Handgrip Strength and Cognitive Recovery in Older Stroke Survivors: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Setting
2.2. Sample Size Calculation
2.3. Low HGS Group vs. Normal HGS Group
2.4. Data Collection
2.5. Neuropsychological Evaluation
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics According to HGS
3.2. Generalized Linear Mixed Model
3.3. Post Hoc Power Analysis
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michel, J.P.; Leonardi, M.; Martin, M.; Prina, M. WHO’s report for the decade of healthy ageing 2021–30 sets the stage for globally comparable data on healthy ageing. Lancet Healthy Longev. 2021, 2, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Rajati, F.; Rajati, M.; Rasulehvandi, R.; Kazeminia, M. Prevalence of stroke in the elderly: A systematic review and meta-analysis. Interdiscip. Neurosurg. 2023, 32, 101746. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.B.; Lawrence, E.; Hillis, A.E.; Chen, K.; Gottesman, R.F.; Llinas, R.H. Pre-stroke employment results in better patient-reported outcomes after minor stroke: Short title: Functional outcomes after minor stroke. Clin. Neurol. Neurosurg. 2018, 165, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Rohde, D.; Gaynor, E.; Large, M.; Mellon, L.; Hall, P.; Brewer, L.; Bennett, K.; Williams, D.; Dolan, E.; Callaly, E.; et al. The impact of cognitive impairment on poststroke outcomes: A 5-year follow-up. J. Geriatr. Psychiatry Neurol. 2019, 32, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Hamed, S.A.; El-Shereef, H.K.; Shawky, O.A.; Mohamed, K.A.; Awad, E.M.; Ahmed, M.A.; Shehata, G.A.; Eltahtawy, M.A. Cognitive impairment after cerebrovascular stroke: Relationship to vascular risk factors. Neuropsychiatr. Dis. Treat. 2009, 5, 103–116. [Google Scholar]
- Douiri, A.; Rudd, A.G.; Wolfe, C.D. Prevalence of poststroke cognitive impairment: South London stroke register 1995–2010. Stroke 2013, 44, 138–145. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Zou, P.; He, G.; Zeng, Y.; Yang, J. Prevalence and factors influencing cognitive impairment among the older adult stroke survivors: A cross-sectional study. Front. Public Health 2023, 11, 1254126. [Google Scholar] [CrossRef]
- Vaishya, R.; Misra, A.; Vaish, A.; Ursino, N.; D’Ambrosi, R. Hand grip strength as a proposed new vital sign of health: A narrative review of evidences. J. Health Popul. Nutr. 2024, 43, 7. [Google Scholar] [CrossRef]
- Li, G.; Lu, Y.; Shao, L.; Wu, L.; Qiao, Y.; Ding, Y.; Ke, C. Handgrip strength is associated with risks of new-onset stroke and heart disease: Results from 3 prospective cohorts. BMC Geriatr. 2023, 23, 268. [Google Scholar] [CrossRef]
- Lopez-Jaramillo, P.; Lopez-Lopez, J.P.; Tole, M.C.; Cohen, D.D. Muscular strength in risk factors for cardiovascular disease and mortality: A narrative review. Anatol. J. Cardiol. 2022, 26, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Syddall, H.E.; Sparkes, J.; Ritchie, J.; Butchart, J.; Kerr, A.; Cooper, C.; Sayer, A.A. Grip strength and its determinants among older people in different healthcare settings. Age Ageing 2014, 43, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Rijk, J.M.; Roos, P.R.; Deckx, L.; van den Akker, M.; Buntinx, F. Prognostic value of handgrip strength in people aged 60 years and older: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2016, 16, 5–20. [Google Scholar] [CrossRef]
- Alfaro-Acha, A.; Snih, S.A.; Raji, M.A.; Kuo, Y.F.; Markides, K.S.; Ottenbacher, K.J. Handgrip strength and cognitive decline in older Mexican Americans. J. Gerontol. A Biol. Sci. Med. Sc. 2006, 61, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Stessman, J.; Rottenberg, Y.; Fischer, M.; Hammerman-Rozenberg, A.; Jacobs, J.M. Handgrip strength in old and very old adults: Mood, cognition, function, and mortality. J. Am. Geriatr. Soc. 2017, 65, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Trevisan, C.; Bolzetta, F.; De Rui, M.; Solmi, M.; Sartori, L.; Musacchio, E.; Zambon, S.; Perissinotto, E.; et al. What physical performance measures predict incident cognitive decline among intact older adults? A 4.4year follow up study. Exp. Gerontol. 2016, 81, 110–118. [Google Scholar] [CrossRef]
- Turunen, K.E.; Laari, S.P.; Kauranen, T.V.; Uimonen, J.; Mustanoja, S.; Tatlisumak, T.; Poutiainen, E. Domain-specific cognitive recovery after first-ever stroke: A 2-year follow-up. J. Int. Neuropsychol. Soc. 2018, 24, 117–127. [Google Scholar] [CrossRef]
- Nys, G.; Van Zandvoort, M.; De Kort, P.; Jansen, B.; Van der Worp, H.; Kappelle, L.; De Haan, E. Domain-specific cognitive recovery after first-ever stroke: A follow-up study of 111 cases. J. Int. Neuropsychol. Soc. 2005, 11, 795–806. [Google Scholar] [CrossRef]
- Julious, S.A. Sample Sizes for Clinical Trials, 2nd ed.; CRC Press: Boca Raton, FL, 2023. [Google Scholar]
- Chow, S.C.; Shao, J.; Wang, H.; Lokhnygina, Y. Sample Size Calculations in Clinical Research, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Machin, D.; Campbell, M.J.; Tan, S.B.; Tan, S.H. Sample Size Tables for Clinical Studies; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Pearson Education: London, UK, 1999. [Google Scholar]
- Nishiguchi, S.; Yamada, M.; Shirooka, H.; Nozaki, Y.; Fukutani, N.; Tashiro, Y.; Hirata, H.; Yamaguchi, M.; Tasaka, S.; Matsushita, T.; et al. Sarcopenia as a risk factor for cognitive deterioration in community-dwelling older adults: A 1-year prospective study. J. Am. Med. Dir. Assoc. 2016, 17, 372.e5–372.e8. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Na, D.; Hahn, S. Seoul Neuropsychological Screening Battery; Human Brain Research & Consulting, Co.: Incheon, Republic of Korea, 2003. [Google Scholar]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Shaughnessy, K.A.; Hackney, K.J.; Clark, B.C.; Kraemer, W.J.; Terbizan, D.J.; Bailey, R.R.; McGrath, R. A narrative review of handgrip strength and cognitive functioning: Bringing a new characteristic to muscle memory. J. Alzheimers Dis. 2020, 73, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Proust-Lima, C.; Amieva, H.; Dartigues, J.F.; Jacqmin-Gadda, H. Sensitivity of four psychometric tests to measure cognitive changes in brain aging-population–based studies. Am. J. Epidemiol. 2007, 165, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, J. Digit symbol substitution test: The case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Y.; Nishita, Y.; Nakagawa, T.; Tange, C.; Tomida, M.; Shimokata, H.; Otsuka, R.; Chen, L.K.; Arai, H. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019, 19, 186. [Google Scholar] [CrossRef]
- Yang, L.; Koyanagi, A.; Smith, L.; Hu, L.; Colditz, G.A.; Toriola, A.T.; López Sánchez, G.F.; Vancampfort, D.; Hamer, M.; Stubbs, B.; et al. Hand grip strength and cognitive function among elderly cancer survivors. PLoS ONE 2018, 13, e0197909. [Google Scholar] [CrossRef]
- Chen, W.L.; Peng, T.C.; Sun, Y.S.; Yang, H.F.; Liaw, F.Y.; Wu, L.W.; Chang, Y.W.; Kao, T.W. Examining the association between quadriceps strength and cognitive performance in the elderly. Medicine 2015, 94, e1335. [Google Scholar] [CrossRef]
- Rabin, L.A.; Paolillo, E.; Barr, W.B. Stability in test-usage practices of clinical neuropsychologists in the United States and Canada over a 10-year period: A follow-up survey of INS and NAN members. Arch. Clin. Neuropsychol. 2016, 31, 206–230. [Google Scholar] [CrossRef]
- Sachs, A.; Rising, K.; Beeson, P.M. A retrospective study of long-term improvement on the Boston Naming test. Am. J. Speech Lang. Pathol. 2020, 29, 425–436. [Google Scholar] [CrossRef]
- Ballard, C.; Rowan, E.; Stephens, S.; Kalaria, R.; Kenny, R.A. Prospective follow-up study between 3 and 15 months after stroke: Improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke 2003, 34, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.W.; Crawford, J.D.; Desmond, D.W.; Bae, H.J.; Lim, J.S.; Godefroy, O.; Roussel, M.; Kang, Y.; Jahng, S.; Köhler, S.; et al. Long-term cognitive decline after stroke: An individual participant data meta-analysis. Stroke 2022, 53, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Newton, J.M.; Swayne, O.B.; Lee, L.; Frackowiak, R.S.; Thompson, A.J.; Greenwood, R.J.; Rothwell, J.C. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur. J. Neurosci. 2007, 25, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.S.; Mohamad, M.; Syazarina, S.O.; Nafisah, W.Y. Hand grips strength effect on motor function in human brain using fMRI: A pilot study. J. Phys. Conf. Ser. 2014, 546, 012005. [Google Scholar] [CrossRef]
- Heuninckx, S.; Wenderoth, N.; Debaere, F.; Peeters, R.; Swinnen, S.P. Neural basis of aging: The penetration of cognition into action control. J. Neurosci. 2005, 25, 6787–6796. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Davare, M.; Andres, M.; Fadiga, L. Precision grasping in humans: From motor control to cognition. Curr. Opin. Neurobiol. 2007, 17, 644–648. [Google Scholar] [CrossRef]
- Girgenti, S.G.; Brunson, A.O.; Marsh, E.B. Baseline function and rehabilitation are as important as stroke severity as long-term predictors of cognitive performance post-stroke. Am. J. Phys. Med. Rehabil. 2023, 102, S43–S50. [Google Scholar] [CrossRef]
| Total (N = 50) | |
|---|---|
| Age (years) | 74.96 (6.78) |
| Sex | |
| Male | 18 (36.00%) |
| Female | 32 (64.00%) |
| BMI (kg/m2) | 23.81 (3.92) |
| mRS (points) | 0.46 (1.13) |
| CCI (points) | |
| ≤4 | 34 (68.00%) |
| 5–6 | 14 (28.00%) |
| ≥7 | 2 (4.00%) |
| Education (years) | 7.74 (4.41) |
| Lesion | |
| Ischemic | 39 (78.00%) |
| Hemorrhagic | 11 (22.00%) |
| NIHSS (points) | 4.82 (4.72) |
| MoCA at discharge (points) | 16.16 (7.32) |
| Low HGS (N = 29) | Normal HGS (N = 21) | p-Value | |
|---|---|---|---|
| Age (years) | 76.76 (6.11) | 72.48 (7.01) | 0.03 (T) |
| Sex | 0.15 (C) | ||
| Male | 8 (27.59%) | 10 (47.62%) | |
| Female | 21 (72.41%) | 11 (52.38%) | |
| BMI (kg/m2) | 24.15 (4.57) | 23.34 (2.82) | 0.45 (T) |
| mRS (points) | 0.4 (0.8) | 0.1 (0.4) | 0.12 (W) |
| CCI (points) | 0.49 (F) | ||
| ≤4 | 18 (62.1%) | 16 (76.2%) | |
| 5–6 | 10 (34.5%) | 4 (19%) | |
| ≥7 | 1 (3.4%) | 1 (4.8%) | |
| Education (years) | 6.83 (4.36) | 9.00 (4.25) | 0.08 (W) |
| Lesion | 0.32 (F) | ||
| Ischemic | 21 (72.41%) | 18 (85.71%) | |
| Hemorrhagic | 8 (27.59%) | 3 (14.29%) | |
| NIHSS (points) | 5.38 (5.44) | 4.05 (3.46) | 0.71 (W) |
| MoCA at discharge (points) | 14.00 (7.02) | 19.14 (6.81) | 0.01 (T) |
| Low HGS | Normal HGS | F-Value | p-Value | Post Hoc p-Value | ||
|---|---|---|---|---|---|---|
| MoCA | Initial, LSM (SE) | 15.38 (1.23) | 17.08 (1.42) | |||
| 1Y, LSM (SE) | 14.73 (1.23) | 18.08 (1.42) | ||||
| Group | 2.30 | 0.14 | ||||
| Time | 0.03 | 0.86 | ||||
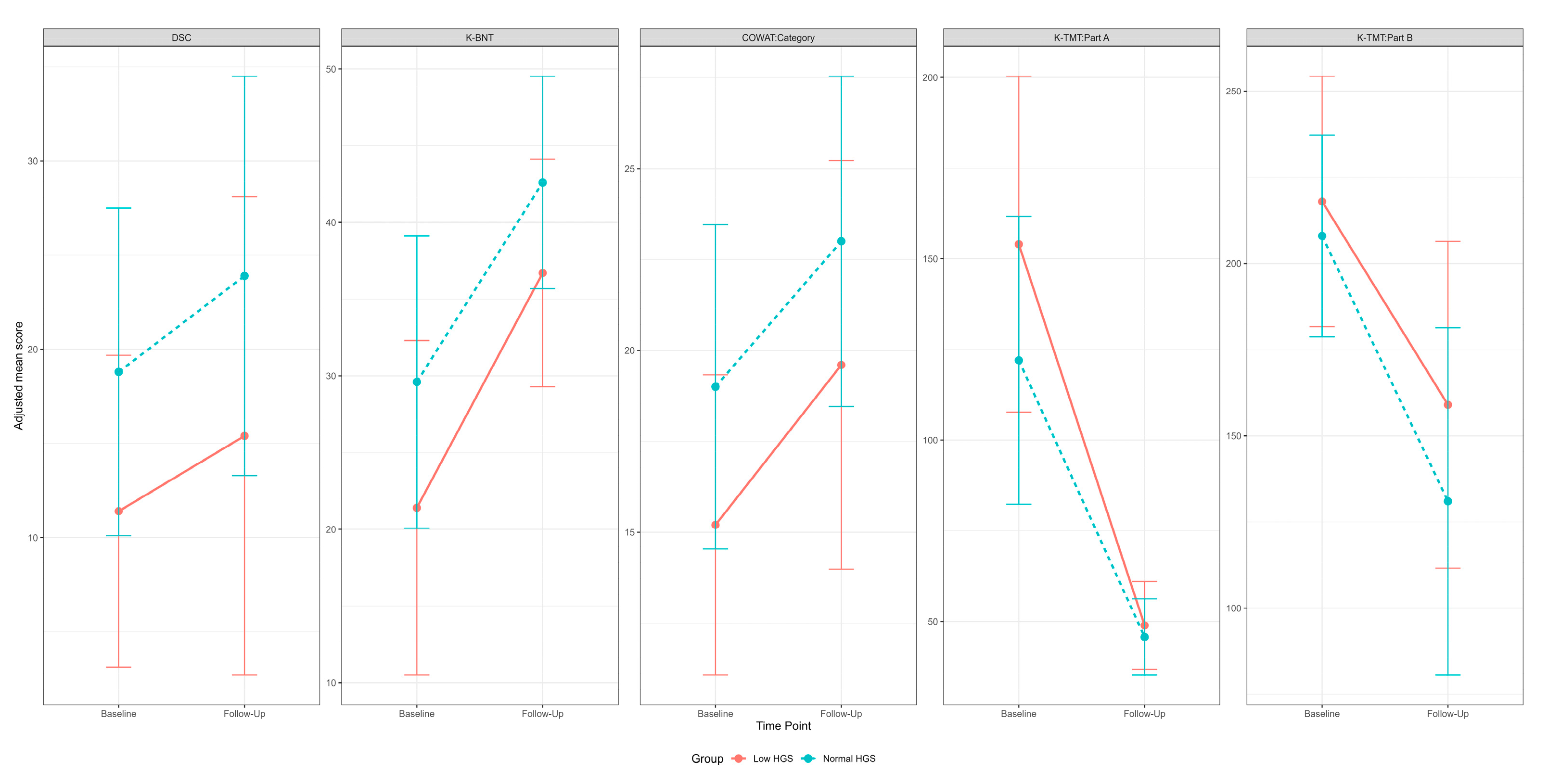
| DSC | Initial, LSM (SE) | 9.01 (2.81) | 21.47 (3.25) | |||
| 1Y, LSM (SE) | 15.49 (2.81) | 23.09 (3.25) | ||||
| Group | 7.48 | 0.01 | 0.03 (B) | |||
| 0.45 (F) | ||||||
| Time | 2.76 | 0.10 | ||||
| DST:Forward | Initial, LSM (SE) | 5.95 (0.30) | 5.61 (0.35) | |||
| 1Y, LSM (SE) | 5.84 (0.30) | 5.75 (0.35) | ||||
| Group | 0.27 | 0.60 | ||||
| Time | 0.01 | 0.94 | ||||
| DST:Backward | Initial, LSM (SE) | 2.42 (0.25) | 2.96 (0.29) | |||
| 1Y, LSM (SE) | 2.49 (0.25) | 3.20 (0.29) | ||||
| Group | 3.60 | 0.06 | ||||
| Time | 0.50 | 0.48 | ||||
| K-BNT | Initial, LSM (SE) | 21.93 (2.21) | 27.54 (2.56) | |||
| 1Y, LSM (SE) | 38.38 (2.21) | 39.06 (2.56) | ||||
| Group | 1.21 | 0.28 | ||||
| Time | 50.93 | <0.0001 | <0.0001 (L) | |||
| 0.002 (N) | ||||||
| RCFT:Copy | Initial, LSM (SE) | 9.82 (1.84) | 14.05 (2.13) | |||
| 1Y, LSM (SE) | 12.67 (1.84) | 13.09 (2.13) | ||||
| Group | 0.95 | 0.33 | ||||
| Time | 0.34 | 0.56 | ||||
| SVLT:DR | Initial, LSM (SE) | 1.11 (0.35) | 0.93 (0.41) | |||
| 1Y, LSM (SE) | 1.83 (0.35) | 2.17 (0.41) | ||||
| Group | 0.03 | 0.86 | ||||
| Time | 8.17 | 0.01 | 0.55 (L) | |||
| 0.11 (N) | ||||||
| RCFT:DR | Initial, LSM (SE) | 2.99 (1.06) | 3.18 (1.23) | |||
| 1Y, LSM (SE) | 2.67 (1.06) | 4.99 (1.23) | ||||
| Group | 0.83 | 0.37 | ||||
| Time | 0.63 | 0.43 | ||||
| COWAT:Category | Initial, LSM (SE) | 15.31 (1.55) | 18.65 (1.79) | |||
| 1Y, LSM (SE) | 20.35 (1.55) | 21.70 (1.79) | ||||
| Group | 1.31 | 0.26 | ||||
| Time | 9.61 | 0.01 | 0.02 (L) | |||
| 0.66 (N) | ||||||
| COWAT:Letter | Initial, LSM (SE) | 5.75 (1.57) | 6.00 (1.81) | |||
| 1Y, LSM (SE) | 7.23 (1.57) | 10.19 (1.81) | ||||
| Group | 0.60 | 0.44 | ||||
| Time | 4.70 | 0.04 | >0.99 (L) | |||
| 0.20 (N) | ||||||
| K-TMT:Part A | Initial, LSM (SE) | 168.22 (16.38) | 111.24 (18.98) | |||
| 1Y, LSM (SE) | 52.98 (16.38) | 49.48 (18.98) | ||||
| Group | 2.45 | 0.12 | ||||
| Time | 27.75 | <0.001 | <0.001 (L) | |||
| 0.10 (N) | ||||||
| K-TMT:Part B | Initial, LSM (SE) | 234.20 (23.05) | 203.25 (26.58) | |||
| 1Y, LSM (SE) | 166.44 (23.05) | 138.25 (26.58) | ||||
| Group | 0.88 | 0.35 | ||||
| Time | 14.16 | <0.001 | 0.02 (L) | |||
| 0.10 (N) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-A. Handgrip Strength and Cognitive Recovery in Older Stroke Survivors: A Prospective Study. Medicina 2024, 60, 1697. https://doi.org/10.3390/medicina60101697
Choi Y-A. Handgrip Strength and Cognitive Recovery in Older Stroke Survivors: A Prospective Study. Medicina. 2024; 60(10):1697. https://doi.org/10.3390/medicina60101697
Chicago/Turabian StyleChoi, Young-Ah. 2024. "Handgrip Strength and Cognitive Recovery in Older Stroke Survivors: A Prospective Study" Medicina 60, no. 10: 1697. https://doi.org/10.3390/medicina60101697
APA StyleChoi, Y.-A. (2024). Handgrip Strength and Cognitive Recovery in Older Stroke Survivors: A Prospective Study. Medicina, 60(10), 1697. https://doi.org/10.3390/medicina60101697






