on 4 August 2024).
- Nicholson, K.; Liu, W.; Fitzpatrick, D.; Hardacre, K.A.; Roberts, S.; Salerno, J.; Stranges, S.; Fortin, M.; Mangin, D. Prevalence of multimorbidity and polypharmacy among adults and older adults: A systematic review. Lancet Healthy Longev 2024, 5, e287–e296. [Google Scholar] [CrossRef]
- Borda, M.G.; Castellanos-Perilla, N.; Tovar-Rios, D.A.; Oesterhus, R.; Soennesyn, H.; Aarsland, D. Polypharmacy is associated with functional decline in Alzheimer’s disease and Lewy body dementia. Arch. Gerontol. Geriatr. 2021, 96, 104459. [Google Scholar] [CrossRef]
- Mohammad, D.; Chan, P.; Bradley, J.; Lanctôt, K.; Herrmann, N. Acetylcholinesterase inhibitors for treating dementia symptoms—A safety evaluation. Expert Opin. Drug. Saf. 2017, 16, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug. Saf. 2018, 9, 171–178. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kishi, T.; Nomura, I.; Sakuma, K.; Okuya, M.; Ikuta, T.; Iwata, N. The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin. Drug. Saf. 2018, 17, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.B.; Schleret, T.R.; Reilly, B.M.; Chen, W.Y.; Abagyan, R. Adverse Effects of Cholinesterase Inhibitors in Dementia, According to the Pharmacovigilance Databases of the United-States and Canada. PLoS ONE 2015, 10, e0144337. [Google Scholar] [CrossRef] [PubMed]
- Esumi, S.; Ushio, S.; Zamami, Y. Polypharmacy in Older Adults with Alzheimer’s Disease. Medicina (Kaunas) 2022, 58, 1445. [Google Scholar] [CrossRef]
- Shi, X.; Lin, X.; Hu, R.; Sun, N.; Hao, J.; Gao, C. Toxicological Differences Between NMDA Receptor Antagonists and Cholinesterase Inhibitors. Am. J. Alzheimers Dis. Other. Demen. 2016, 31, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Mano, T.; Iwata, A.; Toda, T. Safety of Memantine in Combination with Potentially Interactive Drugs in the Real World: A Pharmacovigilance Study Using the Japanese Adverse Drug Event Report (JADER) Database. J. Alzheimers Dis. 2021, 82, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sugama, N.; Nagano, H.; Miyamori, A.; Takahashi, M.; Kushiyama, A. Analysis of Adverse Events of Cholinesterase Inhibitors and NMDA Receptor Antagonists on Arrhythmias Using the Japanese Adverse Drug Event Report Database. Drugs Real World Outcomes 2023, 10, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kose, E.; Yamamoto, T.; Tate, N.; Ando, A.; Enomoto, H.; Yasuno, N. Adverse Drug Event Profile Associated with Anti-dementia Drugs: Analysis of a Spontaneous Reporting Database. Pharmazie 2023, 78, 42–46. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Li, Y. Safety and efficacy of acetylcholinesterase inhibitors for Alzheimer’s disease: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lampela, P.; Tolppanen, A.M.; Tanskanen, A.; Tiihonen, J.; Lavikainen, P.; Hartikainen, S.; Taipale, H. Use of antidementia drugs and risk of pneumonia in older persons with Alzheimer’s disease. Ann. Med. 2017, 49, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Akishita, M.; Kameyama, Y.; Yamaguchi, K.; Yamamoto, H.; Eto, M.; Ouchi, Y. High risk of adverse drug reactions in elderly patients taking six or more drugs: Analysis of inpatient database. Geriatr. Gerontol. Int. 2012, 12, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Akishita, M.; Nakamura, T.; Nomura, K.; Ogawa, S.; Iijima, K.; Eto, M.; Ouchi, Y. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr. Gerontol. Int. 2012, 12, 425–430. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Health Labour and Welfare, Health Labour. Guidelines for the Appropriate Use of Medications in the Elderly. The Ministry of Health Labour and Welfare 2018. Available online: https://www.mhlw.go.jp/content/11121000/kourei-tekisei_web.pdf (accessed on 15 August 2024).
- Maust, D.T.; Strominger, J.; Kim, H.M.; Langa, K.M.; Bynum, J.P.W.; Chang, C.H.; Kales, H.C.; Zivin, K.; Solway, E.; Marcus, S.C. Prevalence of Central Nervous System-Active Polypharmacy Among Older Adults With Dementia in the US. JAMA 2021, 325, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, J.W.; Song, H.J.; Sohn, H.S.; Kwon, J.W. The Association between Polypharmacy and Dementia: A Nested Case-Control Study Based on a 12-Year Longitudinal Cohort Database in South Korea. PLoS ONE 2017, 12, e0169463. [Google Scholar] [CrossRef] [PubMed]
- Delara, M.; Murray, L.; Jafari, B.; Bahji, A.; Goodarzi, Z.; Kirkham, J.; Chowdhury, M.; Seitz, D.P. Prevalence and factors associated with polypharmacy: A systematic review and Meta-analysis. BMC Geriatr. 2022, 22, 601. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ishizaki, T.; Masui, Y.; Hori, N.; Inagaki, H.; Ito, K.; Ogawa, M.; Yasumoto, S.; Arai, Y.; Kamide, K.; et al. Effect of number of medications on the risk of falls among community-dwelling older adults: A 3-year follow-up of the SONIC study. Geriatr. Gerontol. Int. 2024, 24, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.E.; Talbot, D.; Tremblay, F.; Desforges, K.; Sirois, C. Polypharmacy and risk of fractures in older adults: A systematic review. J. Evid. Based Med. 2024, 17, 145–171. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
| Alzheimer’s disease |
| Frontotemporal lobar degeneration |
| Lewy body disease |
| Vascular disease |
| Traumatic brain injury |
| Substance/medication use |
| HIV infection |
| Prion disease |
| Parkinson’s disease |
| Huntington’s disease |
| Another medical condition |
| Multiple etiologies |
| Unspecified |
| Drug | Mechanism | Pharmacokinetics |
|---|---|---|
| Donepezil | AChE inhibitor | Absorbed orally, reaching peak plasma concentration in about 3–5 h. Metabolized primarily by CYP2D6 and CYP3A4 in the liver. Excreted mainly in the urine. Half-life is 70–80 h. |
| Galantamine | AChE inhibitor APL action on nAchR | Absorbed orally, reaching peak plasma concentration in 1 h. Metabolized by CYP2D6 and CYP3A4 in the liver. Excreted mainly in the urine. Half-life is 5–7 h. |
| Rivastigmine | AChE inhibitor BuChE inhibitor | Administered orally or via patch. Metabolized by esterases. The half-life is about 1.5 h for oral administration and 3.4 h for patches. Excreted primarily in urine. |
| Memantine | Glutamate NMDA receptor antagonist | Absorbed orally, reaching peak plasma concentration in 1–7 h. Excreted primarily in urine. The half-life is 60–80 h. |
| Variables | n | % | |
|---|---|---|---|
| Sex | Male | 1055 | 39.8% |
| Female | 1598 | 60.2% | |
| Age | 60–69 | 131 | 4.9% |
| 70–79 | 786 | 29.6% | |
| 80–89 | 1420 | 53.5% | |
| 90+ | 316 | 11.9% | |
| Combination with Alzheimer’s disease therapeutics | Memantine | 207 | 7.8% |
| Donepezil | 1089 | 41.0% | |
| Rivastigmine | 664 | 25.0% | |
| Galantamine | 458 | 17.3% | |
| Memantine+AChEI | 235 | 8.9% | |
| The number of concomitant medications used | 0 drugs | 463 | 17.5% |
| 1 drug | 271 | 10.2% | |
| 2 drugs | 227 | 8.6% | |
| 3 drugs | 264 | 10.0% | |
| 4 drugs | 162 | 6.1% | |
| 5 drugs or more | 1266 | 47.7% | |
| Comorbidity | Diabetes mellitus | 325 | 12.3% |
| Hypertension | 935 | 35.2% | |
| Hyperlipidemia | 360 | 13.6% | |
| Ischemic heart diseases | 162 | 6.1% | |
| Cerebrovascular diseases | 283 | 10.7% | |
| Malignant neoplasms | 82 | 3.1% | |
| Depression | 124 | 4.7% | |
| Parkinson’s disease | 113 | 4.3% | |
| Sleep disorders | 197 | 7.4% |
| Number of Cases | Percentage | Cumulative Percentage | |
|---|---|---|---|
| Bradycardia | 171 | 6.4% | 6.4% |
| Pneumonia | 122 | 4.6% | 11.0% |
| Altered state of consciousness | 96 | 3.6% | 14.7% |
| Seizures | 93 | 3.5% | 18.2% |
| Decreased appetite | 93 | 3.5% | 21.7% |
| Vomiting | 92 | 3.5% | 25.1% |
| Loss of consciousness | 91 | 3.4% | 28.6% |
| Fractures | 91 | 3.4% | 32.0% |
| Cardiac failure | 84 | 3.2% | 35.2% |
| Falls | 80 | 3.0% | 38.2% |
| Bradycardia | Pneumonia | ||||||||||
| Bivariate | Multivariate | Bivariate | Multivariate | ||||||||
| Total | n | OR | p | AOR | p | n | OR | p | AOR | p | |
| Sex | |||||||||||
| Male | 1055 | 66 | ref | 0.746 | ref | 0.920 | 72 | ref | <0.0001 | ref | <0.0001 |
| Female | 1598 | 105 | 1.05 | 1.02 | 50 | 0.44 | 0.40 | ||||
| Age | |||||||||||
| 60–69 | 131 | 7 | ref | 0.032 | ref | 0.063 | 3 | ref | 0.071 | ref | 0.067 |
| 70–79 | 786 | 39 | 0.92 | 0.89 | 28 | 1.58 | 1.48 | ||||
| 80–89 | 1420 | 110 | 1.49 | 1.38 | 70 | 2.21 | 2.22 | ||||
| 90 and older | 316 | 15 | 0.88 | 0.84 | 21 | 3.04 | 3.00 | ||||
| Combination with Alzheimer’s disease therapeutics | |||||||||||
| Memantine | 201 | 6 | ref | 0.018 | ref | 0.016 | 13 | ref | <0.0001 | ref | <0.0001 |
| Donepezil | 1006 | 83 | 2.76 | 3.07 | 25 | 0.35 | 0.37 | ||||
| Rivastigmine | 632 | 32 | 1.70 | 2.00 | 60 | 1.48 | 1.43 | ||||
| Galantamine | 423 | 35 | 2.77 | 3.12 | 21 | 0.72 | 0.73 | ||||
| Memantine + AChEI | 220 | 15 | 2.28 | 2.49 | 3 | 0.19 | 0.19 | ||||
| The number of concomitant medications used | |||||||||||
| 0 drugs | 163 | 20 | ref | 0.201 | ref | 0.207 | 31 | ref | 0.201 | ref | 0.845 |
| 1 drug | 271 | 20 | 1.76 | 1.65 | 13 | 0.70 | 0.79 | ||||
| 2 drugs | 227 | 15 | 1.57 | 1.36 | 8 | 0.51 | 0.82 | ||||
| 3 drugs | 264 | 24 | 2.22 | 2.16 | 10 | 0.55 | 0.75 | ||||
| 4 drugs | 162 | 9 | 1.30 | 1.03 | 4 | 0.35 | 0.64 | ||||
| 5 drugs or more | 1266 | 83 | 1.55 | 1.38 | 56 | 0.64 | 1.02 | ||||
| Comorbidities | |||||||||||
| Diabetes mellitus | 325 | 23 | 1.12 | 0.625 | 0.93 | 0.828 | 11 | 0.70 | 0.246 | 0.72 | 0.319 |
| Hypertension | 935 | 86 | 1.95 | <0.0001 | 2.00 | <0.0001 | 27 | 0.51 | 0.001 | 0.55 | 0.013 |
| Hyperlipidemia | 360 | 21 | 0.89 | 0.611 | 0.66 | 0.083 | 14 | 0.82 | 0.480 | 1.45 | 0.257 |
| Ischemic heart diseases | 162 | 16 | 1.65 | 0.069 | 1.48 | 0.196 | 6 | 0.79 | 0.563 | 0.90 | 0.803 |
| Cerebrovascular diseases | 283 | 15 | 0.79 | 0.408 | 0.67 | 0.141 | 11 | 0.82 | 0.536 | 0.97 | 0.937 |
| Malignant neoplasms | 82 | 2 | 0.36 | 0.170 | 0.31 | 0.056 | 9 | 2.68 | 0.016 | 2.16 | 0.066 |
| Depression | 124 | 4 | 0.47 | 0.186 | 0.48 | 0.125 | 6 | 1.06 | 0.897 | 1.55 | 0.356 |
| Parkinson’s disease | 113 | 4 | 0.52 | 0.206 | 0.57 | 0.235 | 11 | 2.36 | 0.019 | 2.29 | 0.031 |
| Sleep disorders | 197 | 9 | 0.68 | 0.268 | 0.67 | 0.235 | 4 | 0.41 | 0.047 | 0.43 | 0.077 |
| Altered State of Consciousness | Seizures | ||||||||||
| Bivariate | Multivariate | Bivariate | Multivariate | ||||||||
| Total | n | OR | p | AOR | p | n | OR | p | AOR | p | |
| Sex | |||||||||||
| Male | 1055 | 36 | ref | 0.643 | ref | 0.612 | 42 | ref | 0.282 | ref | 0.234 |
| Female | 1598 | 60 | 1.10 | 1.12 | 51 | 0.80 | 0.77 | ||||
| Age | |||||||||||
| 60–69 | 131 | 7 | ref | 0.386 | ref | 0.419 | 6 | ref | 0.881 | ref | 0.793 |
| 70–79 | 786 | 25 | 0.58 | 0.58 | 29 | 0.80 | 0.78 | ||||
| 80–89 | 1420 | 56 | 0.73 | 0.71 | 48 | 0.73 | 0.68 | ||||
| 90 and older | 316 | 8 | 0.46 | 0.45 | 10 | 0.68 | 0.62 | ||||
| Combination with Alzheimer’s disease therapeutics | |||||||||||
| Memantine | 201 | 13 | ref | 0.096 | ref | 0.502 | 11 | ref | 0.058 | ref | 0.103 |
| Donepezil | 1006 | 43 | 0.61 | 0.74 | 45 | 0.77 | 0.84 | ||||
| Rivastigmine | 632 | 15 | 0.34 | 0.51 | 15 | 0.41 | 0.46 | ||||
| Galantamine | 423 | 17 | 0.58 | 0.75 | 11 | 0.44 | 0.47 | ||||
| Memantine + AChEI | 220 | 8 | 0.53 | 0.57 | 11 | 0.88 | 0.96 | ||||
| The number of concomitant medications used | |||||||||||
| 0 drugs | 163 | 2 | ref | <0.0001 | ref | <0.0001 | 14 | ref | 0.856 | ref | 0.839 |
| 1 drug | 271 | 8 | 7.01 | 6.93 | 10 | 1.23 | 1.20 | ||||
| 2 drugs | 227 | 6 | 6.26 | 5.90 | 7 | 1.02 | 0.85 | ||||
| 3 drugs | 264 | 19 | 17.88 | 17.53 | 8 | 1.00 | 0.90 | ||||
| 4 drugs | 162 | 5 | 7.34 | 6.80 | 4 | 0.81 | 0.63 | ||||
| 5 drugs or more | 1266 | 56 | 10.67 | 10.45 | 50 | 1.32 | 1.13 | ||||
| Comorbidities | |||||||||||
| Diabetes mellitus | 325 | 9 | 0.73 | 0.364 | 0.61 | 0.153 | 5 | 0.40 | 0.023 | 0.34 | 0.008 |
| Hypertension | 935 | 37 | 1.16 | 0.494 | 0.96 | 0.874 | 35 | 1.11 | 0.625 | 1.13 | 0.621 |
| Hyperlipidemia | 360 | 15 | 1.19 | 0.557 | 1.02 | 0.944 | 11 | 0.85 | 0.611 | 0.79 | 0.482 |
| Ischemic heart diseases | 162 | 7 | 1.22 | 0.631 | 1.09 | 0.829 | 7 | 1.26 | 0.573 | 1.26 | 0.589 |
| Cerebrovascular diseases | 283 | 11 | 1.09 | 0.800 | 0.94 | 0.861 | 12 | 1.25 | 0.489 | 1.23 | 0.533 |
| Malignant neoplasms | 82 | 3 | 1.13 | 1.000 | 0.88 | 0.835 | 1 | 0.33 | 0.367 | 0.32 | 0.175 |
| Depression | 124 | 5 | 1.53 | 0.803 | 0.93 | 0.881 | 2 | 0.44 | 0.321 | 0.38 | 0.123 |
| Parkinson’s disease | 113 | 6 | 1.53 | 0.300 | 1.43 | 0.437 | 2 | 0.48 | 0.435 | 0.44 | 0.208 |
| Sleep disorders | 197 | 7 | 0.98 | 0.959 | 0.78 | 0.537 | 11 | 1.71 | 0.126 | 1.68 | 0.149 |
| Decreased Appetite | Vomiting | ||||||||||
| Bivariate | Multivariate | Bivariate | Multivariate | ||||||||
| Total | n | OR | p | AOR | p | n | OR | p | AOR | p | |
| Sex | |||||||||||
| Male | 1055 | 35 | ref | 0.668 | ref | 0.647 | 18 | ref | <0.0001 | ref | <0.0001 |
| Female | 1598 | 58 | 1.10 | 1.11 | 74 | 2.80 | 2.71 | ||||
| Age | |||||||||||
| 60–69 | 131 | 1 | ref | 0.351 | ref | 0.244 | 3 | ref | 0.680 | ref | 0.216 |
| 70–79 | 786 | 30 | 5.16 | 5.00 | 31 | 1.75 | 1.71 | ||||
| 80–89 | 1420 | 51 | 4.84 | 4.25 | 49 | 1.52 | 1.22 | ||||
| 90 and older | 316 | 11 | 4.69 | 4.32 | 9 | 1.25 | 0.80 | ||||
| Combination with Alzheimer’s disease therapeutics | |||||||||||
| Memantine | 201 | 5 | ref | 0.270 | ref | 0.126 | 5 | ref | 0.001 | ref | <0.0001 |
| Donepezil | 1006 | 48 | 1.86 | 2.22 | 25 | 0.95 | 1.01 | ||||
| Rivastigmine | 632 | 22 | 1.38 | 2.06 | 39 | 2.52 | 3.56 | ||||
| Galantamine | 423 | 12 | 1.09 | 1.41 | 19 | 1.75 | 2.10 | ||||
| Memantine + AChEI | 220 | 6 | 1.06 | 0.99 | 4 | 0.70 | 0.63 | ||||
| The number of concomitant medications used | |||||||||||
| 0 drugs | 163 | 3 | ref | 0.000 | ref | 0.000 | 6 | ref | 0.011 | ref | 0.000 |
| 1 drug | 271 | 9 | 5.27 | 5.06 | 9 | 2.62 | 3.11 | ||||
| 2 drugs | 227 | 6 | 4.16 | 4.31 | 15 | 5.39 | 7.87 | ||||
| 3 drugs | 264 | 6 | 3.57 | 3.66 | 9 | 2.69 | 3.78 | ||||
| 4 drugs | 162 | 9 | 9.02 | 9.72 | 8 | 3.96 | 6.85 | ||||
| 5 drugs or more | 1266 | 60 | 7.63 | 7.92 | 45 | 2.81 | 4.74 | ||||
| Comorbidities | |||||||||||
| Diabetes mellitus | 325 | 14 | 1.28 | 0.415 | 0.99 | 0.970 | 10 | 0.87 | 0.676 | 0.89 | 0.725 |
| Hypertension | 935 | 44 | 1.68 | 0.015 | 1.18 | 0.468 | 36 | 1.19 | 0.430 | 1.09 | 0.714 |
| Hyperlipidemia | 360 | 17 | 1.45 | 0.195 | 1.06 | 0.831 | 13 | 1.05 | 0.874 | 0.89 | 0.724 |
| Ischemic heart diseases | 162 | 11 | 2.14 | 0.035 | 1.59 | 0.198 | 6 | 1.08 | 0.867 | 1.16 | 0.741 |
| Cerebrovascular diseases | 283 | 12 | 1.25 | 0.489 | 0.90 | 0.752 | 7 | 0.68 | 0.311 | 0.59 | 0.173 |
| Malignant neoplasms | 82 | 4 | 1.43 | 0.532 | 1.32 | 0.616 | 1 | 0.34 | 0.367 | 0.37 | 0.255 |
| Depression | 124 | 3 | 0.67 | 0.800 | 0.48 | 0.183 | 0 | - | - | - | - |
| Parkinson’s disease | 113 | 4 | 1.01 | 1.000 | 0.84 | 0.729 | 2 | 0.49 | 0.434 | 0.38 | 0.126 |
| Sleep disorders | 197 | 9 | 1.35 | 0.418 | 1.05 | 0.890 | 7 | 1.03 | 0.946 | 0.93 | 0.865 |
| Loss of Consciousness | Fractures | ||||||||||
| Bivariate | Multivariate | Bivariate | Multivariate | ||||||||
| Total | n | OR | p | AOR | p | n | OR | p | AOR | p | |
| Sex | |||||||||||
| Male | 1055 | 34 | ref | 0.632 | ref | 0.613 | 18 | ref | <0.0001 | ref | 0.000 |
| Female | 1598 | 57 | 1.11 | 1.11 | 73 | 2.76 | 2.60 | ||||
| Age | |||||||||||
| 60–69 | 131 | 7 | ref | 0.230 | ref | 0.171 | 1 | ref | 0.057 | ref | 0.523 |
| 70–79 | 786 | 24 | 0.56 | 0.55 | 22 | 3.74 | 3.24 | ||||
| 80–89 | 1420 | 44 | 0.57 | 0.44 | 52 | 4.94 | 3.47 | ||||
| 90 and older | 316 | 16 | 0.94 | 0.75 | 16 | 6.93 | 3.47 | ||||
| Combination with Alzheimer’s disease therapeutics | |||||||||||
| Memantine | 201 | 16 | ref | <0.0001 | ref | <0.0001 | 12 | ref | <0.0001 | ref | 0.000 |
| Donepezil | 1006 | 13 | 0.14 | 0.15 | 16 | 0.24 | 0.28 | ||||
| Rivastigmine | 632 | 16 | 0.29 | 0.34 | 36 | 0.93 | 0.99 | ||||
| Galantamine | 423 | 31 | 0.87 | 0.96 | 19 | 0.70 | 0.80 | ||||
| Memantine + AChEI | 220 | 15 | 0.81 | 0.83 | 8 | 0.57 | 0.62 | ||||
| The number of concomitant medications used | |||||||||||
| 0 drugs | 163 | 7 | ref | 0.126 | ref | 0.318 | 18 | ref | 0.143 | ref | 0.184 |
| 1 drug | 271 | 8 | 1.98 | 2.27 | 11 | 1.05 | 1.21 | ||||
| 2 drugs | 227 | 10 | 3.00 | 3.18 | 3 | 0.33 | 0.42 | ||||
| 3 drugs | 264 | 9 | 2.30 | 2.18 | 5 | 0.48 | 0.59 | ||||
| 4 drugs | 162 | 6 | 2.51 | 2.28 | 4 | 0.63 | 0.87 | ||||
| 5 drugs or more | 1266 | 51 | 2.73 | 2.25 | 50 | 1.02 | 1.31 | ||||
| Comorbidities | |||||||||||
| Diabetes mellitus | 325 | 8 | 0.68 | 0.285 | 0.65 | 0.244 | 5 | 0.41 | 0.027 | 0.50 | 0.108 |
| Hypertension | 935 | 36 | 1.21 | 0.384 | 1.00 | 0.998 | 35 | 1.15 | 0.516 | 1.25 | 0.363 |
| Hyperlipidemia | 360 | 14 | 1.16 | 0.613 | 0.97 | 0.928 | 7 | 0.52 | 0.074 | 0.45 | 0.033 |
| Ischemic heart diseases | 162 | 13 | 2.70 | 0.004 | 3.01 | 0.003 | 5 | 0.89 | 0.801 | 1.02 | 0.975 |
| Cerebrovascular diseases | 283 | 16 | 1.83 | 0.044 | 1.87 | 0.050 | 7 | 0.69 | 0.328 | 0.71 | 0.386 |
| Malignant neoplasms | 82 | 2 | 0.70 | 1.000 | 0.74 | 0.678 | 1 | 0.34 | 0.528 | 0.37 | 0.258 |
| Depression | 124 | 2 | 0.45 | 0.442 | 0.51 | 0.308 | 1 | 0.22 | 0.127 | 0.22 | 0.059 |
| Parkinson’s disease | 113 | 3 | 0.76 | 1.000 | 0.82 | 0.733 | 4 | 1.03 | 0.794 | 1.05 | 0.931 |
| Sleep disorders | 197 | 4 | 0.56 | 0.228 | 0.47 | 0.118 | 10 | 1.57 | 0.191 | 1.57 | 0.240 |
| Cardiac Failure | Falls | ||||||||||
| Bivariate | Multivariate | Bivariate | Multivariate | ||||||||
| Total | n | OR | p | AOR | p | n | OR | p | AOR | p | |
| Sex | |||||||||||
| Male | 1055 | 32 | ref | 0.751 | ref | 0.850 | 24 | ref | 0.065 | ref | 0.129 |
| Female | 1598 | 52 | 1.08 | 1.05 | 56 | 1.56 | 1.47 | ||||
| Age | |||||||||||
| 60–69 | 131 | 2 | ref | 0.007 | ref | 0.006 | 3 | ref | 0.587 | ref | 0.749 |
| 70–79 | 786 | 23 | 1.94 | 2.00 | 19 | 1.06 | 0.88 | ||||
| 80–89 | 1420 | 38 | 1.77 | 1.78 | 48 | 1.49 | 1.02 | ||||
| 90 and older | 316 | 21 | 4.59 | 4.80 | 10 | 1.39 | 0.70 | ||||
| Combination with Alzheimer’s disease therapeutics | |||||||||||
| Memantine | 201 | 4 | ref | 0.708 | ref | 0.607 | 12 | ref | <0.0001 | ref | 0.000 |
| Donepezil | 1006 | 36 | 1.74 | 2.04 | 14 | 0.21 | 0.28 | ||||
| Rivastigmine | 632 | 20 | 1.58 | 1.64 | 25 | 0.64 | 0.98 | ||||
| Galantamine | 423 | 14 | 1.60 | 1.80 | 15 | 0.55 | 0.76 | ||||
| Memantine + AChEI | 220 | 10 | 2.26 | 2.27 | 14 | 1.03 | 1.16 | ||||
| The number of concomitant medications used | |||||||||||
| 0 drug | 163 | 14 | ref | 0.670 | ref | 0.594 | 4 | ref | 0.004 | ref | 0.002 |
| 1 drug | 271 | 7 | 0.85 | 0.82 | 10 | 4.40 | 5.33 | ||||
| 2 drugs | 227 | 6 | 0.87 | 0.92 | 5 | 2.58 | 3.04 | ||||
| 3 drugs | 264 | 5 | 0.62 | 0.61 | 8 | 3.59 | 4.49 | ||||
| 4 drugs | 162 | 6 | 1.23 | 1.37 | 2 | 1.43 | 1.72 | ||||
| 5 drugs or more | 1266 | 46 | 1.21 | 1.28 | 51 | 4.82 | 5.95 | ||||
| Comorbidities | |||||||||||
| Diabetes mellitus | 325 | 13 | 1.32 | 0.375 | 1.41 | 0.301 | 4 | 0.37 | 0.025 | 0.35 | 0.022 |
| Hypertension | 935 | 29 | 0.97 | 0.888 | 0.84 | 0.486 | 30 | 1.11 | 0.669 | 1.00 | 0.986 |
| Hyperlipidemia | 360 | 10 | 0.86 | 0.645 | 0.79 | 0.511 | 9 | 0.80 | 0.528 | 0.70 | 0.322 |
| Ischemic heart diseases | 162 | 6 | 1.19 | 0.694 | 1.06 | 0.893 | 2 | 0.39 | 0.234 | 0.39 | 0.142 |
| Cerebrovascular diseases | 283 | 10 | 1.14 | 0.713 | 1.15 | 0.698 | 7 | 0.80 | 0.562 | 0.76 | 0.502 |
| Malignant neoplasms | 82 | 4 | 1.60 | 0.329 | 1.62 | 0.396 | 1 | 0.39 | 0.516 | 0.37 | 0.259 |
| Depression | 124 | 3 | 0.75 | 0.797 | 0.70 | 0.540 | 1 | 0.25 | 0.181 | 0.22 | 0.056 |
| Parkinson’s disease | 113 | 1 | 0.26 | 0.264 | 0.28 | 0.117 | 1 | 0.28 | 0.259 | 0.24 | 0.077 |
| Sleep disorders | 197 | 7 | 1.14 | 0.751 | 0.97 | 0.939 | 12 | 2.28 | 0.002 | 2.15 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otani, N.; Kanda, K.; Ngatu, N.R.; Murakami, A.; Yamadori, Y.; Hirao, T. Association between Polypharmacy and Adverse Events in Patients with Alzheimer’s Disease: An Analysis of the Japanese Adverse Drug Event Report Database (JADER). Medicina 2024, 60, 1633. https://doi.org/10.3390/medicina60101633
Otani N, Kanda K, Ngatu NR, Murakami A, Yamadori Y, Hirao T. Association between Polypharmacy and Adverse Events in Patients with Alzheimer’s Disease: An Analysis of the Japanese Adverse Drug Event Report Database (JADER). Medicina. 2024; 60(10):1633. https://doi.org/10.3390/medicina60101633
Chicago/Turabian StyleOtani, Nobuhiro, Kanae Kanda, Nlandu Roger Ngatu, Akitsu Murakami, Yusuke Yamadori, and Tomohiro Hirao. 2024. "Association between Polypharmacy and Adverse Events in Patients with Alzheimer’s Disease: An Analysis of the Japanese Adverse Drug Event Report Database (JADER)" Medicina 60, no. 10: 1633. https://doi.org/10.3390/medicina60101633
APA StyleOtani, N., Kanda, K., Ngatu, N. R., Murakami, A., Yamadori, Y., & Hirao, T. (2024). Association between Polypharmacy and Adverse Events in Patients with Alzheimer’s Disease: An Analysis of the Japanese Adverse Drug Event Report Database (JADER). Medicina, 60(10), 1633. https://doi.org/10.3390/medicina60101633






