A Prospective Study of the Medication Regimen Complexity Index and Hospitalization Due to Adverse Drug Reactions Among People Living with HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of the MRCI
2.2. Hospitalization
2.3. ADR Recording
2.4. Statistical Analysis
3. Results
3.1. Demographic Analysis
3.2. Association of MRCI in PLHIV with Hospitalization Due to ADRs
3.3. Non-ADR-Related Hospitalization
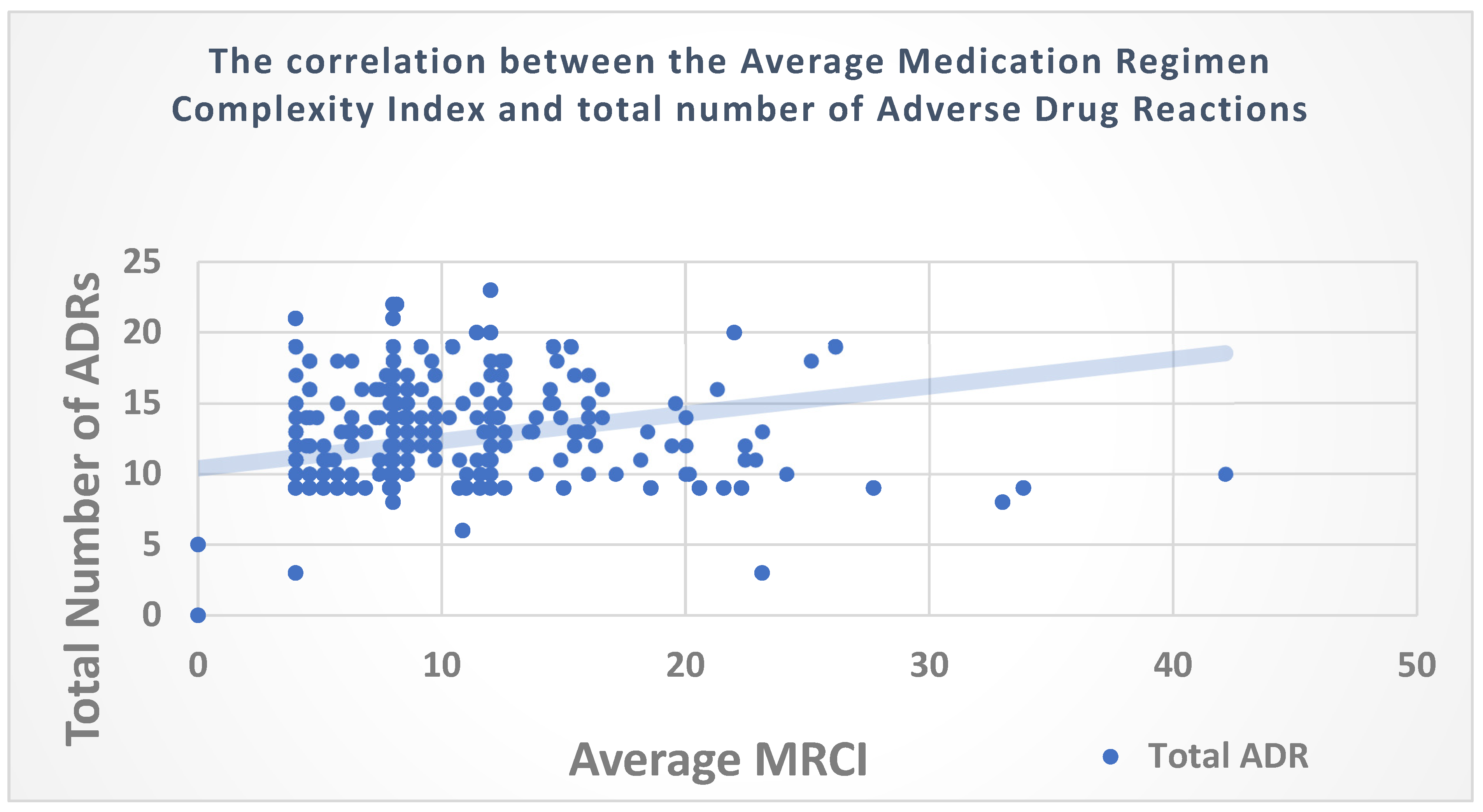
3.4. Correlation of the MRCI with the Number of ADRs among PLHIV
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, J.; Phun, Y.T.; Bailey, M.J.; Kong, D.C.; Stewart, K. Development and Validation of the Medication Regimen Complexity Index. Ann. Pharmacother. 2004, 38, 1369–1376. [Google Scholar] [CrossRef]
- Advinha, A.M.; de Oliveira-Martins, S.; Mateus, V.; Pajote, S.G.; Lopes, M.J. Medication Regimen Complexity in Institutionalized Elderly People in an Aging Society. Int. J. Clin. Pharm. 2014, 36, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Willson, M.N.; Greer, C.L.; Weeks, D.L. Medication Regimen Complexity and Hospital Readmission for an Adverse Drug Event. Ann. Pharmacother. 2014, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, B.C.; Dent, E.; Visvanathan, R.; Wiese, M.D.; Johnell, K.; Chapman, I.; Bell, J.S. Polypharmacy and Medication Regimen Complexity as Factors Associated with Hospital Discharge Destination among Older People: A Prospective Cohort Study. Drugs Aging 2014, 31, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Lepelley, M.; Genty, C.; Lecoanet, A.; Allenet, B.; Bedouch, P.; Mallaret, M.R.; Gillois, P.; Bosson, J.L. Electronic Medication Regimen Complexity Index at Admission and Complications During Hospitalization in Medical Wards: A Tool to Improve Quality of Care? Int. J. Qual. Health Care 2018, 30, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Colavecchia, A.C.; Putney, D.R.; Johnson, M.L.; Aparasu, R.R. Discharge Medication Complexity and 30-Day Heart Failure Readmissions. Res. Soc. Adm. Pharm. 2017, 13, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.K.; Gupte, N.; Kinikar, A.; Bharadwaj, R.; Sastry, J.; Suryavanshi, N.; Nayak, U.; Tripathy, S.; Paranjape, R.; Jamkar, A.; et al. High Rates of All-Cause and Gastroenteritis-Related Hospitalization Morbidity and Mortality among HIV-Exposed Indian Infants. BMC Infect. Dis. 2011, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Poojar, B.; Shenoy, A.; Kamath, A.; Ramapuram, J.; Rao, S.B. Evaluation of Health Literacy and Medication Regimen Complexity Index among Patients with Human Immunodeficiency Virus Infection: A Single-Center, Prospective, Cross-Sectional Study. Clin. Epidemiol. Global Health 2023, 19, 101206. [Google Scholar] [CrossRef]
- Leporini, C.; De Sarro, G.; Russo, E. Adherence to Therapy and Adverse Drug Reactions: Is There a Link? Expert Opin. Drug Saf. 2014, 13 (Suppl. S1), 41–55. [Google Scholar] [CrossRef]
- Saedder, E.A.; Lisby, M.; Nielsen, L.P.; Bonnerup, D.K.; Brock, B. Number of Drugs Most Frequently Found to Be Independent Risk Factors for Serious Adverse Reactions: A Systematic Literature Review. Br. J. Clin. Pharmacol. 2015, 80, 808–817. [Google Scholar] [CrossRef]
- Ofotokun, I.; Pomeroy, C. Sex Differences in Adverse Reactions to Antiretroviral Drugs. Topics HIV Med. 2003, 11, 55–59. [Google Scholar]
- Alomar, M.J. Factors Affecting the Development of Adverse Drug Reactions. Saudi Pharm. J. 2014, 22, 83–94. [Google Scholar] [CrossRef]
- Wester, K.; Jönsson, A.K.; Spigset, O.; Druid, H.; Hägg, S. Incidence of Fatal Adverse Drug Reactions: A Population-Based Study. Br. J. Clin. Pharmacol. 2008, 65, 573–579. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications-detail-redirect/9789241549684 (accessed on 3 March 2024).
- Division of AIDS (DAIDS). Table for Grading the Severity of Adult and Pediatric Adverse Events. Available online: https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables (accessed on 26 March 2024).
- Schumock, G.T.; Thornton, J.P. Focusing on the Preventability of Adverse Drug Reactions. Hosp. Pharm. 1992, 27, 538–544. [Google Scholar] [PubMed]
- Aronson, J.K.; Ferner, R.E. Joining the DoTS: New Approach to Classifying Adverse Drug Reactions. BMJ 2003, 327, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Ferner, R.E. Clarification of Terminology in Drug Safety. Drug Saf. 2005, 28, 851–870. [Google Scholar] [CrossRef]
- Wimmer, B.C.; Bell, J.S.; Fastbom, J.; Wiese, M.D.; Johnell, K. Medication Regimen Complexity and Number of Medications as Factors Associated with Unplanned Hospitalizations in Older People: A Population-Based Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 831–837. [Google Scholar] [CrossRef]
- Schoonover, H.; Corbett, C.F.; Weeks, D.L.; Willson, M.N.; Setter, S.M. Predicting Potential Postdischarge Adverse Drug Events and 30-Day Unplanned Hospital Readmissions from Medication Regimen Complexity. J. Patient Saf. 2014, 10, 186–191. [Google Scholar] [CrossRef]
- Curtain, C.M.; Chang, J.Y.; Cousins, J.; Parameswaran Nair, N.; Bereznicki, B.; Bereznicki, L. Medication Regimen Complexity Index Prediction of Adverse Drug Reaction–Related Hospital Admissions. Ann. Pharmacother. 2020, 54, 996–1000. [Google Scholar] [CrossRef]
- Morillo-Verdugo, R.; de las Aguas Robustillo-Cortés, M.; Martín, L.A.; de Sotomayor Paz, M.Á.; de León Naranjo, F.L.; Almeida-González, C.V. Determination of a Cutoff Value for Medication Regimen Complexity Index to Predict Polypharmacy in HIV+ Older Patients. Rev. Esp. Quimioter. 2019, 32, 458. [Google Scholar]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Compagnucci, P.; MacDonald, B.; Mayedo, A.; Torlapati, P.G.; Bassiouny, M.; et al. Catheter ablation approach and outcome in HIV+ patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2023, 34, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M.; Abegaz, W.E.; Shewamare, A.; Medhin, G.; Belay, M. Prevalence and Correlates of Anemia Among HIV-Infected Patients on Highly Active Anti-Retroviral Therapy at Zewditu Memorial Hospital, Ethiopia. BMC Hematol. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Volberding, P.A.; Levine, A.M.; Dieterich, D.; Mildvan, D.; Mitsuyasu, R.; Saag, M.; Anemia in HIV Working Group. Anemia in HIV Infection: Clinical Impact and Evidence-Based Management Strategies. Clin. Infect. Dis. 2004, 38, 1454–1463. [Google Scholar] [CrossRef]
- Breitbart, W.; McDonald, M.V.; Rosenfeld, B.; Monkman, N.D.; Passik, S. Fatigue in Ambulatory AIDS Patients. J. Pain Symptom Manag. 1998, 15, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Manaye, Y.; Asrat, A.; Mengesha, E.W. Time to Development of Anemia and Predictors Among HIV-Infected Patients Initiating ART at Felege Hiwot Referral Hospital, Northwest Ethiopia: A Retrospective Follow-Up Study. BioMed Res. Int. 2020, 2020, 7901241. [Google Scholar] [CrossRef] [PubMed]
- Harding, B.N.; Whitney, B.M.; Nance, R.M.; Crane, H.M.; Burkholder, G.; Moore, R.D.; Mathews, W.C.; Eron, J.J.; Hunt, P.W.; Volberding, P.; et al. Antiretroviral drug class and anaemia risk in the current treatment era among people living with HIV in the USA: A clinical cohort study. BMJ Open 2020, 10, e031487. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, M.; Manrique, G.; Bernier, L.; Moussa, L.; Cresson, J.; Gutzeit, A.; Froehlich, J.M.; Koh, D.M.; Chartrand-Lefebvre, C.; Matoori, S. Gender differences in pharmacokinetics: A perspective on contrast agents. ACS Pharmacol. Transl. Sci. 2023, 7, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ammassari, A.; Murri, R.; Pezzotti, P.; Trotta, M.P.; Ravasio, L.; De Longis, P.; Caputo, S.L.; Narciso, P.; Pauluzzi, S.; Carosi, G.; et al. Self-Reported Symptoms and Medication Side Effects Influence Adherence to Highly Active Antiretroviral Therapy in Persons with HIV Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2001, 28, 445–449. [Google Scholar] [CrossRef]
- Agrawal, M.; Shrivastava, S.; Khare, R.L.; Jaiswal, S.; Singh, P.; Hishikar, R. Nephrotoxicity in Patients on Tenofovir vs Non-Tenofovir Containing ART Regimen: An Observational Study. Pharmacophore 2022, 13, 23–31. [Google Scholar] [CrossRef]
- Agbaji, O.O.; Abah, I.O.; Ebonyi, A.O.; Gimba, Z.M.; Abene, E.E.; Gomerep, S.S.; Falang, K.D.; Anejo-Okopi, J.; Agaba, P.A.; Ugoagwu, P.O.; et al. Long Term Exposure to Tenofovir Disoproxil Fumarate-Containing Antiretroviral Therapy Is Associated with Renal Impairment in an African Cohort of HIV-Infected Adults. J. Int. Assoc. Provid. AIDS Care 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; He, Y.H.; Yang, T.T.; Yan, D.M.; Wang, Y.; Zhao, X.; Liu, H.X.; He, S.H.; Zhou, Z.H. Effects of Long-Term Exposure to Tenofovir Disoproxil Fumarate-Containing Antiretroviral Therapy on Renal Function in HIV-Positive Chinese Patients. J. Microbiol. Immunol. Infect. 2019, 52, 710–719. [Google Scholar] [CrossRef] [PubMed]
- National Guidelines for HIV Care and Treatment, 2021. [Internet]. Available online: https://www.aidsdatahub.org/resource/national-guidelines-hiv-care-and-treatment-2021 (accessed on 13 February 2024).
- Shivakumar, Y.M.; Burra, E.; Shahid, K.; Tamene, Y.; Mody, S.P.; Sadiq, K.O.; Penumetcha, S.S. Tenofovir-Induced Renal Dysfunction Among HIV-Infected Patients: A Systematic Review. Cureus 2023, 15, e47358. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, S.; Mao, Z.; Li, Y.; Zhang, H.; Yang, K.; Zhang, H.; Liu, R.; Qian, X.; Li, L.; et al. Dyslipidemia Prevalence, Awareness, Treatment, Control, and Risk Factors in Chinese Rural Population: The Henan Rural Cohort Study. Lipids Health Dis. 2018, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, H.; Shokoohi, M.; Yousefzadeh, G.; Sarvar Azimzadeh, B.; Moshtaghi Kashanian, G.; Bagheri, M.M.; Mirzazadeh, A. Prevalence of Dyslipidemia and Its Association with Other Coronary Artery Disease Risk Factors Among Urban Population in Southeast of Iran: Results of the Kerman Coronary Artery Disease Risk Factors Study (KERCADRS). J. Diabetes Metab. Disord. 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed]

| Mode of Transmission | ||
|---|---|---|
| Heterosexual intercourse | 271 | 95.1% |
| Mother to Child | 12 | 4.2% |
| Unknown | 2 | 0.7% |
| Partner’s HIV status | ||
| HIV-positive partners | 126 | 44.2% |
| HIV-negative partners | 61 | 21.4 |
| Mother is-positive or Both parents are-positive | 8 | 1.4% |
| Missing information (Unknown status) | 90 | 31.6% |
| Family size | ||
| 3-Member Families | 60 | 21.1% |
| 4-Member Families | 79 | 27.7% |
| >5-Member Families | 35 | 12.4% |
| Not Recorded | 43 | 15.1% |
| ≤2 * | 68 | 23.9% |
| Entry Point to healthcare system | ||
| VCTC | 168 | 58.94% |
| OPD | 32 | 11.22% |
| Pvt Practitioner | 78 | 27.36 |
| PPTCT | 3 | 1.5% |
| NGO | 3 | 1.5% |
| IP | 1 | 0.35% |
| Previous antiretroviral (ARV) usage prior to enrollment in the NACO Program | ||
| Yes | 105 | 36.8% |
| No | 137 | 48.1% |
| Unspecified or Missing information | 43 | 15.1% |
| World Health Organization (WHO) Clinical Stage at the initiation of ART | N | % |
| Stage-I | 194 | 68.1% |
| Stage-II | 18 | 6.3% |
| Stage-III | 22 | 7.7% |
| Stage-IV | 51 | 17.9% |
| History of TB | 76 | 26.7% |
| 61 15 | 21.4% |
| TB Treatment Status | ||
| Ongoing | ||
| Cure | ||
| Completed | ||
| Baseline CD4 at the time of study enrollment (in cells/mm3) | ||
| >500 | 145 | 50.9% |
| 200–499 | 115 | 40.4% |
| <200 | 25 | 8.8% |
| >500 | 145 | 50.9% |
| End of the study CD4 (current status) | ||
| >500 | 139 | 48.8% |
| 200–499 | 102 | 35.8% |
| <200 | 18 | 6.3% |
| Missing | 26 | 9.1% |
| Baseline HIV Viral Load (HIV RNA copies) at the time of study enrollment | ||
| Target Not Detected | 172 | 60.4% |
| Below 20 copies/mL | 36 | 12.6% |
| >20–1,000,000 copies/mL | 66 | 23.2% |
| Above 1,000,000 copies/mL | 11 | 3.9% |
| End of the study Viral Load (HIV RNA copies) | ||
| Target Not Detected | 156 | 54.7% |
| Below 20 copies/mL | 31 | 10.9% |
| >20–1,000,000 copies/mL | 67 | 23.5% |
| Above 1,000,000 copies/mL | 4 | 1.4% |
| Initiated regimen after the diagnosis of HIV infection | ||
| Protease inhibitor-Based Regimen | 5 | 1.75% |
| Nucleoside RT Inhibitors-Based Regimen | 203 | 71.22% |
| Non-Nucleoside RT Inhibitors-Based Regimen | 77 | 27.01% |
| Integrase Inhibitors-Based Regimen | 0 | 0% |
| Regimen at the time of study enrollment (Baseline) | ||
| Protease inhibitor-Based Regimen | 62 | 21.75% |
| Nucleoside RT Inhibitors-Based Regimen | 147 | 51.57% |
| Non-Nucleoside RT Inhibitors-Based Regimen | 00.00 | 00.00 |
| Integrase Inhibitors-Based Regimen | 76 | 26.66% |
| 223 | 78.24% |
| 62 | 21.75% |
| Adverse Reaction | Male (n = 147) | Female (n = 138) | Total |
|---|---|---|---|
| Alanine aminotransferase increased | 72 | 27 | 99 |
| Aspartate aminotransferase increased | 20 | 4 | 24 |
| Hepatic enzyme increased (Combined AST and ALT) | 45 | 6 | 51 |
| Hyperbilirubinemia | 20 | 21 | 41 |
| Hypobilirubinemia | 0 | 1 | 1 |
| Blood alkaline phosphatase increased | 1 | 9 | 10 |
| Hyperamylasemia | 2 | 0 | 2 |
| Blood lactate dehydrogenase increased | 2 | 0 | 2 |
| Anemia | 106 | 203 | 309 |
| Neutropenia | 3 | 4 | 7 |
| Thrombocytopenia | 7 | 15 | 22 |
| Leukopenia | 3 | 6 | 9 |
| Dyslipidemia | 67 | 72 | 139 |
| Blood creatinine increased | 114 | 23 | 137 |
| Blood urea increased | 19 | 6 | 25 |
| Hyperglycemia | 21 | 13 | 34 |
| Hyponatremia | 0 | 1 | 1 |
| Hypersensitivity | 0 | 1 | 1 |
| Toxic optic neuropathy | 1 | 0 | 1 |
| 503 | 412 | 915 | |
| Suspected Drugs | |||
| Abacavir/Lamivudine | 13 (2.58%) | 0 | 13 (1.42%) |
| Atazanavir/Ritonavir | 26 (5.16%) | 28 (6.79%) | 54 (5.90%) |
| Dolutegravir | 67 (13.32%) | 27 (6.55%) | 94 (10.27%) |
| Efavirenz | 18 (3.57%) | 7 (1.69%) | 25 (2.73%) |
| Efavirenz/TLE | 6 (1.19%) | 3 (0.72%) | 9 (0.98%) |
| Lamivudine | 86 (17.09%) | 42 (10.19%) | 128 (13.98%) |
| Lamivudine/Abacavir | 2 (0.39%) | 0 | 2 (0.21%) |
| Nevirapine | 1 (0.19%) | 0 | 1 (0.10%) |
| Tenofovir | 234 (46.52%) | 276 (66.99%) | 510 (55.73%) |
| Zidovudine | 32 (6.36%) | 20 (4.85%) | 52 (5.68%) |
| Zidovudine/Lamivudine | 14 (2.78%) | 2 (0.48%) | 16 (1.74%) |
| Ritonavir | 3 (0.59%) | 6 (1.45%) | 9 (0.98%) |
| Amoxicillin | 0 | 1 (0.24%) | 1 (0.10%) |
| Ethambutol | 1 (0.19%) | 0 | 1 (0.10%) |
| 503 | 412 | 915 | |
| Frequency | Percent | |
|---|---|---|
| Abnormal sensation of limbs | 6 | 1.63% |
| Hypoesthesia | 8 | 2.18% |
| Paresthesia | 17 | 4.64% |
| Paresthesia generalized | 14 | 3.81% |
| Paresthesia lower limb | 11 | 3.00% |
| Skin hypoesthesia | 4 | 1.09% |
| Anxiety state, unspecified | 32 | 8.74% |
| Fatigue | 48 | 13.11% |
| Intermittent headache | 11 | 3.00% |
| Muscle contraction headache | 3 | 0.81% |
| Headache | 3 | 0.81% |
| Headache transient | 4 | 1.09% |
| Throbbing headache | 2 | 0.54% |
| Muscle pain | 8 | 2.18% |
| Myalgia | 34 | 9.28% |
| Arthromyalgia | 8 | 2.18% |
| Arthralgia | 11 | 3.00% |
| Muscular weakness | 2 | 0.54% |
| Sleeplessness | 47 | 12.84% |
| Dizziness | 4 | 1.09% |
| Giddiness | 2 | 0.54% |
| Depressed mood | 7 | 1.91% |
| Weight gain | 45 | 12.29% |
| Weight loss | 35 | 9.56% |
| Total | 366 | 100% |
| Baseline Medication Regimen Complexity Index | ||||
|---|---|---|---|---|
| Hospitalization due to ADR | 0–8 | >8 | Total | |
| No | 164 | 111 | 275 | |
| Yes | 2 | 8 | 10 | |
| Total | 58.2% | 41.8% | 100.0% | |
| Average Medication Regimen Complexity Index | ||||
| Hospitalization due to ADR | 0–8 | >8 | Total | |
| No | 161 | 114 | 275 | |
| Yes | 2 | 8 | 10 | |
| Total | 57.1% | 42.8% | 100.0% | |
| Follow-up Visit Number | Number of Patients Hospitalized (N = 285%) | Causes for Hospitalization | Number of Days of Hospitalization Median (IQR) |
|---|---|---|---|
| 1st | 2 (22.22) |
| 6.5 (4–6.5) |
| 2nd | 1 (8.33) |
| 5 (5–5) |
| 3rd | 2 (15.38) |
| 6 (4–6) |
| 4th | 2 (16.66) |
| 3 (2–3) |
| 5th | 0 | Not applicable | |
| 6th | 3 (37.5) |
| 4 (4–4) |
| Follow-Up Visit Number | Male | Female | First Episode of Hospitalization (n-%) | Second Episode of Hospitalization (n-%) | Number of Days of Hospitalization Median (IQR) |
|---|---|---|---|---|---|
| 1st | 7 | 2 | 9 (3.15) | 1 (0.4) | 3 (2–7) |
| 2nd | 8 | 4 | 12 (4.21) | 0 | 5 (3–6) |
| 3rd | 9 | 4 | 13 (4.56) | 0 | 4 (2–6) |
| 4th | 7 | 5 | 12 (4.21) | 1 (0.4) | 7 (3.75–8.25) |
| 5th | 3 | 4 | 7 (2.6) | 0 | 4 (3–6) |
| 6th | 3 | 7 | 10 (3.7) | 0 | 5 (3–8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poojar, B.; Kamath, A.; Rao, S.B.; Ullal, S.D.; Ramapuram, J.; Yadiyal, M.B.; Shenoy, A.K. A Prospective Study of the Medication Regimen Complexity Index and Hospitalization Due to Adverse Drug Reactions Among People Living with HIV. Medicina 2024, 60, 1705. https://doi.org/10.3390/medicina60101705
Poojar B, Kamath A, Rao SB, Ullal SD, Ramapuram J, Yadiyal MB, Shenoy AK. A Prospective Study of the Medication Regimen Complexity Index and Hospitalization Due to Adverse Drug Reactions Among People Living with HIV. Medicina. 2024; 60(10):1705. https://doi.org/10.3390/medicina60101705
Chicago/Turabian StylePoojar, Basavaraj, Ashwin Kamath, Sathish B. Rao, Sheetal Dinkar Ullal, John Ramapuram, Muralidhar B. Yadiyal, and Ashok K. Shenoy. 2024. "A Prospective Study of the Medication Regimen Complexity Index and Hospitalization Due to Adverse Drug Reactions Among People Living with HIV" Medicina 60, no. 10: 1705. https://doi.org/10.3390/medicina60101705
APA StylePoojar, B., Kamath, A., Rao, S. B., Ullal, S. D., Ramapuram, J., Yadiyal, M. B., & Shenoy, A. K. (2024). A Prospective Study of the Medication Regimen Complexity Index and Hospitalization Due to Adverse Drug Reactions Among People Living with HIV. Medicina, 60(10), 1705. https://doi.org/10.3390/medicina60101705





