Age-Related Changes in Insulin Resistance and Muscle Mass: Clinical Implications in Obese Older Adults
Abstract
:1. Introduction
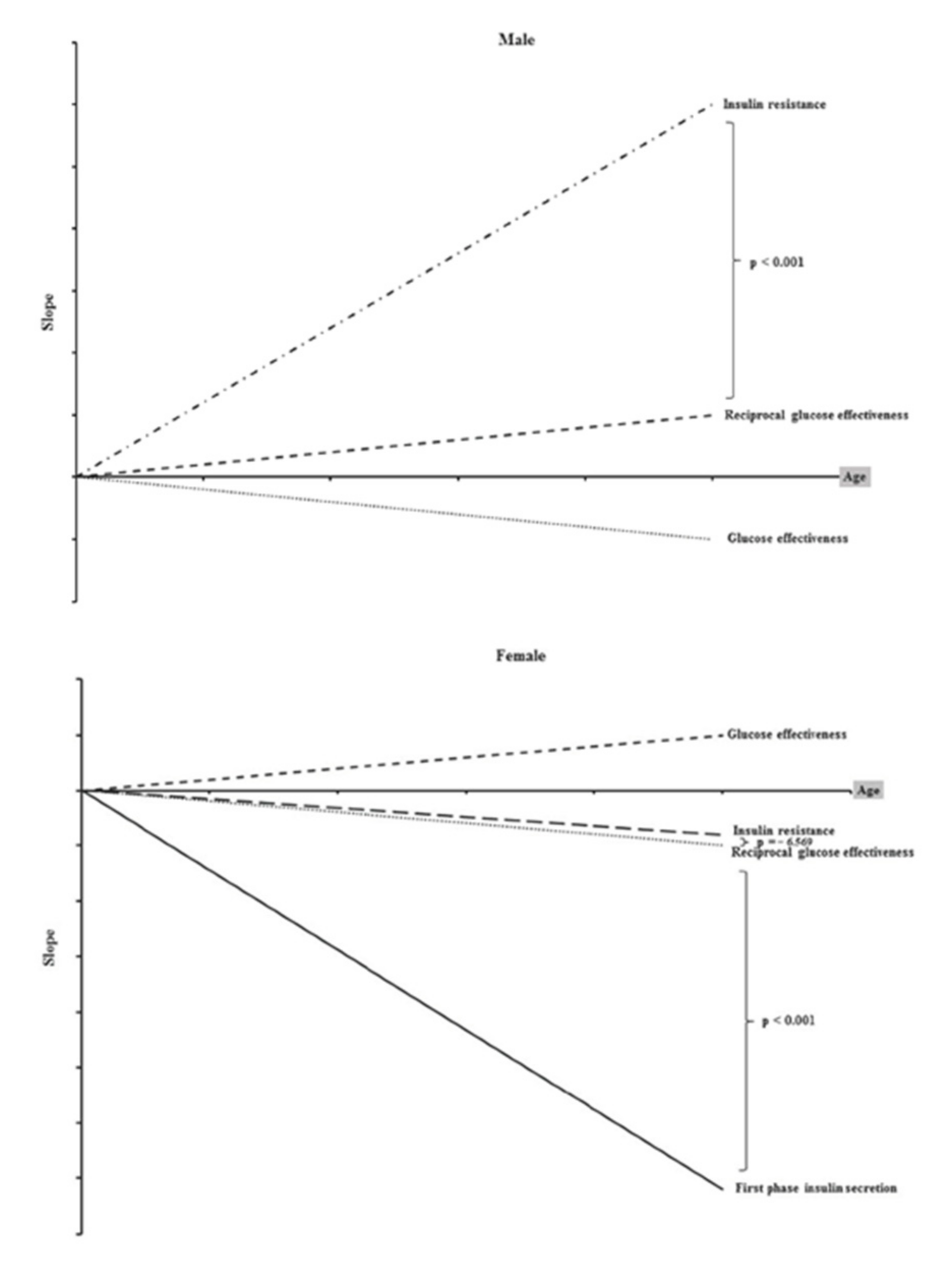
2. Age-Related Changes in Insulin Action
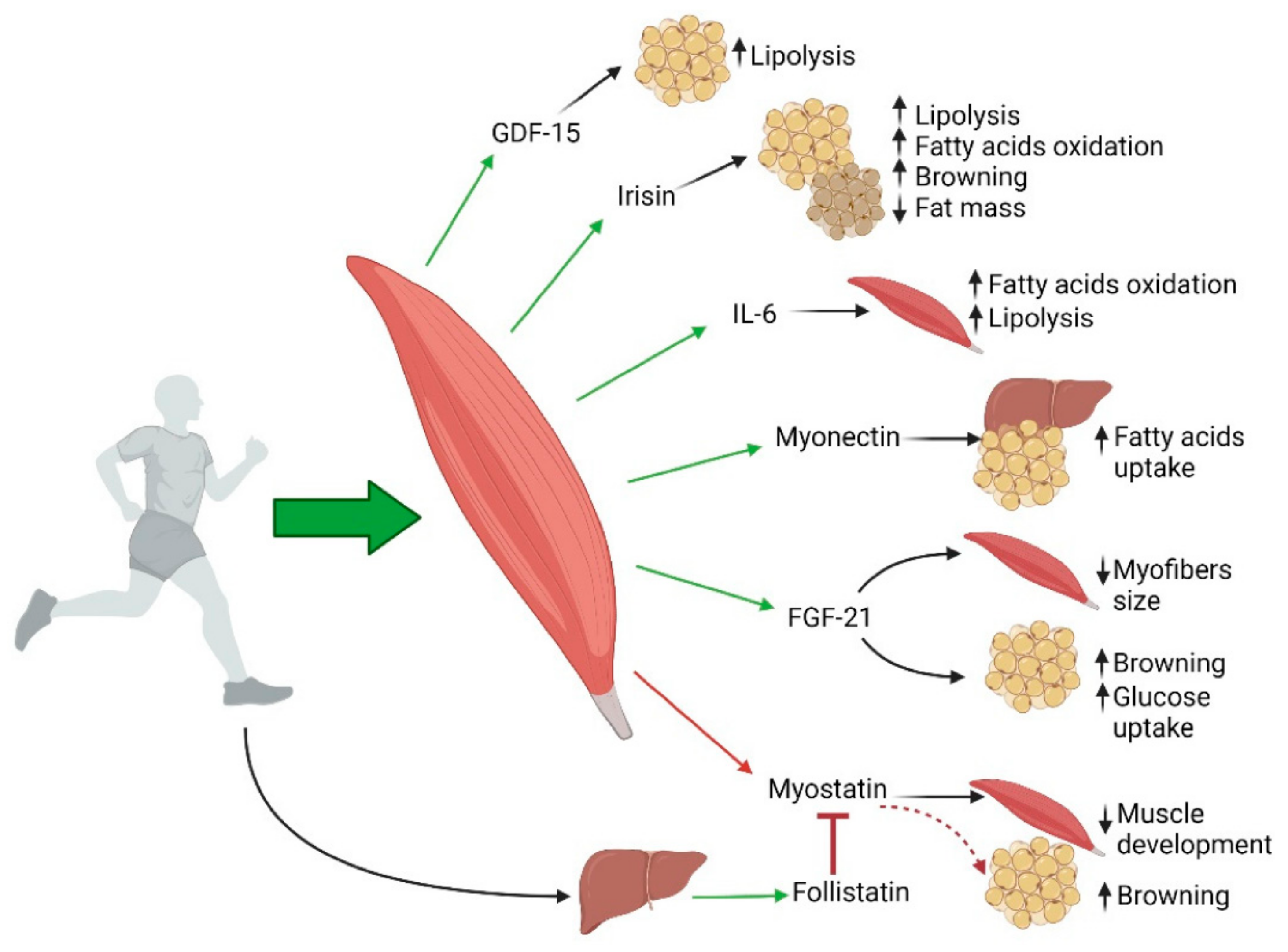
3. Aging, Sarcopenia and Obesity
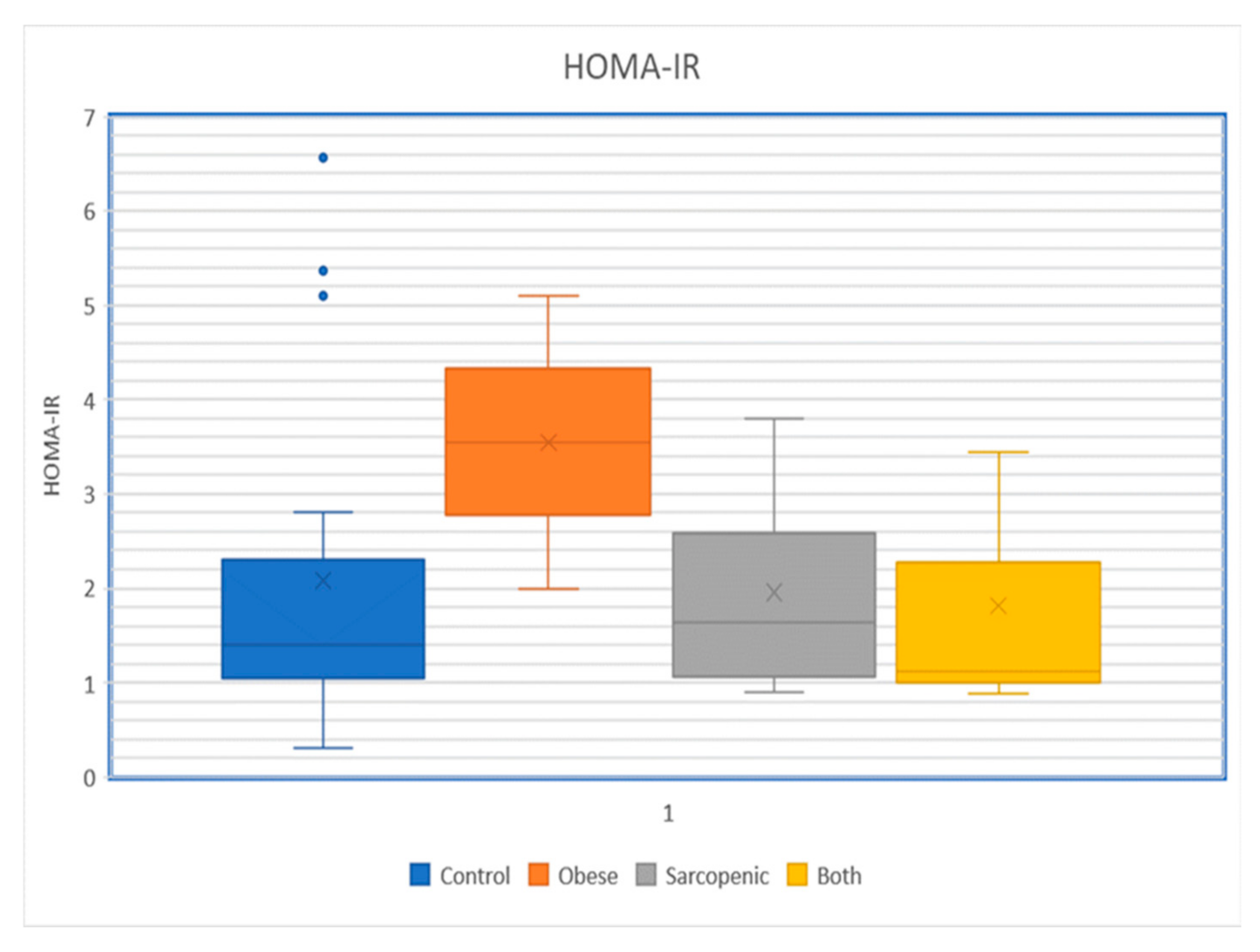
4. Association of Sarcopenic Obesity with Insulin Resistance
5. Changes in Sarcopenic Obesity and Functional Capacity with Aging
6. Clinical Implications of Sarcopenic Obesity in Older Adults
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Q.; Xiao, G.L.; Fan, Y.B.; He, M.; Lv, S.; Li, Y.S. Sarcopenic obesity: Research advances in pathogenesis and diagnostic criteria. Aging Clin. Exp. Res. 2021, 33, 247–252. [Google Scholar] [CrossRef]
- Zamboni, M.; Rubele, S.; Rossi, A.P. Sarcopenia and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 13–19. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Obes. Facts 2018, 11, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Evans, K.; Abdelhafiz, D.; Abdelhafiz, A.H. Sarcopenic obesity as a determinant of cardiovascular disease risk in older people: A systematic review. Postgrad. Med. 2021, 133, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Anton, S.D.; Hida, A.; Mankowski, R.; Layne, A.; Solberg, L.M.; Mainous, A.G.; Buford, T. Nutrition and Exercise in Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 649–667. [Google Scholar] [CrossRef]
- Gierach, M.; Junik, R. Insulin resistance in metabolic syndrome depending on the occurrence of its components. Endokrynol. Pol. 2021, 72, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Bokemark, L.; Frödén, A.; Attvall, S.; Wikstrand, J.; Fagerberg, B. The euglycemic hyperinsulinemic clamp examination: Variability and reproducibility. Scand. J. Clin. Lab. Investig. 2000, 60, 27–36. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Himsworth, H.P. The syndrome of diabetes mellitus and its causes. Lancet 1949, 1, 465–473. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharmacol. 2017, 15, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Kurauti, M.A.; Soares, G.M.; Marmentini, C.; Bronczek, G.A.; Branco, R.C.S.; Boschero, A.C. Insulin and aging. Vitam. Horm. 2021, 115, 185–219. [Google Scholar]
- Zhao, Y.; Yue, R. Aging adipose tissue, insulin resistance, and type 2 diabetes. Biogerontology 2024, 25, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Pabla, P.; Jones, E.J.; Piasecki, M.; Phillips, B.E. Skeletal muscle dysfunction with advancing age. Clin. Sci. 2024, 138, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tocino, M.L.; Cigarrán, S.; Ureña, P.; González-Casaus, M.L.; Mas-Fontao, S.; Gracia-Iguacel, C.; Ortíz, A.; Gonzalez-Parra, E. Definition and evolution of the concept of sarcopenia. Nefrologia (Engl. Ed.) 2024, 44, 323–330. [Google Scholar] [CrossRef]
- Faam, B.; Zarkesh, M.; Daneshpour, M.S.; Azizi, F.; Hedayati, M. The association between inflammatory markers and obesity-related factors in Tehranian adults: Tehran lipid and glucose study. Iran. J. Basic Med. Sci. 2014, 17, 577–582. [Google Scholar]
- Motie, M.; Evangelista, L.S.; Horwich, T.; Lombardo, D.; Zaldivar, F.; Hamilton, M.; Fonarow, G.C. Association between inflammatory biomarkers and adiposity in obese patients with heart failure and metabolic syndrome. Exp. Ther. Med. 2014, 8, 181–186. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Kumar, S.; Hossain, J.; Inge, T.; Balagopal, P.B. Changes in Myokines in Youths with Severe Obesity Following Roux-en-Y Gastric Bypass Surgery. JAMA Surg. 2019, 154, 668–669. [Google Scholar] [CrossRef]
- RAND Health Care. 36-Item Short Form Survey Instrument (SF-36). Available online: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/survey-instrument.html (accessed on 14 March 2024).
- Zhang, J.; Wang, L.; Deng, X.; Fei, G.; Jin, L.; Pan, X.; Cai, L.; Albano, A.D.; Zhong, C. Five-Minute Cognitive Test as A New Quick Screening of Cognitive Impairment in The Elderly. Aging Dis. 2019, 10, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Sääksjärvi, K.; Härkänen, T.; Stenholm, S.; Schaap, L.; Lundqvist, A.; Koskinen, S.; Borodulin, K.; Visser, M. Probable Sarcopenia, Obesity, and Risk of All-Cause Mortality: A Pooled Analysis of 4612 Participants. Gerontology 2023, 69, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Purnamasari, D.; Tetrasiwi, E.N.; Kartiko, G.J.; Astrella, C.; Husam, K.; Laksmi, P.W. Sarcopenia and Chronic Complications of Type 2 Diabetes Mellitus. Rev. Diabet. Stud. 2022, 18, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Greig, C.; Masud, T.; Wilson, D.; Jackson, T.A. COVID-19 and Acute Sarcopenia. Aging Dis. 2020, 11, 1345–1351. [Google Scholar] [CrossRef]
- López-Sampalo, A.; Cobos-Palacios, L.; Vilches-Pérez, A.; Sanz-Cánovas, J.; Vargas-Candela, A.; Mancebo-Sevilla, J.J.; Hernández-Negrín, H.; Gómez-Huelgas, R.; Bernal-López, M.R. COVID-19 in Older Patients: Assessment of Post-COVID-19 Sarcopenia. Biomedicines 2023, 11, 733. [Google Scholar] [CrossRef]
- Yakti, F.A.Z.; Abusalah, L.; Ganji, V. Sarcopenia and Mortality in Critically Ill COVID-19 Patients. Life 2023, 14, 24. [Google Scholar] [CrossRef]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- Boyer, W.R.; Johnson, T.M.; Fitzhugh, E.C.; Richardson, M.R.; Churilla, J.R. The Associations Between Increasing Degrees of HOMA-IR and Measurements of Adiposity Among Euglycemic U.S. Adults. Metab. Syndr. Relat. Disord. 2016, 14, 108–113. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Sergi, G.; Coin, A.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Sarcopenic Obesity and Metabolic Syndrome in Adult Caucasian Subjects. J. Nutr. Health Aging 2016, 20, 958–963. [Google Scholar] [CrossRef]
- Ma, J.; Hwang, S.J.; McMahon, G.M.; Curhan, G.C.; Mclean, R.R.; Murabito, J.M.; Fox, C.S. Mid-adulthood cardiometabolic risk factor profiles of sarcopenic obesity. Obesity 2016, 24, 526–534. [Google Scholar] [CrossRef]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Crimmins, E.M. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity 2012, 20, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Goulet, E.D.; Lord, C.; Chaput, J.P.; Aubertin-Leheudre, M.; Brochu, M.; Dionne, I.J. No difference in insulin sensitivity between healthy postmenopausal women with or without sarcopenia: A pilot study. Appl. Physiol. Nutr. Metab. 2007, 32, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Seibel, M.; Waite, L.M.; Hirani, V. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: The Concord Health and Ageing in Men Project. Exp. Gerontol. 2018, 108, 99–105. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef]
- Maliszewska, K.; Adamska-Patruno, E.; Kretowski, A. The interplay between muscle mass decline, obesity, and type 2 diabetes. Pol. Arch. Intern. Med. 2019, 129, 809–816. [Google Scholar] [CrossRef]
- Feraco, A.; Gorini, S.; Armani, A.; Camajani, E.; Rizzo, M.; Caprio, M. Exploring the Role of Skeletal Muscle in Insulin Resistance: Lessons from Cultured Cells to Animal Models. Int. J. Mol. Sci. 2021, 22, 9327. [Google Scholar] [CrossRef]
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef]
- Gielen, E.; Dupont, J.; Dejaeger, M.; Laurent, M.R. Sarcopenia, osteoporosis and frailty. Metabolism 2023, 145, 155638. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Amin, V.; Fraser, M.A.; Periasamy, M. Relationship between Obesity, Sarcopenia, and Insulin Resistance in Older Adults. Diabetes 2024, 73 (Suppl. S1), 158-OR. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016, 17, 467–483. [Google Scholar] [CrossRef]
- Tardif, N.; Salles, J.; Guillet, C.; Patrac, V.; Bertrand-Michel, J.; Migne, C.; Collin, M.L. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2α activation. Aging Cell 2014, 13, 1001–1011. [Google Scholar] [CrossRef]
- Bouchonville, M.F.; Villareal, D.T. Sarcopenic obesity: How do we treat it? Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients 2020, 12, 3367. [Google Scholar] [CrossRef]
- Hsu, K.J.; Liao, C.D.; Tsai, M.W.; Chen, C.N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef]
- Kim, Y.J.; Moon, S.; Yu, J.M.; Chung, H.S. Implication of diet and exercise on the management of age-related sarcopenic obesity in Asians. Geriatr. Gerontol. Int. 2022, 22, 695–704. [Google Scholar] [CrossRef]
- Yin, Y.H.; Liu, J.Y.W.; Välimäki, M. Effectiveness of non-pharmacological interventions on the management of sarcopenic obesity: A systematic review and meta-analysis. Exp. Gerontol. 2020, 135, 110937. [Google Scholar] [CrossRef]
- Hurley, B.F.; Roth, S.M. Strength training in the elderly: Effects on risk factors for age-related diseases. Sports Med. 2000, 30, 249–268. [Google Scholar] [CrossRef]
- Li, Z.; Heber, D. Sarcopenic obesity in the elderly and strategies for weight management. Nutr. Rev. 2012, 70, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Abiri, B.; Hosseinpanah, F.; Seifi, Z.; Amini, S.; Valizadeh, M. The Implication of Nutrition on the Prevention and Improvement of Age-Related Sarcopenic Obesity: A Systematic Review. J. Nutr. Health Aging 2023, 27, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; D’Introno, A.; Rubele, S.; Caliari, C.; Gattazzo, S.; Zoico, E.; Mazzali, G.; Fantin, F.; Zamboni, M. The Potential of β-Hydroxy-β-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity. Drugs Aging 2017, 34, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J. Sarcopenic Obesity: Involvement of Oxidative Stress and Beneficial Role of Antioxidant Flavonoids. Antioxidants 2023, 12, 1063. [Google Scholar] [CrossRef]
- Darmon, P. Intentional weight loss in older adults: Useful or wasting disease generating strategy? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 284–289. [Google Scholar] [CrossRef]
- de Sousa, M.V.; da Silva Soares, D.B.; Caraça, E.R.; Cardoso, R. Dietary protein and exercise for preservation of lean mass and perspectives on type 2 diabetes prevention. Exp. Biol. Med. 2019, 244, 992–1004. [Google Scholar] [CrossRef]
- Lim, S.T.; Kang, S. Exercise therapy for sarcopenia and diabetes. World J. Diabetes 2023, 14, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Ji, X.; Zhang, Y.; Li, K.; Huang, F. Non-Pharmacological Strategies for Managing Sarcopenia in Chronic Diseases. Clin. Interv. Aging 2024, 19, 827–841. [Google Scholar] [CrossRef]
- Chiu, S.C.; Yang, R.S.; Yang, R.J.; Chang, S.F. Effects of resistance training on body composition and functional capacity among sarcopenic obese residents in long-term care facilities: A preliminary study. BMC Geriatr. 2018, 18, 21. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; Von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.; et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Results of the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos. Int. 2016, 27, 3261–3270. [Google Scholar]
- Mastino, D.; Robert, M.; Betry, C.; Laville, M.; Gouillat, C.; Disse, E. Bariatric Surgery Outcomes in Sarcopenic Obesity. Obes. Surg. 2016, 26, 2355–2362. [Google Scholar] [CrossRef]

| Test | Men | Women |
|---|---|---|
| Grip strength using calibrated handheld dynamometer | <27 kg | <16 kg |
| Chair stand for leg muscle strength | >15 s for five rises | |
| Appendicular skeletal muscle mass (ASM) (with MRI, CT, or DEXA) | <20 kg | <15 kg |
| ASM/height2 (to adjust for body size) | <7.0 kg/m2 | <5.5 kg/m2 |
| Gait speed | ≤0.8 m/s | |
| Short physical performance battery (SPPB) | ≤8-point score | |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizvi, A.A.; Rizzo, M. Age-Related Changes in Insulin Resistance and Muscle Mass: Clinical Implications in Obese Older Adults. Medicina 2024, 60, 1648. https://doi.org/10.3390/medicina60101648
Rizvi AA, Rizzo M. Age-Related Changes in Insulin Resistance and Muscle Mass: Clinical Implications in Obese Older Adults. Medicina. 2024; 60(10):1648. https://doi.org/10.3390/medicina60101648
Chicago/Turabian StyleRizvi, Ali A., and Manfredi Rizzo. 2024. "Age-Related Changes in Insulin Resistance and Muscle Mass: Clinical Implications in Obese Older Adults" Medicina 60, no. 10: 1648. https://doi.org/10.3390/medicina60101648
APA StyleRizvi, A. A., & Rizzo, M. (2024). Age-Related Changes in Insulin Resistance and Muscle Mass: Clinical Implications in Obese Older Adults. Medicina, 60(10), 1648. https://doi.org/10.3390/medicina60101648







