Evaluation of Wear on Primary Tooth Enamel and Fracture Resistance of Esthetic Pediatric Crowns Manufactured from Different Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Study Design
2.3. Crown Manufacturing and Cementation
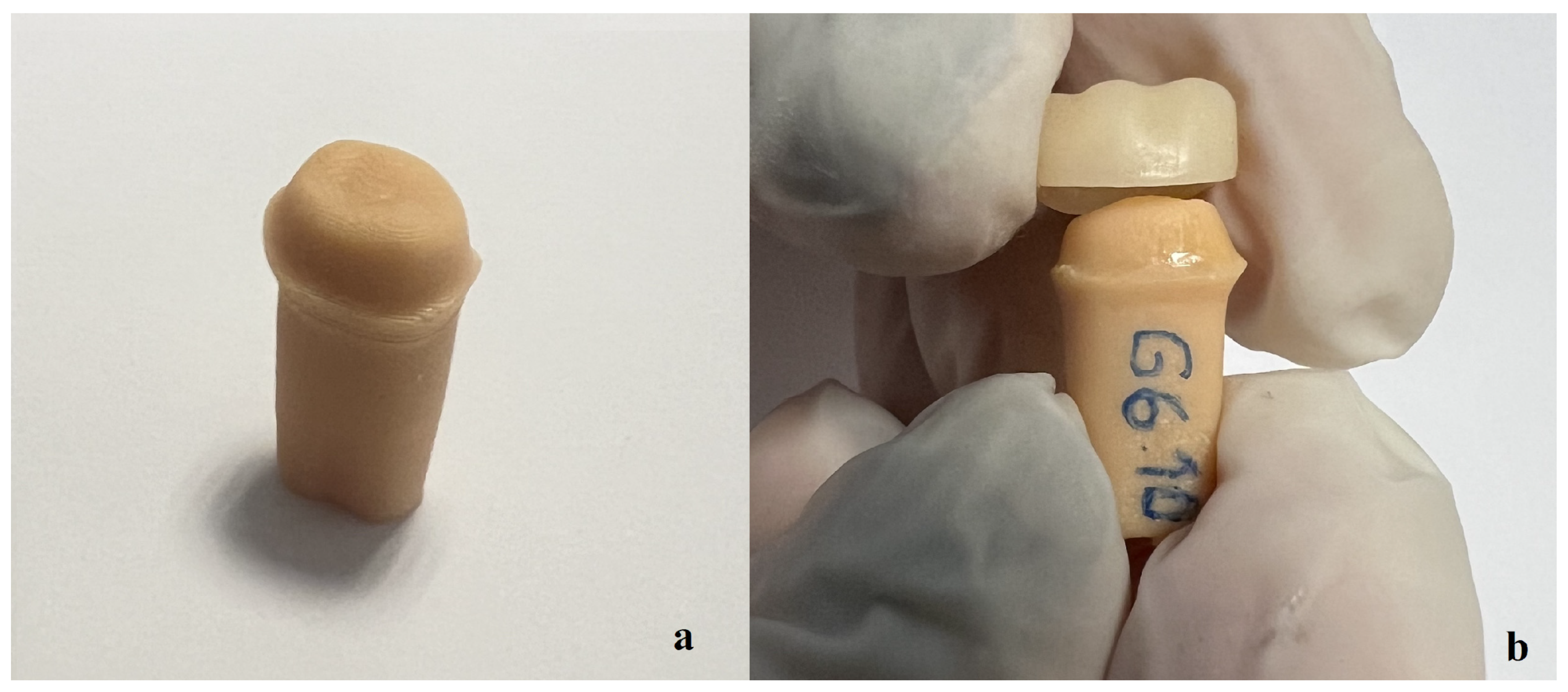
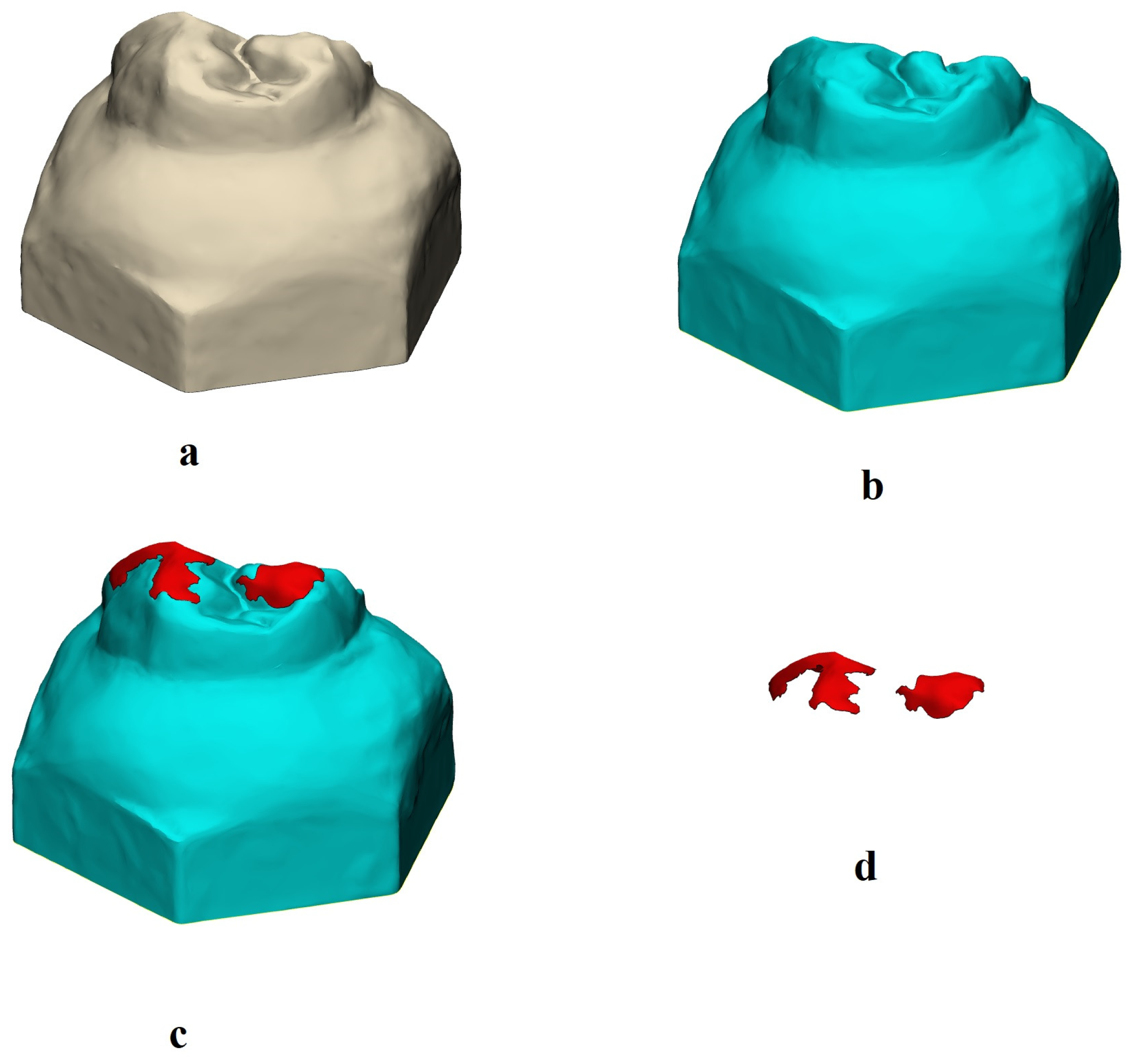
2.4. Wear Evaluation
2.5. Fracture Resistance Test
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
- The restoration type did not have a significant effect on the 2D and 3D wear of the primary tooth enamel.
- The fracture resistance of the tested materials differed according to the material type. Although the milled PEEK group showed the highest fracture resistance, all tested materials could withstand chewing forces in children.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padma Kumari, B.; Retnakumari, N. Loss of space and changes in the dental arch after premature loss of the lower primary molar: A longitudinal study. Indian. Soc. Pedod. Prev. Dent. 2006, 24, 90–96. [Google Scholar]
- Habib, F.; Chaly, P.E.; Junaid, M.; Musthafa, H.M. Caries experience, clinical consequences of untreated dental caries and associated factors among school going children—A cross-sectional study. Indian J. Dent. Res. 2020, 31, 180–185. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Pediatric restorative dentistry. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2023; pp. 443–456. [Google Scholar]
- Almajed, O.S. Shaping Smiles: A Narrative Review of Crown Advancements in Pediatric Dentistry. Cureus 2024, 16, e52997. [Google Scholar] [CrossRef] [PubMed]
- Sztyler, K.; Wiglusz, R.J.; Dobrzynski, M. Review on Preformed Crowns in Pediatric Dentistry-The Composition and Application. Materials 2022, 15, 2081. [Google Scholar] [CrossRef]
- Choi, J.W.; Bae, I.H.; Noh, T.H.; Ju, S.W.; Lee, T.K.; Ahn, J.S.; Jeong, T.S.; Huh, J.B. Wear of primary teeth caused by opposed all-ceramic or stainless-steel crowns. J. Adv. Prosthodont. 2016, 8, 43–52. [Google Scholar] [CrossRef]
- Çiftçi, Z.Z.; Şahin, İ.; Karayılmaz, H. Comparative evaluation of the fracture resistance of newly developed prefabricated fibreglass crowns and zirconium crowns. Int. J. Paediatr. Dent. 2022, 32, 756–763. [Google Scholar] [CrossRef]
- Alzanbaqi, S.D.; Alogaiel, R.M.; Alasmari, M.A.; Al Essa, A.M.; Khogeer, L.N.; Alanazi, B.S.; Hawsah, E.S.; Shaikh, A.M.; Ibrahim, M.S. Zirconia Crowns for Primary Teeth: A Systematic Review and Meta-Analyses. Int. J. Environ. Res. Public Health 2022, 19, 2838. [Google Scholar] [CrossRef]
- Bolaca, A.; Erdogan, Y. In vitro evaluation of the wear of primary tooth enamel against different ceramic and composite resin materials. Niger. J. Clin. Pract. 2019, 22, 313–319. [Google Scholar] [CrossRef]
- Abushanan, A.; Sharanesha, R.B.; Aljuaid, B.; Alfaifi, T.; Aldurayhim, A. Fracture resistance of primary zirconia crowns: An in vitro study. Children 2022, 9, 77. [Google Scholar] [CrossRef]
- Townsend, J.A.; Knoell, P.; Yu, Q.; Zhang, J.F.; Wang, Y.; Zhu, H.; Beattie, S.; Xu, X. In vitro fracture resistance of three commercially available zirconia crowns for primary molars. Pediatr. Dent. 2014, 36, 125–129. [Google Scholar]
- Taran, P.K.; Şahinbaş, A.; Bayraktar, G.A. Effect of different restorative materials on primary tooth wear: A quantitative evaluation using microcomputed tomography. Pediatr. Dent. 2021, 43, 395–402. [Google Scholar] [PubMed]
- Signoriello, A.G.; Stellini, E.; Bertolini, R.; Pezzato, L.; Positello, P.; Gracco, A.A.; Mazzoleni, S.; Ludovichetti, F.S. Paedodontic preformed crowns in primary teeth and relative degree of dental wear. Eur. J. Paediatr. Dent. 2024, 25, 1. [Google Scholar] [PubMed]
- Kessler, A.; Reymus, M.; Hickel, R.; Kunzelmann, K.H. Three-body wear of 3D printed temporary materials. Dent. Mater. 2019, 35, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Rekow, E.D. Digital dentistry: The new state of the art—Is it disruptive or destructive? Dent. Mater. 2020, 36, 9–24. [Google Scholar] [CrossRef]
- Oğuz, E.I.; Bezgin, T.; Orhan, A.I.; Buyuksungur, A.; Orhan, K. Fracture resistance of esthetic prefabricated and custom-made crowns for primary molars after artificial aging. Pediatr. Dent. 2022, 44, 368–374. [Google Scholar]
- El Hayek, J.E.; Tohme, H.; Nasr, L.; El Hachem, R.; Kabbani, N.; Mchayleh, N.F. Fracture strength of preformed zirconia crown and CAD-CAM zirconia, ceramic, and hybrid composite crowns for the restoration of primary molars: An in vitro study. Int. J. Paediatr. Dent. 2024, in press.
- Bolaca, A.; Erdoğan, Y. Fracture resistance evaluation of CAD/CAM zirconia and composite primary molar crowns with different occlusal thicknesses. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241235994. [Google Scholar] [CrossRef]
- Mete, A.; Yilmaz, Y.; Derelioglu, S.S. Fracture resistance force of primary molar crowns milled from polymeric computer-aided design/computer-assisted manufactured resin blocks. Niger. J. Clin. Pract. 2018, 21, 525–530. [Google Scholar]
- Möhn, M.; Frankenberger, R.; Krämer, N. Wear and marginal quality of aesthetic crowns for primary molars. Int. J. Paediatr. Dent. 2022, 32, 273–283. [Google Scholar] [CrossRef]
- Lee, K.E.; Kang, H.S.; Shin, S.Y.; Lee, T.; Lee, H.S.; Song, J.S. Comparison of three-dimensional printed resin crowns and preformed stainless steel crowns for primary molar restorations: A randomized controlled trial. J. Clin. Pediatr. Dent. 2024, 48, 59–67. [Google Scholar]
- Aktaş, N.; Ciftci, V. Current applications of three-dimensional (3D) printing in pediatric dentistry: A literature review. J. Clin. Pediatr. Dent. 2024, 48, 4–13. [Google Scholar]
- Aktas, N.; Bankoglu Güngör, M. Effects of 3D-printing technology and cement type on the fracture resistance of permanent resin crowns for primary teeth. Int. J. Prosthodont. 2024, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Chawla, S.; Perween, N.; Gupta, R. PEEK: A futuristic dental material in pediatric dentistry. J. Pediatr. Dent. 2021, 7, 72–74. [Google Scholar] [CrossRef]
- Vichi, A.; Balestra, D.; Scotti, N.; Louca, C.; Paolone, G. Translucency of CAD/CAM and 3D printable composite materials for permanent dental restorations. Polymers 2023, 15, 1443. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.E.; Hwangbo, N.K.; Kim, J.E. Effects of surface glazing on the mechanical and biological properties of 3D printed permanent dental resin materials. J. Prosthodont. Res. 2024, 68, 273–282. [Google Scholar] [CrossRef]
- Donmez, M.B.; Okutan, Y. Marginal gap and fracture resistance of implant-supported 3D-printed definitive composite crowns: An in vitro study. J. Dent. 2022, 124, 104216. [Google Scholar] [CrossRef]
- Yanar, G.N.; İnal, C.B.; Aktaş, N.; Bankoğlu Güngör, M. A severely damaged premolar tooth restored with coronal pulpotomy and a 3D-printed endocrown. J. Clin. Pediatr. Dent. 2024, 48, 193–199. [Google Scholar]
- Choi, J.W.; Song, E.J.; Shin, J.H.; Jeong, T.S.; Huh, J.B. In vitro investigation of wear of CAD/CAM polymeric materials against primary teeth. Materials 2017, 10, 1410. [Google Scholar] [CrossRef]
- Talekar, A.; Chaudhari, G.; Mallineni, S.K.; Kothare, S.; Patil, A.; Musale, P.; Chunawala, Y.; Choubey, S. Ex vivo assessment of natural teeth wear against zirconia and novel glass-fiber-reinforced composite crowns in primary teeth by a three-dimensional assessment method. Int. J. Dent. 2021, 2021, 9670982. [Google Scholar] [CrossRef]
- Elian El Hayek, J.; El Osta, N.; Farhat Mchayleh, N. Fracture strength of preformed zirconia crown and new custom-made zirconia crown for the restoration of deciduous molars: In vitro study. Eur. Arch. Paediatr. Dent. 2022, 23, 333–339. [Google Scholar] [CrossRef]
- Solá-Ruíz, M.F.; Baima-Moscardó, A.; Selva-Otaolaurruchi, E.; Montiel-Company, J.M.; Agustín-Panadero, R.; Fons-Badal, C.; Fernández-Estevan, L. Wear in Antagonist Teeth Produced by Monolithic Zirconia Crowns: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 997. [Google Scholar] [CrossRef]
- Branco, A.C.; Colaço, R.; Figueiredo-Pina, C.G.; Serro, A.P. A State-of-the-Art Review on the Wear of the Occlusal Surfaces of Natural Teeth and Prosthetic Crowns. Materials 2020, 13, 3525. [Google Scholar] [CrossRef]
- Shin, Y.; Wada, K.; Tsuchida, Y.; Ijbara, M.; Ikeda, M.; Takahashi, H.; Iwamoto, T. Wear behavior of materials for additive manufacturing after simulated occlusion of deciduous dentition. J. Mech. Behav. Biomed. Mater. 2023, 138, 105627. [Google Scholar] [CrossRef]
- Kist, S.; Stawarczyk, B.; Kollmuss, M.; Hickel, R.; Huth, K.C. Fracture load and chewing simulation of zirconia and stainless-steel crowns for primary molars. Eur. J. Oral. Sci. 2019, 127, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, H.; Kim, I.H.; Lee, J.; Lee, K.E.; Lee, H.S.; Kim, J.H.; Song, J.S.; Shin, Y. Novel 3D Printed Resin Crowns for Primary Molars: In Vitro Study of Fracture Resistance, Biaxial Flexural Strength, and Dynamic Mechanical Analysis. Children 2022, 9, 1445. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Rallini, M.; Pagano, S.; Eramo, S.; D’Errico, P.; Torre, L.; Kenny, J.M. Mechanical effect of static loading on endodontically treated teeth restored with fiber-reinforced posts. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 384–394. [Google Scholar] [CrossRef]
- Yahyazadehfar, M.; Mutluay, M.M.; Majd, H.; Ryou, H.; Arola, D. Fatigue of the resin-enamel bonded interface and the mechanisms of failure. J. Mech. Behav. Biomed. Mater. 2013, 21, 121–132. [Google Scholar] [CrossRef]
- Benetti, A.R.; Larsen, L.; Dowling, A.H.; Fleming, G.J. Assessment of wear facets produced by the ACTA wear machine. J. Dent. 2016, 45, 19–25. [Google Scholar] [CrossRef]
- Hahnel, S.; Behr, M.; Handel, G.; Rosentritt, M. Two-body wear of artificial acrylic and composite resin teeth in relation to antagonist material. J. Prosthet. Dent. 2009, 101, 269–278. [Google Scholar] [CrossRef]
- Mello, P.C.; Coppedê, A.R.; Macedo, A.P.; de Mattos, M.d.G.; Rodrigues, R.C.; Ribeiro, R.F. Abrasion wear resistance of different artificial teeth opposed to metal and composite antagonists. J. Appl. Oral. Sci. 2009, 17, 451–456. [Google Scholar] [CrossRef]
- Beleidy, M.; Ziada, A. 3D surface deviation wear analysis of veneered PEEK crowns and its correlation with optical digital profilometry. J. Prosthodont. 2023, 32, 32–39. [Google Scholar] [CrossRef]
- Hao, Z.; Yin, H.; Wang, L.; Meng, Y. Wear behavior of seven artificial resin teeth assessed with three-dimensional measurements. J. Prosthet. Dent. 2014, 112, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Nakase, Y.; Yamaguchi, S.; Okawa, R.; Nakano, K.; Kitagawa, H.; Imazato, S. Physical properties and wear behavior of CAD/CAM resin composite blocks containing S-PRG filler for restoring primary molar teeth. Dent. Mater. 2022, 38, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Walia, T.; Salami, A.A.; Bashiri, R.; Hamood, O.M.; Rashid, F. A randomised controlled trial of three aesthetic full-coronal restorations in primary maxillary teeth. Eur. J. Paediatr. Dent. 2014, 15, 113–118. [Google Scholar] [PubMed]
- Donovan, T.E.; Alraheam, I.A.; Sulaiman, T.A. An evidence-based evaluation of contemporary dental ceramics. Dent. Update 2018, 45, 541–546. [Google Scholar] [CrossRef]
- Graf, T.; Erdelt, K.J.; Güth, J.F.; Edelhoff, D.; Schubert, O.; Schweiger, J. Influence of pre-treatment and artificial aging on the retention of 3D-printed permanent composite crowns. Biomedicines 2022, 10, 186. [Google Scholar] [CrossRef]
- Kamegai, T.; Tatsuki, T.; Nagano, H.; Mitsuhashi, H.; Kumeta, J.; Tatsuki, Y.; Kamegai, T.; Inaba, D. A determination of bite force in northern Japanese children. Eur. J. Orthod. 2005, 27, 53–57. [Google Scholar] [CrossRef]
- Braun, S.; Hnat, W.P.; Freudenthaler, J.W.; Marcotte, M.R.; Hönigle, K.; Johnson, B.E. A study of maximum bite force during growth and development. Angle Orthod. 1996, 66, 261–264. [Google Scholar]
- Owais, A.I.; Shaweesh, M.; Abu Alhaija, E.S. Maximum occusal bite force for children in different dentition stages. Eur. J. Orthod. 2013, 35, 427–433. [Google Scholar] [CrossRef]
- Lu, W.J.; Srimaneepong, V.; Chen, C.S.; Huang, C.H.; Lin, H.C.; Liu, C.F.; Huang, H.H. Influence of aging on the fracture characteristics of polyetheretherketone dental crowns: A preliminary study. Polymers 2022, 14, 4123. [Google Scholar] [CrossRef]
- Matzinger, M.; Hahnel, S.; Preis, V.; Rosentritt, M. Polishing effects and wear performance of chairside CAD/CAM materials. Clin. Oral. Investig. 2019, 23, 725–737. [Google Scholar] [CrossRef]

| Group/Material | Composition | Manufacturer | Production Method |
|---|---|---|---|
| Group 1: Prefabricated Zirconia Crown | 88–96% zirconium oxide, 4–6% yttrium oxide, 5% hafnium oxide, 2–5% organic binders, 1–4% pigments | NuSmile, Houston, TX, USA | Prefabricated Crown |
| Group 2: Prefabricated Composite Crown | 82% inorganic filler (particle size 0.02–3 μm) (barium glass, Bis-GMA-based matrix, pigments, additives, catalysts) | Edelweiss, Wolfurt, Austria | Prefabricated Crown |
| Group 3: Composite Crown | 28.4% cross-linked dimethacrylates, 71.1% barium glass, silicon dioxide | Ivoclar Vivadent, Schaan, Liechtenstein | Subtractive Manufacturing (CAD-CAM; milling) |
| Group 4: Resin Matrix Ceramic Crown | 29% Bis-MEPP, UDMA, DMA, 71% silica (20 nm), barium glass (300 nm) nanoparticles | GC Corporation, Tokyo, Japan | Subtractive Manufacturing (CAD-CAM; milling) |
| Group 5: PEEK Crown | 100% polyetheretherketone (PEEK) | White Peaks Dental Systems GmbH, Essen, Germany | Subtractive Manufacturing (CAD-CAM; milling) |
| Group 6: Permanent Crown Resin | Organic matrix: 50– < 75% wt. Bis-EMA Esterification products of 4.4′-isopropylidiphenol, ethoxylated and 2-methylprop-2enoic acid. Silanized dental glass, methyl benzoylformate, diphenyl [2,4,6-trimethylbenzoyl] phosphine oxide. Inorganic filler: Silanized dental glass (particle size 0.7 μm) (30–50% wt.) | Formlabs Inc., Somerville, MA, USA | Additive Manufacturing (3D-printing) |
| Group/Material | Preparation of the Internal Surface before Cementation |
|---|---|
| Group 1: Prefabricated Zirconia Crown | No preparation is required |
| Group 2: Prefabricated Composite Crown | Sandblasting with Al2O3 particles Cleaning with ethanol in an ultrasonic bath Rinsing and drying with air Application of bonding agent (3M Scotchbond) Drying with air |
| Group 3: Composite Crown | Sandblasting with Al2O3 particles Cleaning with ethanol in an ultrasonic bath Rinsing and drying with air Application of bonding agent (3M Scotchbond) Drying with air |
| Group 4: Resin Matrix Ceramic Crown | Application of 5% hydrofluoric acid (IPS Ceramic Etching Gel; Ivoclar Vivadent) for 60 s Application of bonding agent (3M Scotchbond) |
| Group 5: PEEK Crown | Sandblasting with Al2O3 particles Application of bonding agent (3M Scotchbond) Drying with air |
| Group 6: Permanent Crown Resin | Sandblasting with Al2O3 particles Cleaning with ethanol in an ultrasonic bath Rinsing and drying with air Application of bonding agent (3M Scotchbond) Drying with air |
| Failure Type | Definitions |
|---|---|
| I | Cracks that are not visible to the naked eye but can be seen under a stereomicroscope |
| II | Visible cracks on unseparated margins |
| III | Cracks on separated margins |
| IV | Crown fracture with less than half of the crown displaced, with the supporting structure intact |
| V | Crown fracture with more than half of the crown displaced, with the supporting structure intact |
| VI | Crown fracture involving the supporting structure |
| Material (n = 10) | Mean | Standard Deviation | Standard Error | 95% Confidence Interval | Minimum Value | Maximum Value | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| 3D Wear | |||||||
| Group 1: Prefabricated Zirconia Crown | 1.69 | 0.74 | 0.23 | 1.16 | 2.22 | 0.30 | 2.8 |
| Group 2: Prefabricated Composite Crown | 1.62 | 1.38 | 0.44 | 0.63 | 2.61 | 0.00 | 3.9 |
| Group 3: Composite Crown | 1.74 | 0.95 | 0.3 | 1.06 | 2.42 | 0.10 | 3.5 |
| Group 4: Resin Matrix Ceramic Crown | 0.99 | 0.66 | 0.21 | 0.52 | 1.47 | 0.10 | 2.2 |
| Group 5: PEEK Crown | 1.1 | 0.49 | 0.16 | 0.75 | 1.45 | 0.30 | 2.2 |
| Group 6: Permanent Crown Resin | 1.29 | 0.82 | 0.26 | 0.71 | 1.88 | 0.10 | 3.2 |
| Total | 1.41 | 0.9 | 0.11 | 1.17 | 1.64 | 0.00 | 3.9 |
| 2D Wear | |||||||
| Group 1: Prefabricated Zirconia Crown | 0.59 | 0.23 | 0.07 | 0.43 | 0.75 | 0.2 | 1 |
| Group 2: Prefabricated Composite Crown | 0.97 | 0.53 | 0.17 | 0.59 | 1.35 | 0.1 | 1.7 |
| Group 3: Composite Crown | 0.88 | 0.34 | 0.11 | 0.64 | 1.13 | 0.2 | 1.5 |
| Group 4: Resin Matrix Ceramic Crown | 0.84 | 0.75 | 0.24 | 0.3 | 1.38 | 0.1 | 2.1 |
| Group 5: PEEK Crown | 0.73 | 0.39 | 0.12 | 0.45 | 1 | 0.02 | 1.5 |
| Group 6: Permanent Crown Resin | 1.01 | 0.43 | 0.17 | 0.7 | 1.32 | 0.4 | 1.8 |
| Total | 0.84 | 0.48 | 0.061 | 0.7 | 0.96 | 0.02 | 2.1 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p Value |
|---|---|---|---|---|---|
| 3D Wear | |||||
| Between Groups | 5.181 | 5 | 1.036 | 1.318 | 0.270 |
| Within Groups | 42.467 | 54 | 0.786 | ||
| Total | 47.649 | 5 | |||
| 2D Wear | |||||
| Between Groups | 1.215 | 5 | 0.243 | 1.082 | 0.381 |
| Within Groups | 12.131 | 54 | 0.225 | ||
| Total | 13.346 | 59 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p Value |
|---|---|---|---|---|---|
| Between Groups | 11,958,033.950 | 5 | 2,391,606.790 | 67.797 | 0.000 |
| Within Groups | 1,904,895.700 | 54 | 35,275.846 | ||
| Total | 13,862,929.650 | 59 |
| Material (n = 10) | Mean | Standard Deviation | Standard Error | 95% Confidence Interval | Minimum Value | Maximum Value | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower LIMIT | ||||||
| Group 1: Prefabricated Zirconia Crown | 521.40 C | 63.16 | 19.97 | 476.22 | 566.58 | 430 | 645 |
| Group 2: Prefabricated Composite Crown | 633.70 BC | 103.79 | 32.82 | 559.45 | 707.95 | 428 | 772 |
| Group 3: Composite Crown | 643.80 B | 96.61 | 30.55 | 574.69 | 712.91 | 497 | 793 |
| Group 4: Resin Matrix Ceramic Crown | 596.00 BC | 78.54 | 24.84 | 539.82 | 652.18 | 465 | 757 |
| Group 5: PEEK Crown | 1797.00 A | 412.60 | 130.47 | 1501.85 | 2092.15 | 1315 | 2684 |
| Group 6: Permanent Crown Resin | 625.20 BC | 105.62 | 33.40 | 549.64 | 700.76 | 461 | 835 |
| Total | 802.85 | 484.73 | 62.58 | 677.63 | 928.07 | 428 | 2684 |
| Failure Type/Crown Group | Type I | Type II | Type III | Type IV | Type V | Type VI |
|---|---|---|---|---|---|---|
| Group 1: Prefabricated Zirconia Crown | - | - | - | - | 8 | 2 |
| Group 2: Prefabricated Composite Crown | - | - | - | 2 | - | 8 |
| Group 3: Composite Crown | - | - | - | - | - | 10 |
| Group 4: Resin Matrix Ceramic Crown | - | - | - | - | - | 10 |
| Group 5: PEEK Crown | - | - | - | - | - | 10 |
| Group 6: Permanent Crown Resin | - | - | 1 | - | - | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktaş, N.; Bankoğlu Güngör, M. Evaluation of Wear on Primary Tooth Enamel and Fracture Resistance of Esthetic Pediatric Crowns Manufactured from Different Materials. Medicina 2024, 60, 1678. https://doi.org/10.3390/medicina60101678
Aktaş N, Bankoğlu Güngör M. Evaluation of Wear on Primary Tooth Enamel and Fracture Resistance of Esthetic Pediatric Crowns Manufactured from Different Materials. Medicina. 2024; 60(10):1678. https://doi.org/10.3390/medicina60101678
Chicago/Turabian StyleAktaş, Nagehan, and Merve Bankoğlu Güngör. 2024. "Evaluation of Wear on Primary Tooth Enamel and Fracture Resistance of Esthetic Pediatric Crowns Manufactured from Different Materials" Medicina 60, no. 10: 1678. https://doi.org/10.3390/medicina60101678
APA StyleAktaş, N., & Bankoğlu Güngör, M. (2024). Evaluation of Wear on Primary Tooth Enamel and Fracture Resistance of Esthetic Pediatric Crowns Manufactured from Different Materials. Medicina, 60(10), 1678. https://doi.org/10.3390/medicina60101678





