High-Grade Thalamic Glioma: Case Report with Literature Review
Abstract
1. Introduction
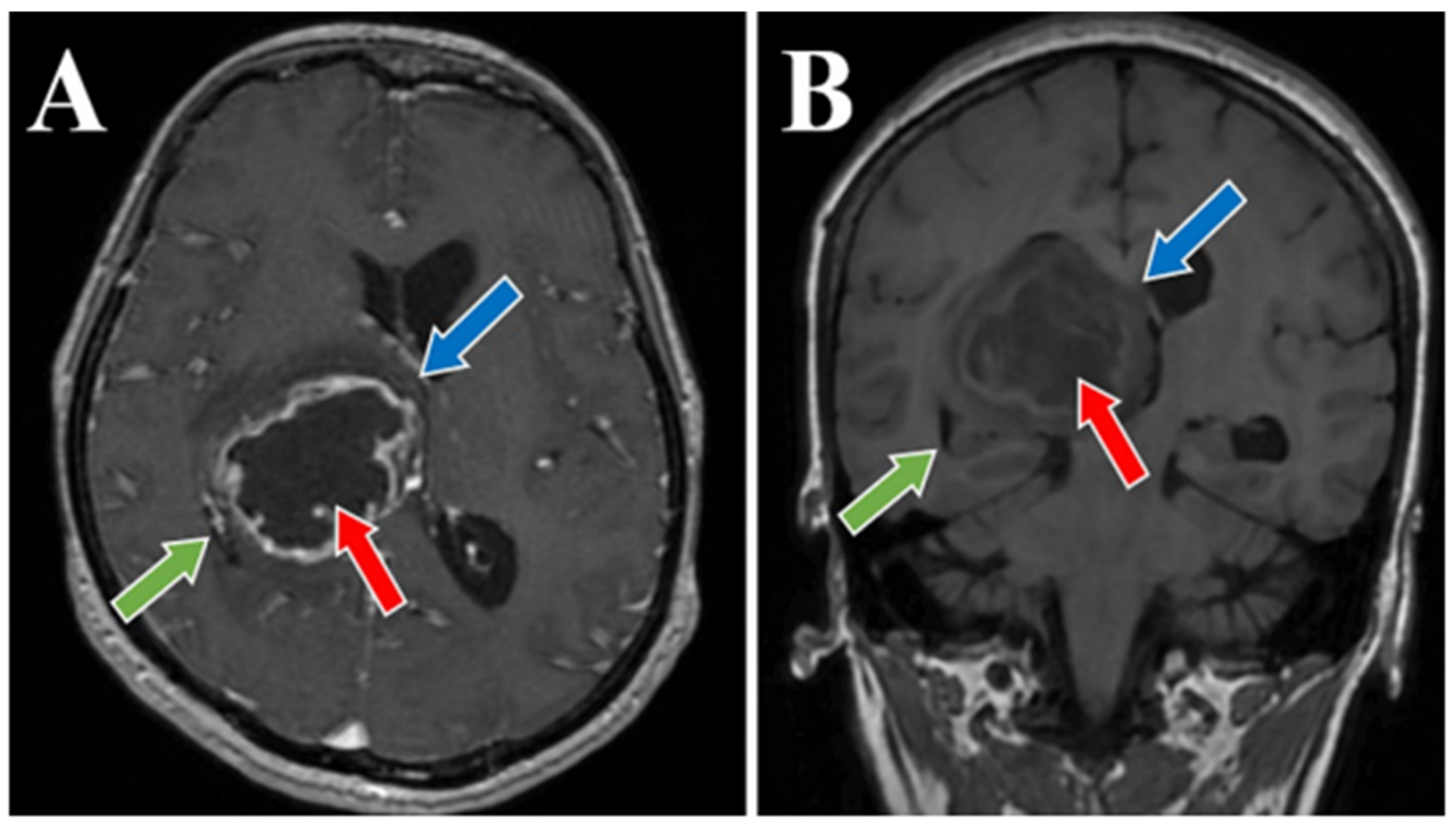
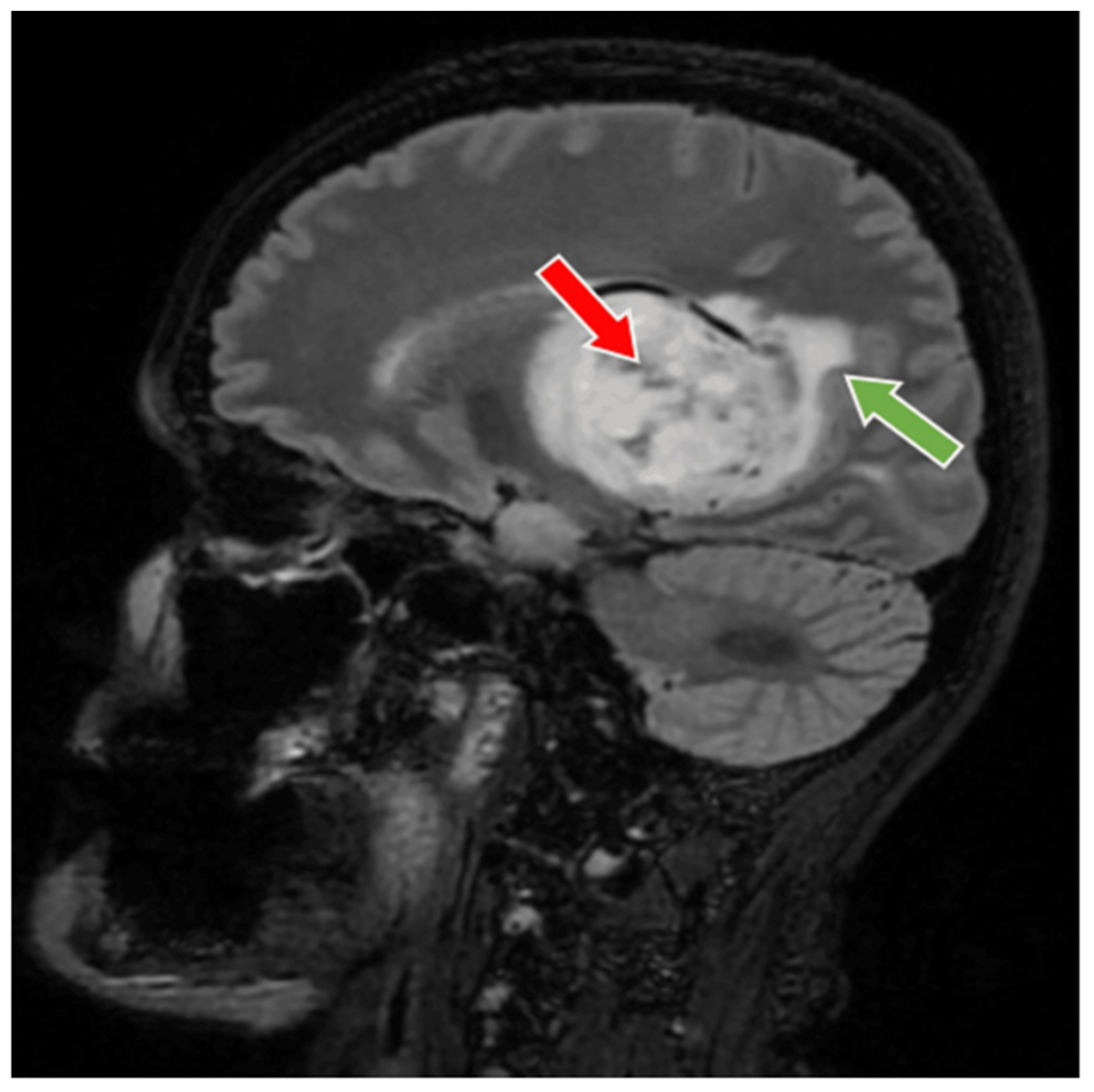
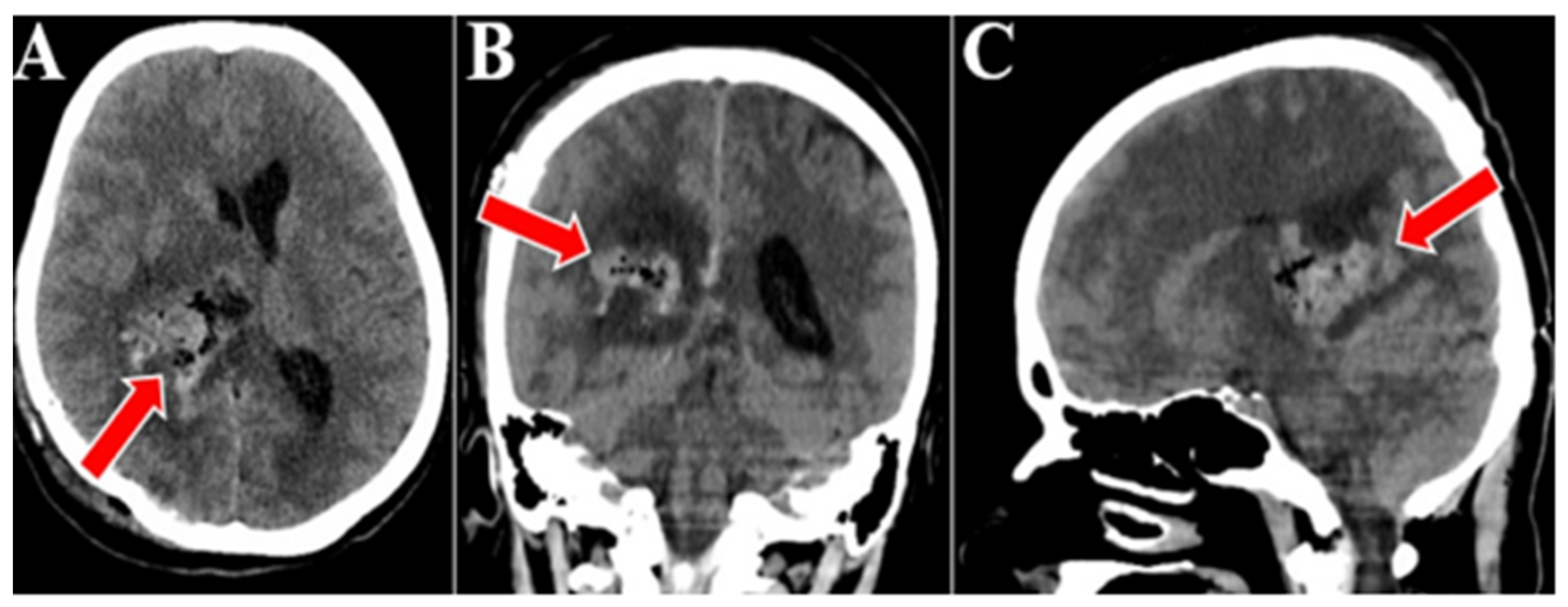
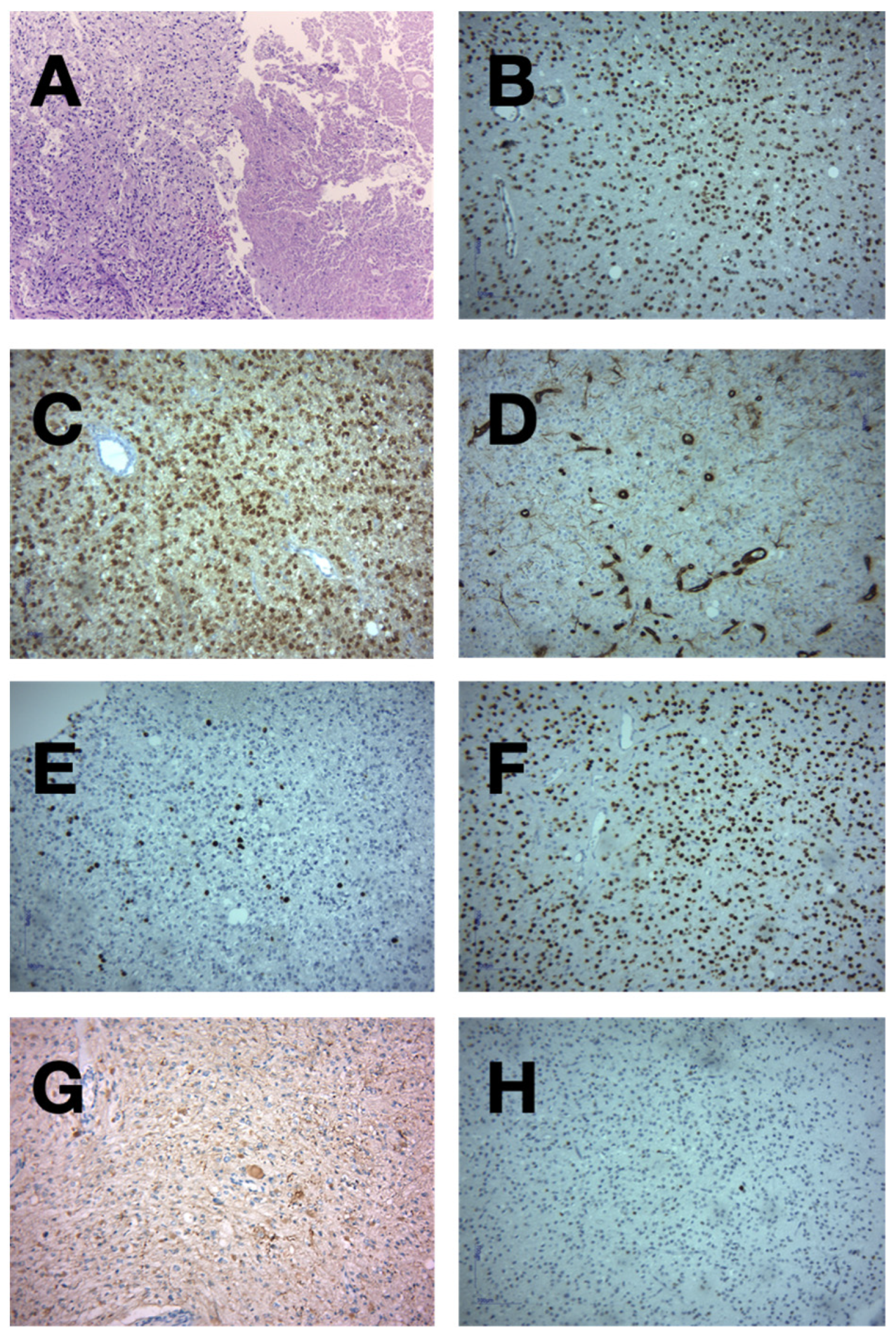
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, T.-T.; Chen, H.-H.; Liang, M.-L.; Hsieh, K.L.-C.; Yang, Y.-S.; Ho, D.M.-T.; Chang, K.-P.; Lee, Y.-Y.; Lin, S.-C.; Hsu, T.-R.; et al. Clinical considerations and surgical approaches for low-grade gliomas in deep hemispheric locations: Thalamic lesions. Child’s Nerv. Syst. 2016, 32, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Palmisciano, P.; El Ahmadieh, T.Y.; Haider, A.S.; Bin Alamer, O.; Robertson, F.C.; Plitt, A.R.; Aoun, S.G.; Yu, K.; Cohen-Gadol, A.; Moss, N.S.; et al. Thalamic gliomas in adults: A systematic review of clinical characteristics, treatment strategies, and survival outcomes. J. Neuro-Oncol. 2021, 155, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, X.; Ji, N.; Xie, J.; Han, J.; Ren, X.; Song, G.; Wu, R.; Zhang, L.; Gao, Z. Clinical, radiological, and pathological features of 33 adult unilateral thalamic gliomas. World J. Surg. Oncol. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Tang, C.; Wang, Y.; Li, Z.; Hu, S.; Hua, W.; Li, W.; Huang, S.; Ma, J.; Zhang, Y. High-grade thalamic gliomas: Microsurgical treatment and prognosis analysis. J. Clin. Neurosci. 2018, 49, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Kumabe, T.; Kanamori, M.; Sonoda, Y.; Tominaga, T. Distant recurrences limit the survival of patients with thalamic high-grade gliomas after successful resection. Neurosurg. Rev. 2017, 40, 469–477. [Google Scholar] [CrossRef]
- Cao, L.; Li, C.; Zhang, Y.; Gui, S. Surgical resection of unilateral thalamic tumors in adults: Approaches and outcomes. BMC Neurol. 2015, 15, 229. [Google Scholar] [CrossRef]
- Kelly, P.J. Stereotactic biopsy and resection of thalamic astrocytomas. Neurosurgery 1989, 25, 185–194, discussion 194–195. [Google Scholar] [CrossRef]
- Greenwood, J. Radical surgery of tumors of the thalamus, hypothalamus, and third ventricle area. Surg. Neurol. 1973, 1, 29–33. [Google Scholar]
- Kurian, K.M.; Zhang, Y.; Haynes, H.R.; Macaskill, N.A.; Bradley, M. Diagnostic challenges of primary thalamic gliomas-identification of a minimally enhancing neuroradiological subtype with aggressive neuropathology and poor clinical outcome. Clin. Neuroradiol. 2014, 24, 231–238. [Google Scholar] [CrossRef]
- Ozek, M.M.; Türe, U. Surgical approach to thalamic tumors. Child’s Nerv. Syst. 2002, 18, 450–456. [Google Scholar] [CrossRef]
- Zuo, M.; Li, M.; Chen, N.; Yu, T.; Kong, B.; Liang, R.; Wang, X.; Mao, Q.; Liu, Y. IDH1 status is significantly different between high-grade thalamic and superficial gliomas. Cancer Biomark. 2017, 20, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Waqar, M.; Hanif, S.; Rathi, N.; Das, K.; Zakaria, R.; Brodbelt, A.R.; Walker, C.; Jenkinson, M.D. Diagnostic challenges, management and outcomes of midline low-grade gliomas. J. Neuro-Oncol. 2014, 120, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Aihara, K.; Mukasa, A.; Gotoh, K.; Saito, K.; Nagae, G.; Tsuji, S.; Tatsuno, K.; Yamamoto, S.; Takayanagi, S.; Narita, Y.; et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro-Oncology 2014, 16, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Meyronet, D.; Esteban-Mader, M.; Bonnet, C.; Joly, M.-O.; Uro-Coste, E.; Amiel-Benouaich, A.; Forest, F.; Rousselot-Denis, C.; Burel-Vandenbos, F.; Bourg, V.; et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro-Oncology 2017, 19, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Niu, X.; Gao, T.; Zheng, L.; Qiu, Y.; Mao, Q. Molecular Characteristics of Thalamic Gliomas in Adults. J. Mol. Neurosci. 2021, 71, 1598–1604. [Google Scholar] [CrossRef]
- Chitturi, R.; Chinnam, A. Pathological Evaluation of Diffuse Gliomas Using IDH1 and ATRX in a Resource-Limited Setting. Cureus 2024, 16, e65551. [Google Scholar] [CrossRef]
- Sharma, N.; Mallela, A.N.; Shi, D.D.; Tang, L.W.; Abou-Al-Shaar, H.; Gersey, Z.C.; Zhang, X.; McBrayer, S.K.; Abdullah, K.G. Isocitrate dehydrogenase mutations in gliomas: A review of current understanding and trials. Neuro-Oncol. Adv. 2023, 5, vdad053. [Google Scholar] [CrossRef]
- Kim, S.-I.; Lee, K.; Bae, J.; Lee, S.; Yun, H.; Park, C.-K.; Choi, S.H.; Maquiling, C.A.; Park, S.-H.; Won, J.-K. Revisiting vimentin: A negative surrogate marker of molecularly defined oligodendroglioma in adult type diffuse glioma. Brain Tumor Pathol. 2021, 38, 271–282. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [CrossRef] [PubMed]
- de Dios, O.; Ramírez-González, M.A.; Gómez-Soria, I.; Segura-Collar, B.; Manosalva, J.; Megías, D.; E De Andrea, C.; Fernández-Rubio, L.; Hernández-Laín, A.; Sepúlveda-Sánchez, J.M.; et al. NKG2C/KLRC2 tumor cell expression enhances immunotherapeutic efficacy against glioblastoma. J. Immunother. Cancer 2024, 12, e009210. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, J.B.; Torre, M.; Harary, M.; Smith, T.R.; Aizer, A.A.; Reardon, D.A.; Barnholtz-Sloan, J.S.; Perry, A. The Misclassification of Diffuse Gliomas: Rates and Outcomes. Clin. Cancer Res. 2019, 25, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, S.; Yu, L.; Gao, Z. Long-term temozolomide might be an optimal choice for patient with multifocal glioblastoma, especially with deep-seated structure involvement: A case report and literature review. World J. Surg. Oncol. 2015, 13, 142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quiñones-Hinojosa, A.; Sanai, N.; Soriano-Navarro, M.; Gonzalez-Perez, O.; Mirzadeh, Z.; Gil-Perotin, S.; Romero-Rodriguez, R.; Berger, M.S.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J. Comp. Neurol. 2006, 494, 415–434. [Google Scholar] [CrossRef]
- Lim, D.A.; Cha, S.; Mayo, M.C.; Chen, M.-H.; Keles, E.; VandenBerg, S.; Berger, M.S. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-Oncology 2007, 9, 424–429. [Google Scholar] [CrossRef]
- Sai Kiran, N.A.; Thakar, S.; Dadlani, R.; Mohan, D.; Furtado, S.V.; Ghosal, N.; Aryan, S.; Hegde, A.S. Surgical management of thalamic gliomas: Case selection, technical considerations, and review of literature. Neurosurg. Rev. 2013, 36, 383–393. [Google Scholar] [CrossRef]
- Rangel-Castilla, L.; Spetzler, R.F. The 6 thalamic regions: Surgical approaches to thalamic cavernous malformations, operative results, and clinical outcomes. J. Neurosurg. 2015, 123, 676–685. [Google Scholar] [CrossRef]
- Lim, J.; Park, Y.; Ahn, J.W.; Hwang, S.J.; Kwon, H.; Sung, K.S.; Cho, K. Maximal surgical resection and adjuvant surgical technique to prolong the survival of adult patients with thalamic glioblastoma. PLoS ONE 2021, 16, e0244325. [Google Scholar] [CrossRef]
- Cinalli, G.; Aguirre, D.T.; Mirone, G.; Ruggiero, C.; Cascone, D.; Quaglietta, L.; Aliberti, F.; Santi, S.D.; Buonocore, M.C.; Nastro, A.; et al. Surgical treatment of thalamic tumors in children. J. Neurosurg. Pediatr. 2018, 21, 247–257. [Google Scholar] [CrossRef]
- Lim, J.; Cho, K. The modified lateral supraorbital approach for tumors of the petroclival junction extending into the anterior cerebellopontine area. J. Neuro-Oncol. 2016, 127, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.-T.; Rendahl, A.K.; Chen, W.-P.; Freund, D.M.; Gray, W.M.; Cohen, J.D.; Hegeman, A.D. Proteome Scale-Protein Turnover Analysis Using High Resolution Mass Spectrometric Data from Stable-Isotope Labeled Plants. J. Proteome Res. 2016, 15, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Toth, E.; Kumar, S.S.; Chaitanya, G.; Riley, K.; Balasubramanian, K.; Pati, S. Machine learning approach to detect focal-onset seizures in the human anterior nucleus of the thalamus. J. Neural Eng. 2020, 17, 066004. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Balañá, C.; Vaz, M.A.; Lopez, D.; de la Peñas, R.; García-Bueno, J.M.; Molina-Garrido, M.J.; Sepúlveda, J.M.; Cano, J.M.; Bugés, C.; Sanz, S.M.; et al. Should we continue temozolomide beyond six cycles in the adjuvant treatment of glioblastoma without an evidence of clinical benefit? A cost analysis based on prescribing patterns in Spain. Clin. Transl. Oncol. 2014, 16, 273–279. [Google Scholar] [CrossRef]
- Darlix, A.; Baumann, C.; Lorgis, V.; Ghiringhelli, F.; Blonski, M.; Chauffert, B.; Zouaoui, S.; Pinelli, C.; Rech, F.; Beauchesne, P.; et al. Prolonged administration of adjuvant temozolomide improves survival in adult patients with glioblastoma. Anticancer Res. 2013, 33, 3467–3474. [Google Scholar]
- van den Bent, M.J.; Taal, W. Are we done with dose-intense temozolomide in recurrent glioblastoma? Neuro-Oncol. 2014, 16, 1161–1163. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef]
- Kamath, A.A.; Friedman, D.D.; Akbari, S.H.A.; Kim, A.H.; Tao, Y.; Luo, J.; Leuthardt, E.C. Glioblastoma Treated With Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy: Safety, Efficacy, and Outcomes. Neurosurgery 2019, 84, 836–843. [Google Scholar] [CrossRef]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.A.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef]
- Korshunov, A.; Ryzhova, M.; Hovestadt, V.; Bender, S.; Sturm, D.; Capper, D.; Meyer, J.; Schrimpf, D.; Kool, M.; Northcott, P.A.; et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015, 129, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Khuong-Quang, D.A.; Buczkowicz, P.; Rakopoulos, P.; Liu, X.-Y.; Fontebasso, A.M.; Bouffet, E.; Bartels, U.; Albrecht, S.; Schwartzentruber, J.; Letourneau, L.; et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 2012, 124, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Hao, S.; Pan, C.; Wang, Y.; Wu, Z.; Zhang, J.; Yan, H.; Zhang, L.; Wan, H. The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum. Pathol. 2015, 46, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.; Witt, H.; Hovestadt, V.; Khuong-Quang, D.-A.; Jones, D.T.; Konermann, C.; Pfaff, E.; Tönjes, M.; Sill, M.; Bender, S.; et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012, 22, 425–437. [Google Scholar] [CrossRef]
- Solomon, D.A.; Wood, M.D.; Tihan, T.; Bollen, A.W.; Gupta, N.; Phillips, J.J.J.; Perry, A. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol. 2015, 26, 569–580. [Google Scholar] [CrossRef]
- Esquenazi, Y.; Moussazadeh, N.; Link, T.W.; E Hovinga, K.; Reiner, A.S.; DiStefano, N.M.; Brennan, C.; Gutin, P.; Tabar, V. Thalamic Glioblastoma: Clinical Presentation, Management Strategies, and Outcomes. Neurosurgery 2018, 83, 76–85. [Google Scholar] [CrossRef]
- Niu, X.; Wang, T.; Zhou, X.; Yang, Y.; Wang, X.; Zhang, H.; Chen, N.; Yue, Q.; Wang, F.; Zhang, Y.; et al. Surgical treatment and survival outcome of patients with adult thalamic glioma: A single institution experience of 8 years. J. Neuro-Oncol. 2020, 147, 377–386. [Google Scholar] [CrossRef]
- Nishio, S.; Morioka, T.; Suzuki, S.; Takeshita, I.; Fukui, M. Thalamic gliomas: A clinicopathologic analysis of 20 cases with reference to patient age. Acta Neurochir. 1997, 139, 336–342. [Google Scholar] [CrossRef]
- Ferroli, P.; Restelli, F.; Bertolini, G.; Monti, E.; Falco, J.; Bonomo, G.; Tramacere, I.; Pollo, B.; Calatozzolo, C.; Patanè, M.; et al. Are Thalamic Intrinsic Lesions Operable? No-Man’s Land Revisited by the Analysis of a Large Retrospective, Mono-Institutional, Cohort. Cancers 2023, 15, 361. [Google Scholar] [CrossRef]

| First Author and Year | Age (Median) | Sex, No of Patients | Tumor Volume (Median) | Extent of Resection | Neurological Complications | Surgical Complications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | GTR | STR | PR | Motor Deficits | Sensory Deficits | Visual Deficit | Hemorrhage | Hydrocephalus | |||
| Sai Kiran NA et al., 2013 [27] | 29 | 15 | 7 | NA | 9 | 13 | 16 | 16 | NA | NA | 0 | 9 |
| Lim J et al., 2021 [29] | 42 | 19 patients | 26 cm3 | 11 | 7 | 6 | 6 | 3 | 3 | 5 | 3 | |
| Esquenazi et al., 2018 [46] | 53 | 31 | 26 | 13 cm3 | 0 | 57 | 35 | 35 | 14 | 14 | 5 | 27 |
| Niu X et al., 2020 [47] | 41 | 56 | 46 | 4 cm3 | 46 | 50 | NA | NA | NA | NA | 4 | 11 |
| Nishio S et al., 1997 [48] | 24 | 11 | 9 | NA | 1 | 0 | 5 | 2 | 3 | 3 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, C.; Radoi, M.P.; Dumitru, A.; Glavan, L.-A.; Covache-Busuioc, R.-A.; Popa, A.A.; Costin, H.-P.; Corlatescu, A.-D.; Ciurea, A.V. High-Grade Thalamic Glioma: Case Report with Literature Review. Medicina 2024, 60, 1667. https://doi.org/10.3390/medicina60101667
Toader C, Radoi MP, Dumitru A, Glavan L-A, Covache-Busuioc R-A, Popa AA, Costin H-P, Corlatescu A-D, Ciurea AV. High-Grade Thalamic Glioma: Case Report with Literature Review. Medicina. 2024; 60(10):1667. https://doi.org/10.3390/medicina60101667
Chicago/Turabian StyleToader, Corneliu, Mugurel Petrinel Radoi, Adrian Dumitru, Luca-Andrei Glavan, Razvan-Adrian Covache-Busuioc, Andrei Adrian Popa, Horia-Petre Costin, Antonio-Daniel Corlatescu, and Alexandru Vladimir Ciurea. 2024. "High-Grade Thalamic Glioma: Case Report with Literature Review" Medicina 60, no. 10: 1667. https://doi.org/10.3390/medicina60101667
APA StyleToader, C., Radoi, M. P., Dumitru, A., Glavan, L.-A., Covache-Busuioc, R.-A., Popa, A. A., Costin, H.-P., Corlatescu, A.-D., & Ciurea, A. V. (2024). High-Grade Thalamic Glioma: Case Report with Literature Review. Medicina, 60(10), 1667. https://doi.org/10.3390/medicina60101667