Arthroscopic Debridement Enhanced by Intra-Articular Antibiotic-Loaded Calcium Sulphate Beads for Septic Arthritis of a Native Knee Following Iatrogenic Joint Injection: A Case Report
Abstract
1. Introduction
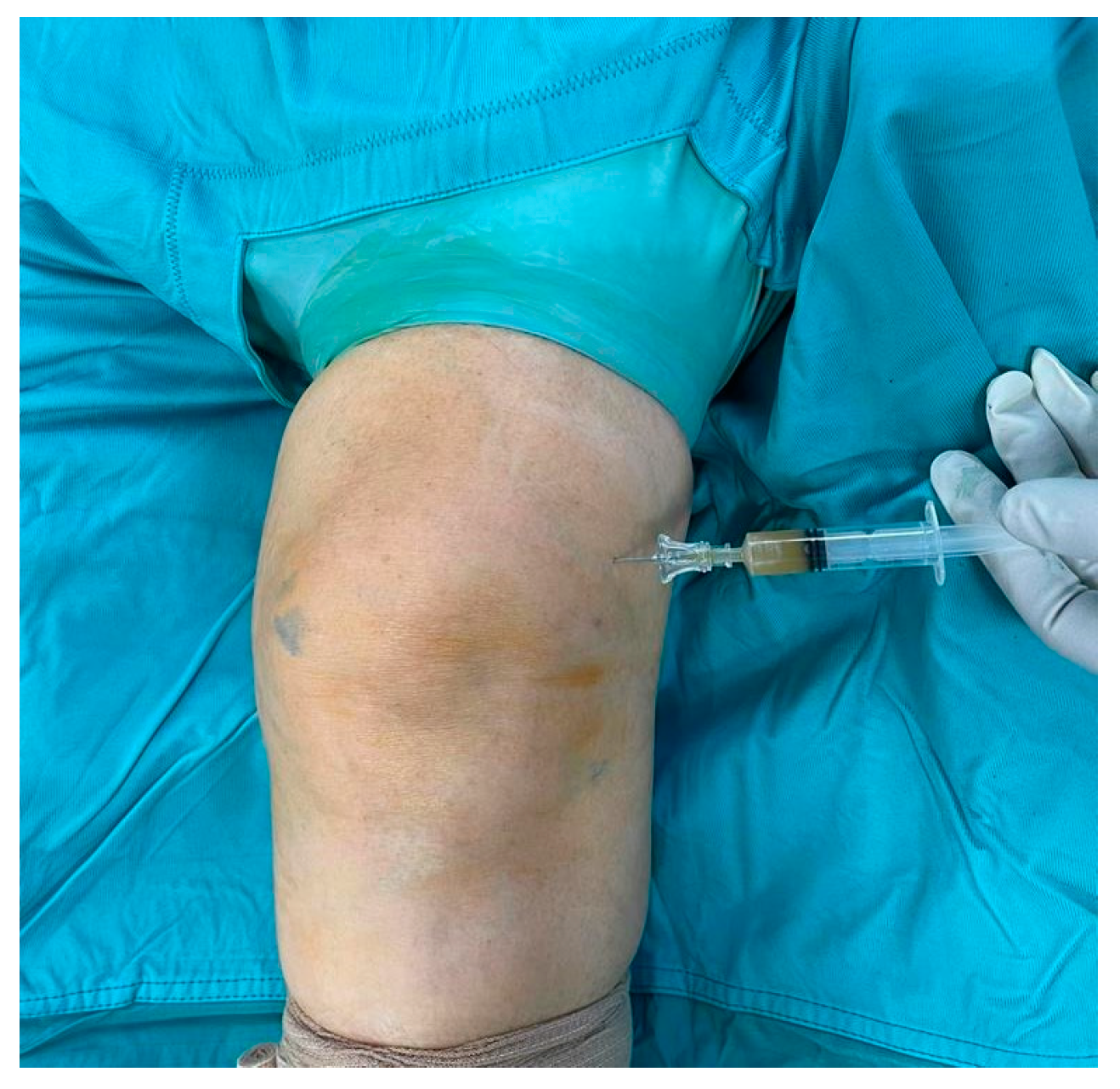
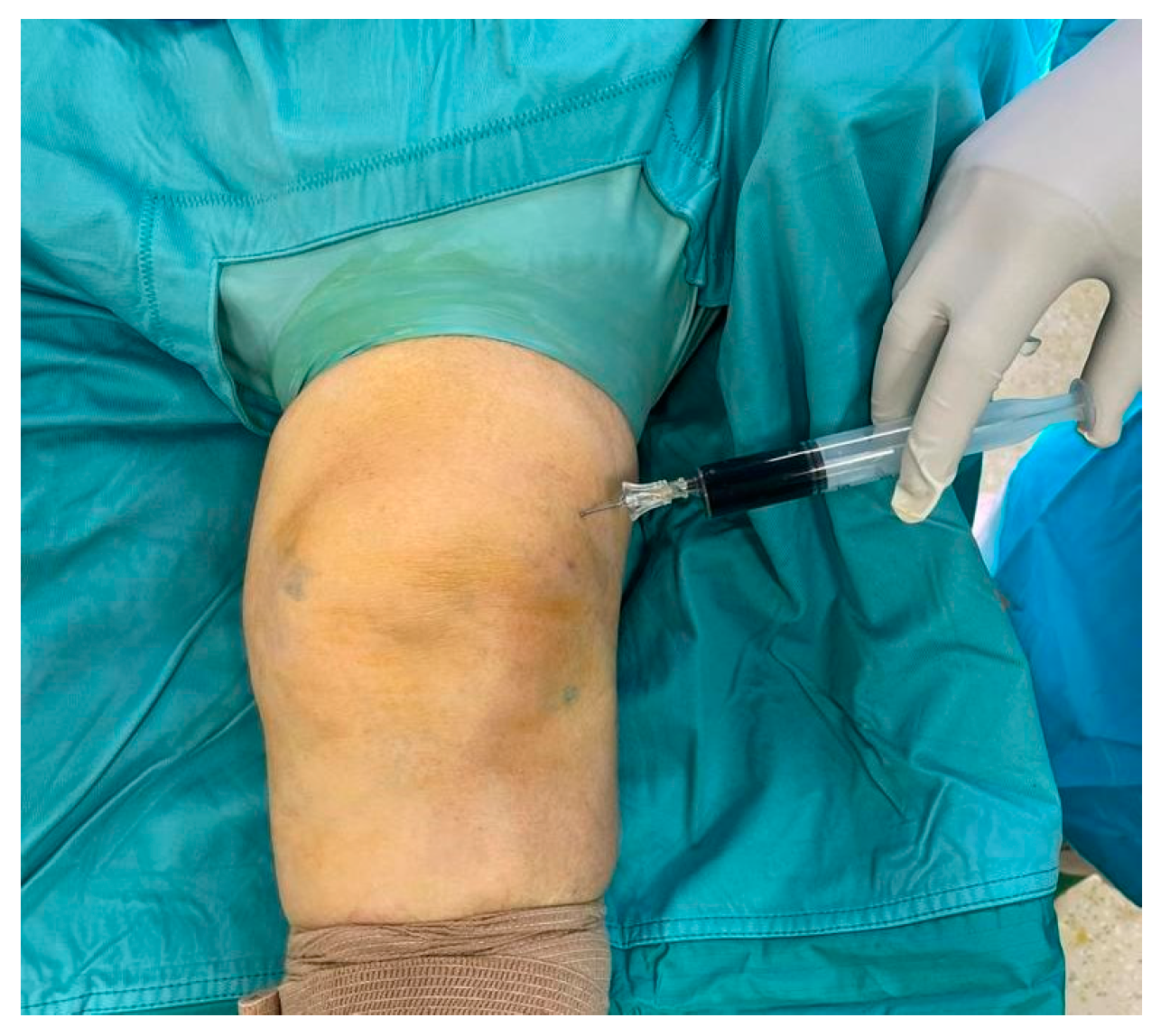
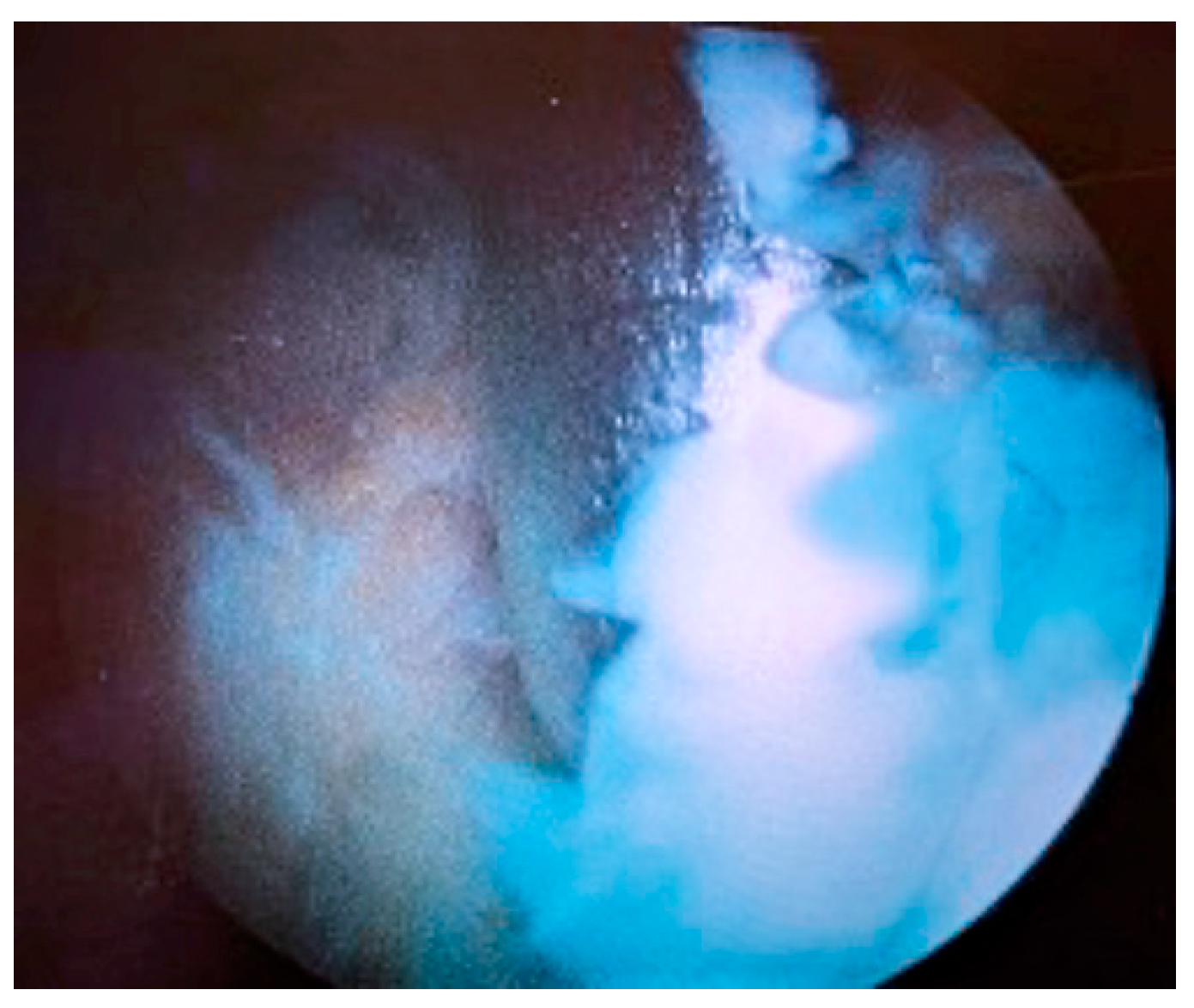
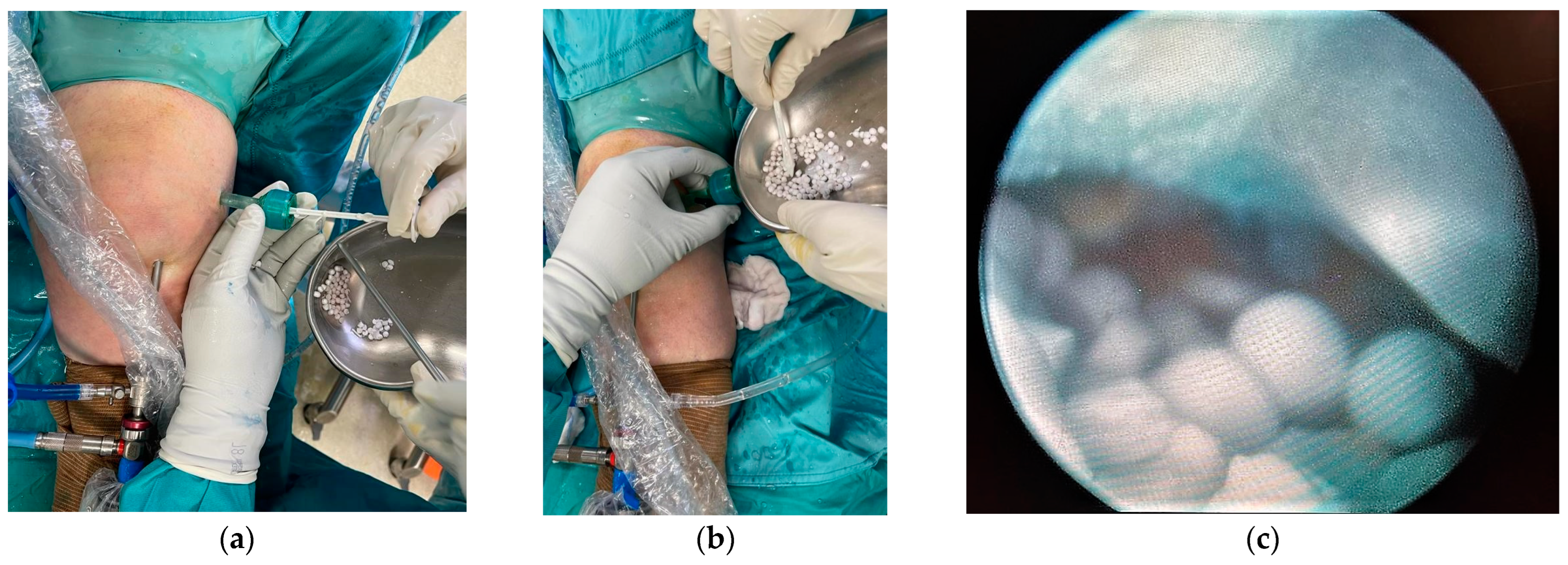
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Z.; Deng, X.; Li, L.; Wang, J. Similar Efficacy of Arthroscopy and Arthrotomy in Infection Eradication in the Treatment of Septic Knee: A Systematic Review and meta-analysis. Front. Surg. 2022, 8, 801911. [Google Scholar] [CrossRef] [PubMed]
- De Franco, C.; Artiaco, S.; de Matteo, V.; Bistolfi, A.; Balato, G.; Vallefuoco, S.; Massè, A.; Rosa, D. The eradication rate of infection in septic knee arthritis according to the Gächter Classification: A systematic review. Orthop. Rev. 2022, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Olivo, C.; Vilchez-Cavazos, F.; Blazquez-Saldana, J.; Villarreal-Villarreal, G.; Pena-Martinez, V.; Simental-Mendia, M. Comparison of open arthrotomy versus arthroscopic surgery for the treatment of septic arthritis in adults: A systematic review and meta-analysis. Int. Orthop. 2021, 45, 1947–1959. [Google Scholar] [CrossRef]
- Darraj, H.; Hakami, K.M.; Zogel, B.; Maghrabi, R.; Khired, Z. Septic Arthritis of the Knee in Children. Cureus 2023, 15, e45659. [Google Scholar] [CrossRef]
- Larghi, M.M.; Grassi, M.; Placenza, E.; Faugno, L.; Cerveri, P.; Manzotti, A. Septic arthritis following joint injections: A 17 years retrospective study in an Academic General Hospital. Acta Bio Medica Atenei Parm. 2021, 92, e2021308. [Google Scholar] [CrossRef]
- Ross, J.J. Septic arthritis of native joints. Infect. Dis. Clin. N. Am. 2017, 31, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Dubost, J.J.; Couderc, M.; Tatar, Z.; Tournadre, A.; Lopez, J.; Mathieu, S.; Soubrier, M. Three-decade trends in the distribution of organisms causing septic arthritis in native joints: Single-center study of 374 cases. Jt. Bone Spine 2014, 81, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.; Zhou, A.; Hussain, H.A.; Thahir, A.; Krkovic, M. Risk factors for septic arthritis and multiple arthroscopic washouts: Minimum 2-year follow-up at a major trauma centre. Clin. Rheumatol. 2022, 41, 2513–2523. [Google Scholar] [CrossRef]
- Momodu, I.I.; Savaliya, V. Septic Arthritis. 2023 Jul 3. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Margaretten, M.E.; Kohlwes, J.; Moore, D.; Bent, S. Does this adult patient have septic arthritis? JAMA 2007, 297, 1478–1488. [Google Scholar] [CrossRef]
- Weaver, J.S.; Omar, I.; Epstein, K.; Brown, A.; Chadwick, N.; Taljanovic, M.S. High-resolution ultrasound in the evaluation of musculoskeletal infections. J. Ultrason. 2023, 23, E272–E284. [Google Scholar] [CrossRef]
- Adam, M.; Ibrahim, B.; Khidir, R.; Elmahdi, E.; Ahmed, S.; Ahmed, A. Usefulness of MRI findings in differentiating between septic arthritis and transient synovitis of hip joint in children: A systematic review and meta-analysis. Eur. J. Radiol. Open 2022, 9, 100439. [Google Scholar] [CrossRef] [PubMed]
- Gamie, Z.; Karthikappallil, D.; Gamie, E.; Stamiris, S.; Kenanidis, E.; Tsiridis, E. Molecular sequencing technologies in the diagnosis and management of prosthetic joint infections. Expert Rev. Mol. Diagn. 2022, 22, 603–624. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Chisari, E.; Snyder, J.; Cooper, C.; Parvizi, J.; Sniffen, J. Next-Generation Sequencing Supports Targeted Antibiotic Treatment for Culture Negative Orthopedic Infections. Clin. Infect. Dis. 2023, 76, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Panjwani, T.; Wong, K.L.; Tan, S.H.S.; Liau, G.; Vaidya, N.; Krishna, L. Arthroscopic debridement has lower re-operation rates than arthrotomy in the treatment of acute septic arthritis of the knee: A meta-analysis. J. ISAKOS 2019, 4, 307–312. [Google Scholar] [CrossRef]
- Stutz, G.; Kuster, M.S.; Kleinstück, F.; Gächter, A. Arthroscopic management of septic arthritis: Stages of infection and results. Knee Surg. Sports Traumatol. Arthrosc. 2000, 8, 270–274. [Google Scholar] [CrossRef]
- Gaechter, A. Arthroscopic Lavage for Joint Infections. Orthop. Traumatol. 1993, 2, 104–106. [Google Scholar] [CrossRef]
- Ateschrang, A.; Albrecht, D.; Schröter, S.; Hirt, B.; Weise, K.; Dolderer, J.H. Septic arthritis of the knee: Presentation of a novel irrigation-suction system tested in a cadaver study. BMC Musculoskelet. Disord. 2011, 12, 10–17. [Google Scholar] [CrossRef]
- Shaw, J.D.; Miller, S.; Plourde, A.; Shaw, D.L.; Wustrack, R.; Hansen, E.N. Methylene Blue–Guided Debridement as an Intraoperative Adjunct for the Surgical Treatment of Periprosthetic Joint Infection. J. Arthroplast. 2017, 32, 3718–3723. [Google Scholar] [CrossRef]
- Abosala, A.; Ali, M. The Use of Calcium Sulphate beads in Periprosthetic Joint Infection, a systematic review. J. Bone Jt. Infect. 2020, 5, 43–49. [Google Scholar] [CrossRef]
- Mereddy, P.; Nallamilli, S.R.; Gowda, V.P.; Kasha, S.; Godey, S.K.; Nallamilli, R.R.; Rohit, G.P.R.K.; Meda, V.G. The use of Stimulan in bone and joint infections. Bone Jt. Open 2023, 4, 516–522. [Google Scholar] [CrossRef]
- Gächter, A. Der Gelenkinfekt. Inf. Arzt. 1985, 6, 35–43. [Google Scholar]
- Calanna, F.; Chen, F.; Risitano, S.; Vorhies, J.S.; Franceschini, M.; Giori, N.J.; Indelli, P.F. Debridement, antibiotic pearls, and retention of the implant (DAPRI): A modified technique for implant retention in total knee arthroplasty PJI treatment. J. Orthop. Surg. 2019, 27, 2309499019874413. [Google Scholar] [CrossRef] [PubMed]
- Grassi, W.; de Angelis, R. Clinical features of gout. Reumatismo 2012, 63, 238–245. [Google Scholar] [CrossRef]
- Wallace, S.L.; Robinson, H.; Masi, A.T.; Decker, J.L.; Mccarty, D.J.; Yü, T.S.F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977, 20, 895–900. [Google Scholar] [CrossRef]
- Yun, S.Y.; Choo, H.J.; Jeong, H.W.; Lee, S.J. Comparison of MR Findings between Patients with Septic Arthritis and Acute Gouty Arthritis of the Knee. J. Korean Soc.Radiol. 2022, 83, 1071–1080. [Google Scholar] [CrossRef]
- Ong, K.L.; Runa, M.; Xiao, Z.; Ngai, W.; Lau, E.; Altman, R.D. Severe Acute Localized Reactions Following Intra-Articular Hyaluronic Acid Injections in Knee Osteoarthritis. Cartilage 2021, 13, 1474S–1486S. [Google Scholar] [CrossRef] [PubMed]
- Fodor, D.; Serban, O.; Macavei, S.G.; Tudoran, L.B.; Bora, P.; Badarinza, M. A new form of severe acute localized reactions following intra-articular hyaluronic acid injections in knee osteoarthritis. A case report. Med. Ultrason. 2024. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, C.L.; Scott, J.L.; Perino, G.; Adler, R.; Fealy, S.; Goldring, M.B. Acute inflammation with induction of anaphylatoxin C5a and terminal complement complex C5b-9 associated with multiple intra-articular injections of hylan G-F 20: A case report. Osteoarthr. Cartil. 2012, 20, 791–795. [Google Scholar] [CrossRef][Green Version]
- Goldberg, V.M.; Coutts, R.D. Pseudoseptic reactions to hylan viscosupplementation: Diagnosis and treatment. Clin. Orthop. Relat. Res. 2004, 419, 130–137. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Engelbrecht, H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg 1970, 41, 511–515. [Google Scholar]
- Lun, D.X.; Li, S.Y.; Li, N.N.; Mou, L.M.; Li, H.Q.; Zhu, W.P.; Li, H.F.; Hu, Y.C. Limitations and modifications in the clinical application of calcium sulfate. Front. Surg. 2024, 11, 1278421. [Google Scholar] [CrossRef] [PubMed]
- Steadman, W.; Chapman, P.R.; Schuetz, M.; Schmutz, B.; Trampuz, A.; Tetsworth, K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Ene, R.; Nica, M.; Ene, D.; Cursaru, A.; Cirstoiu, C. Review of Calcium-Sulphate-Based Ceramics and Synthetic Bone Substitutes Used for Antibiotic Delivery in PJI and Osteomyelitis Treatment. EFORT Open Rev. 2021, 6, 297–304. [Google Scholar] [CrossRef]
- Sheridan, G.A.; Falk, D.P.; Fragomen, A.T.; Rozbruch, S.R. Calcium sulfate in the management of osteomyelitis: A systematic review and meta-analysis of comparative studies. Medicine 2022, 101, E31364. [Google Scholar] [CrossRef] [PubMed]
- Compton, J.; Vander Voort, W.; Willey, M.; Sekar, P. A case of Histoplasma capsulatum variety capsulatum septic arthritis successfully treated with surgery, systemic antifungals, and local amphotericin cement beads. Int. J. Infect. Dis. 2018, 77, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, A.I.; Okroj, K.T.; Rogers, T.; Della Valle, C.J.; Sporer, S.M. Systemic Absorption of Antibiotics From Antibiotic-Loaded Cement Spacers for the Treatment of Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 835–839. [Google Scholar] [CrossRef]
- Selvaratnam, V.; Roche, A.; Narayan, B.; Giotakis, N.; Mukhopadhaya, S.; Aniq, H.; Nayagam, S. Effectiveness of an Antibiotic-impregnated Bioabsorbable Carrier for the Treatment of Chronic Intramedullary and Diffuse Osteomyelitis. Strateg. Trauma Limb Reconstr. 2023, 18, 148–154. [Google Scholar] [CrossRef]
- Dudareva, M.; Kümin, M.; Vach, W.; Kaier, K.; Ferguson, J.; McNally, M.; Scarborough, M. Short or Long Antibiotic Regimes in Orthopaedics (SOLARIO): A randomised controlled open-label non-inferiority trial of duration of systemic antibiotics in adults with orthopaedic infection treated operatively with local antibiotic therapy. Trials 2019, 20, 693. [Google Scholar] [CrossRef]
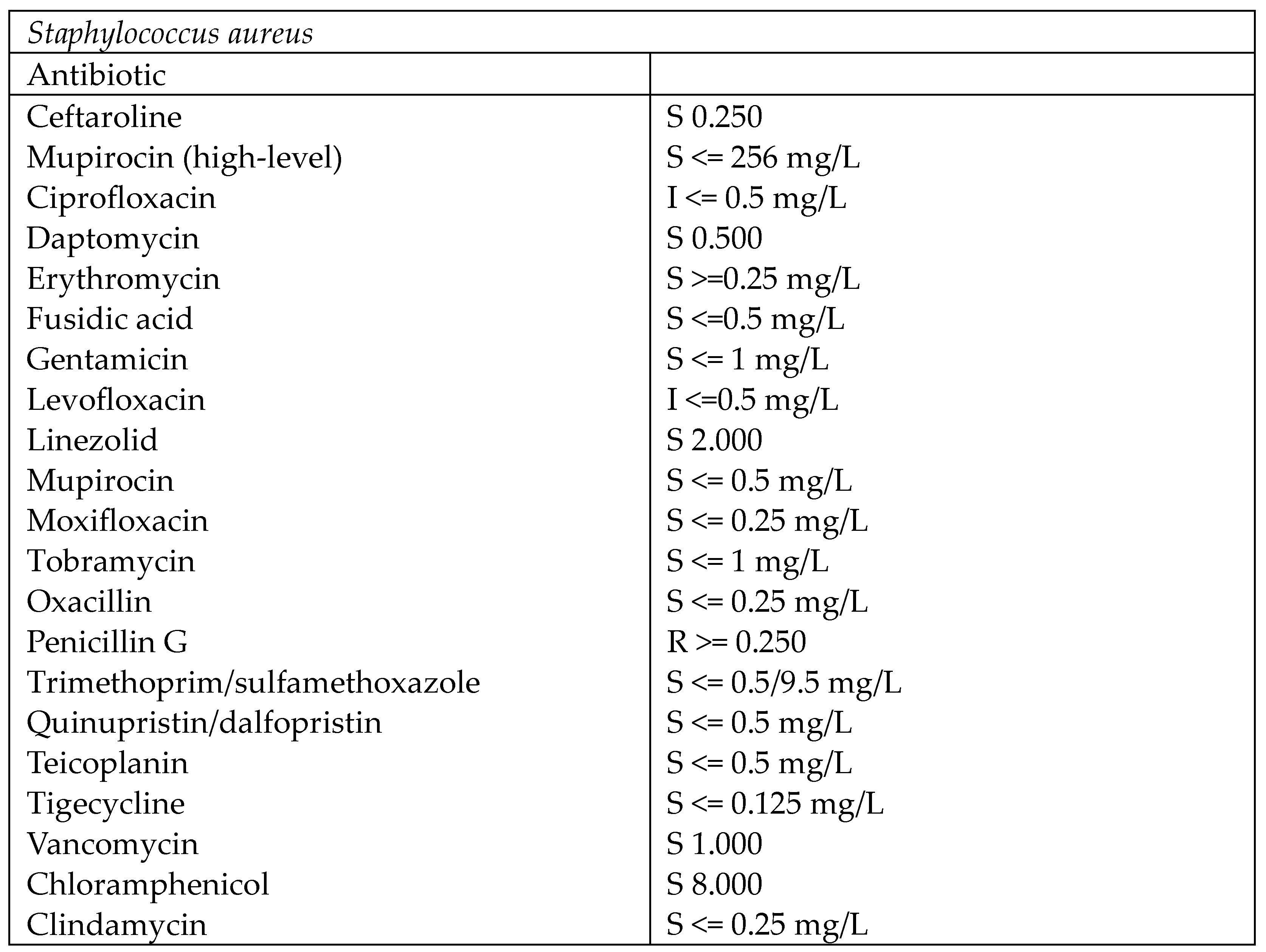
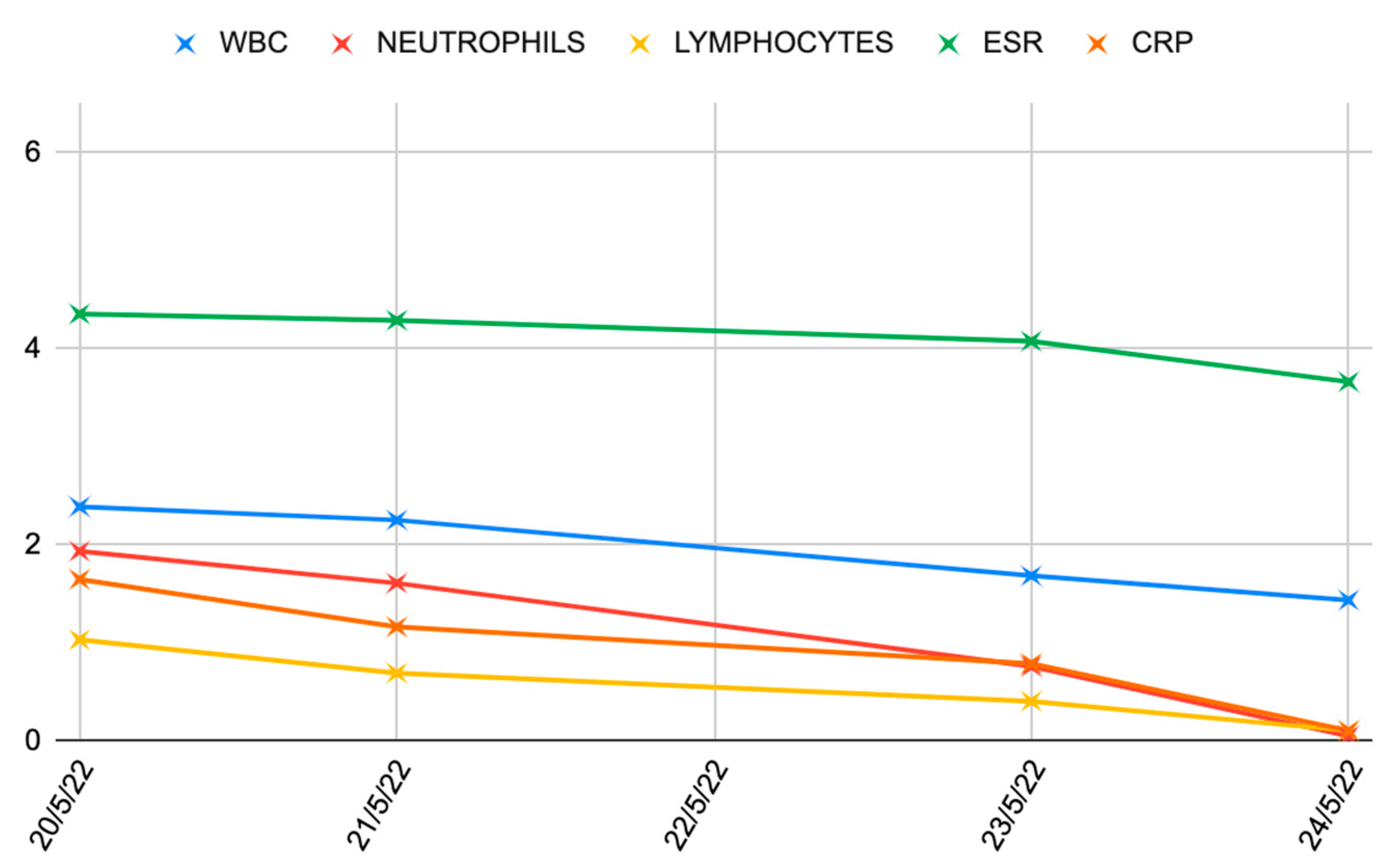
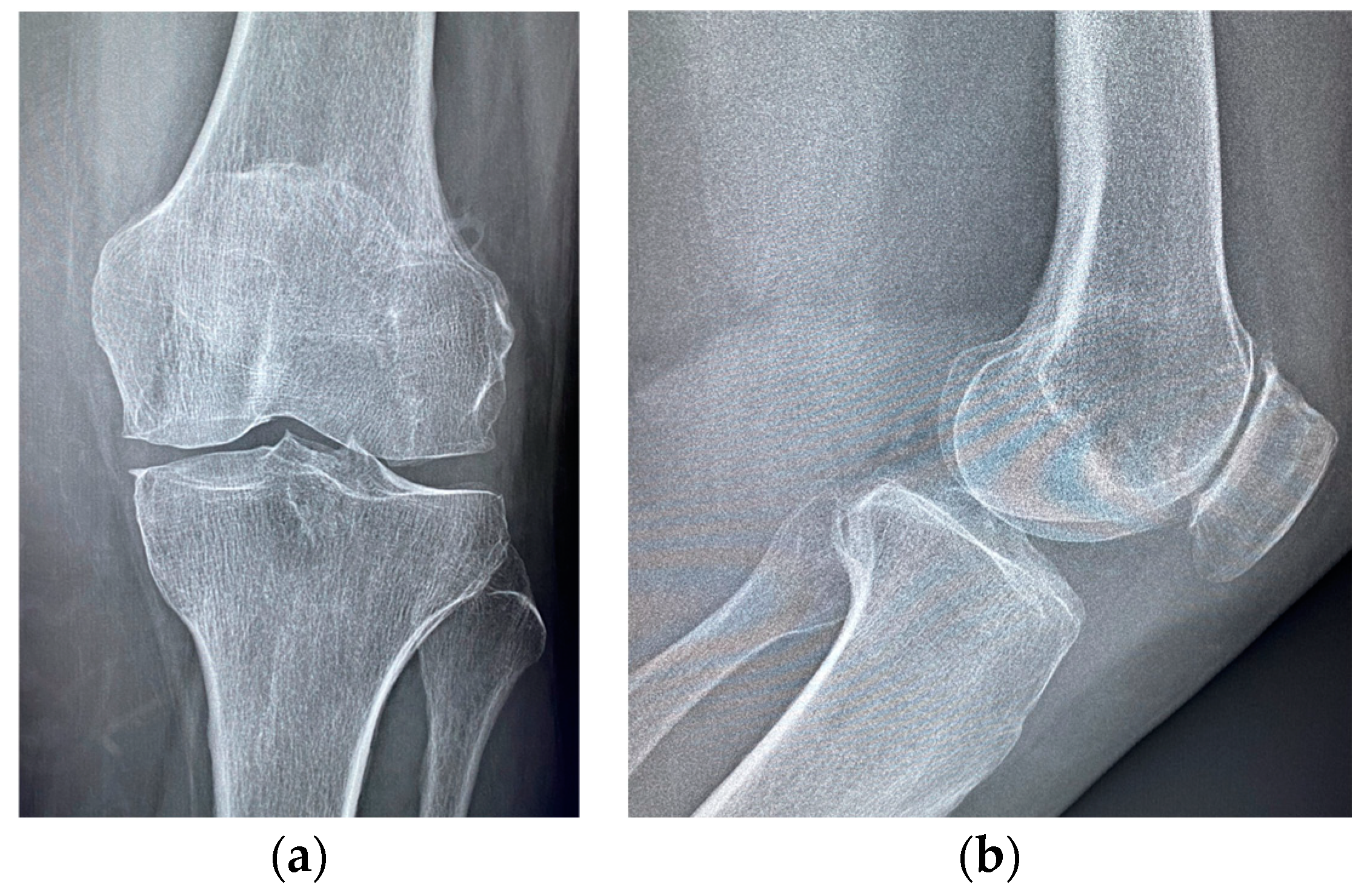

| Stage I | Opacity of fluid, redness of the synovial membrane, possible petechial bleeding, no radiological alterations |
| Stage II | Severe inflammation, fibrinous deposition, pus, no radiological alterations |
| Stage III | Thickening of the synovial membrane, compartment formation (“sponge-like” arthroscopic view, especially in the suprapatellar pouch), no radiological alterations |
| Stage IV | Aggressive pannus with infiltration of the cartilage, possibly undermining the cartilage, radiological signs of subchondral osteolysis, possible osseous erosions and cysts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alongi, S.; Troiano, E.; Latino, C.; Colasanti, G.B.; Greco, T.; Perisano, C.; Mosca, M.; Giannotti, S.; Mondanelli, N. Arthroscopic Debridement Enhanced by Intra-Articular Antibiotic-Loaded Calcium Sulphate Beads for Septic Arthritis of a Native Knee Following Iatrogenic Joint Injection: A Case Report. Medicina 2024, 60, 1636. https://doi.org/10.3390/medicina60101636
Alongi S, Troiano E, Latino C, Colasanti GB, Greco T, Perisano C, Mosca M, Giannotti S, Mondanelli N. Arthroscopic Debridement Enhanced by Intra-Articular Antibiotic-Loaded Calcium Sulphate Beads for Septic Arthritis of a Native Knee Following Iatrogenic Joint Injection: A Case Report. Medicina. 2024; 60(10):1636. https://doi.org/10.3390/medicina60101636
Chicago/Turabian StyleAlongi, Simone, Elisa Troiano, Cristina Latino, Giovanni Battista Colasanti, Tommaso Greco, Carlo Perisano, Massimiliano Mosca, Stefano Giannotti, and Nicola Mondanelli. 2024. "Arthroscopic Debridement Enhanced by Intra-Articular Antibiotic-Loaded Calcium Sulphate Beads for Septic Arthritis of a Native Knee Following Iatrogenic Joint Injection: A Case Report" Medicina 60, no. 10: 1636. https://doi.org/10.3390/medicina60101636
APA StyleAlongi, S., Troiano, E., Latino, C., Colasanti, G. B., Greco, T., Perisano, C., Mosca, M., Giannotti, S., & Mondanelli, N. (2024). Arthroscopic Debridement Enhanced by Intra-Articular Antibiotic-Loaded Calcium Sulphate Beads for Septic Arthritis of a Native Knee Following Iatrogenic Joint Injection: A Case Report. Medicina, 60(10), 1636. https://doi.org/10.3390/medicina60101636









