Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Research Question
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Randomized controlled trials (RCTs);
- Studies published in Spanish and English;
- Studies published between January 2010 and March 2024;
- Adult patients diagnosed with lung cancer participating in pulmonary rehabilitation programs or undergoing exercise training of any intensity in any setting (hospital, community center, or home) before or after surgical procedures.
- Studies reporting at least one of the following outcomes: health-related quality of life, lung function, 6 min walking distance, symptom improvement, postoperative hospital stay, mortality, and adverse effects.
2.3.2. Exclusion Criteria
- Preprint articles and letters to the editor;
- Studies published as conference abstracts;
- Studies not available in accessible formats;
- Patients with metastatic tumors or neoplasms;
- Patients with inoperable lung cancer due to advanced stage;
- Studies reporting the same patient cohort as previously published similar research.
2.4. Data Sources and Search Strategy
2.5. Study Selection and Data Extraction
2.6. Risk of Bias Assessment
2.7. Assessment of Evidence Quality
2.8. Statistical Analysis
3. Results
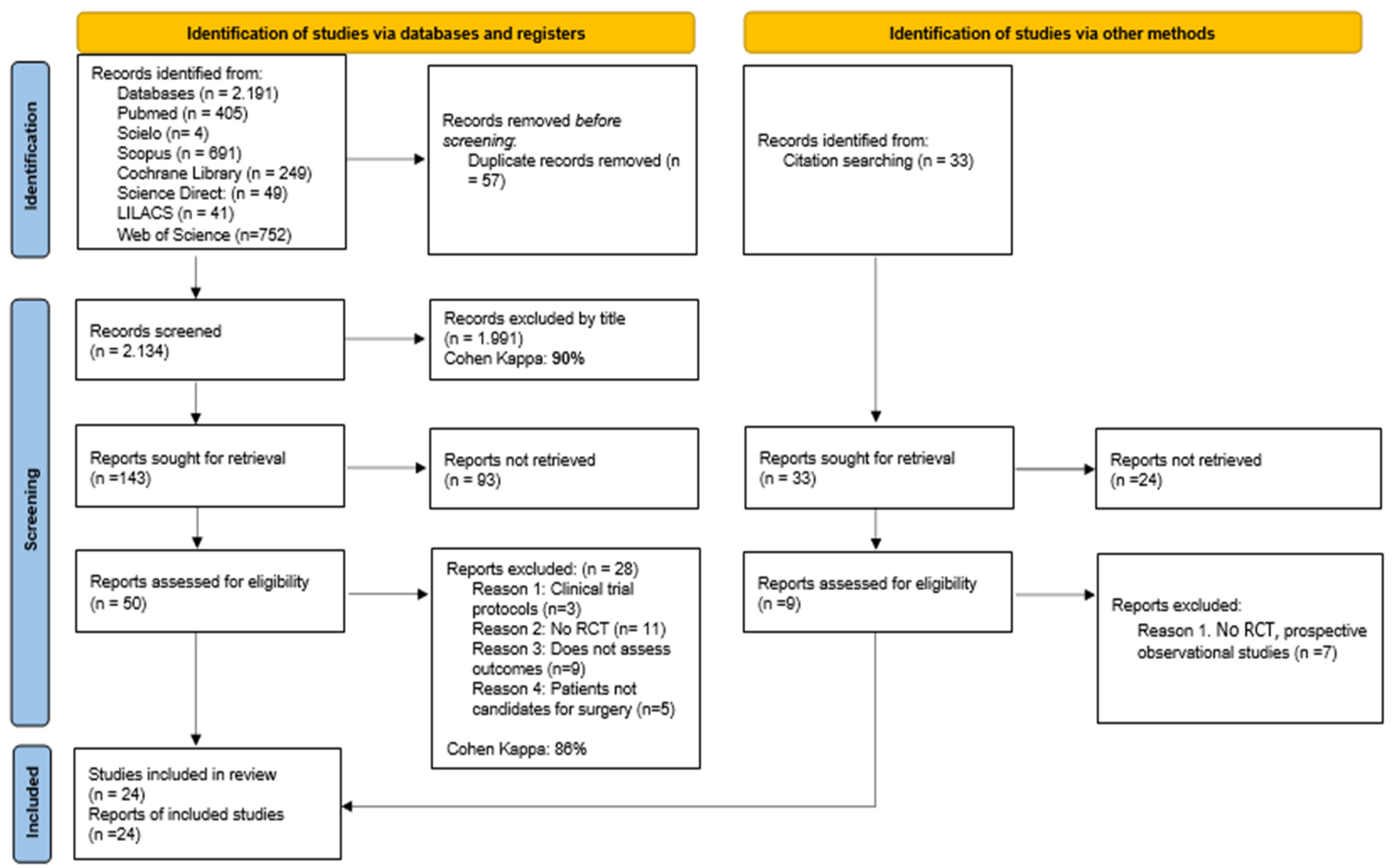
3.1. Studies Identified for the Review
3.2. Characteristics of the Studies Included in the Review
3.3. Summary of Intervention and Results of Studies Included in the Review
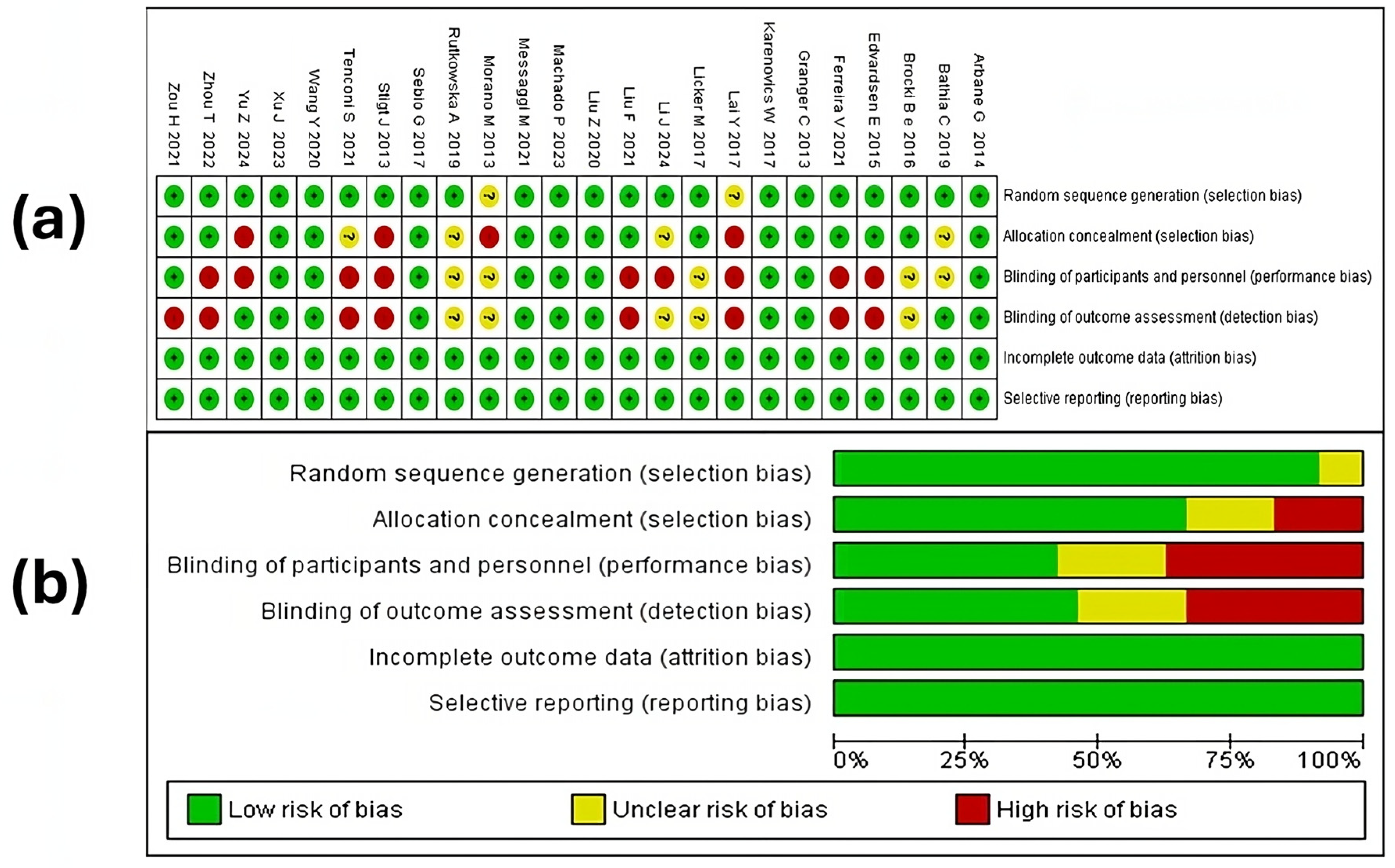
3.4. Risk of Bias Assessment Report
3.4.1. Random Sequence Generation
3.4.2. Allocation Concealment
3.4.3. Blinding of Participants and Personnel
3.4.4. Blinding of Outcome Assessment
3.4.5. Incomplete Outcomes and Selective Reporting
3.5. Qualitative Synthesis of Study Results
3.5.1. Health-Related Quality of Life
3.5.2. Symptom Improvement
3.5.3. Adverse Events
3.5.4. Mortality
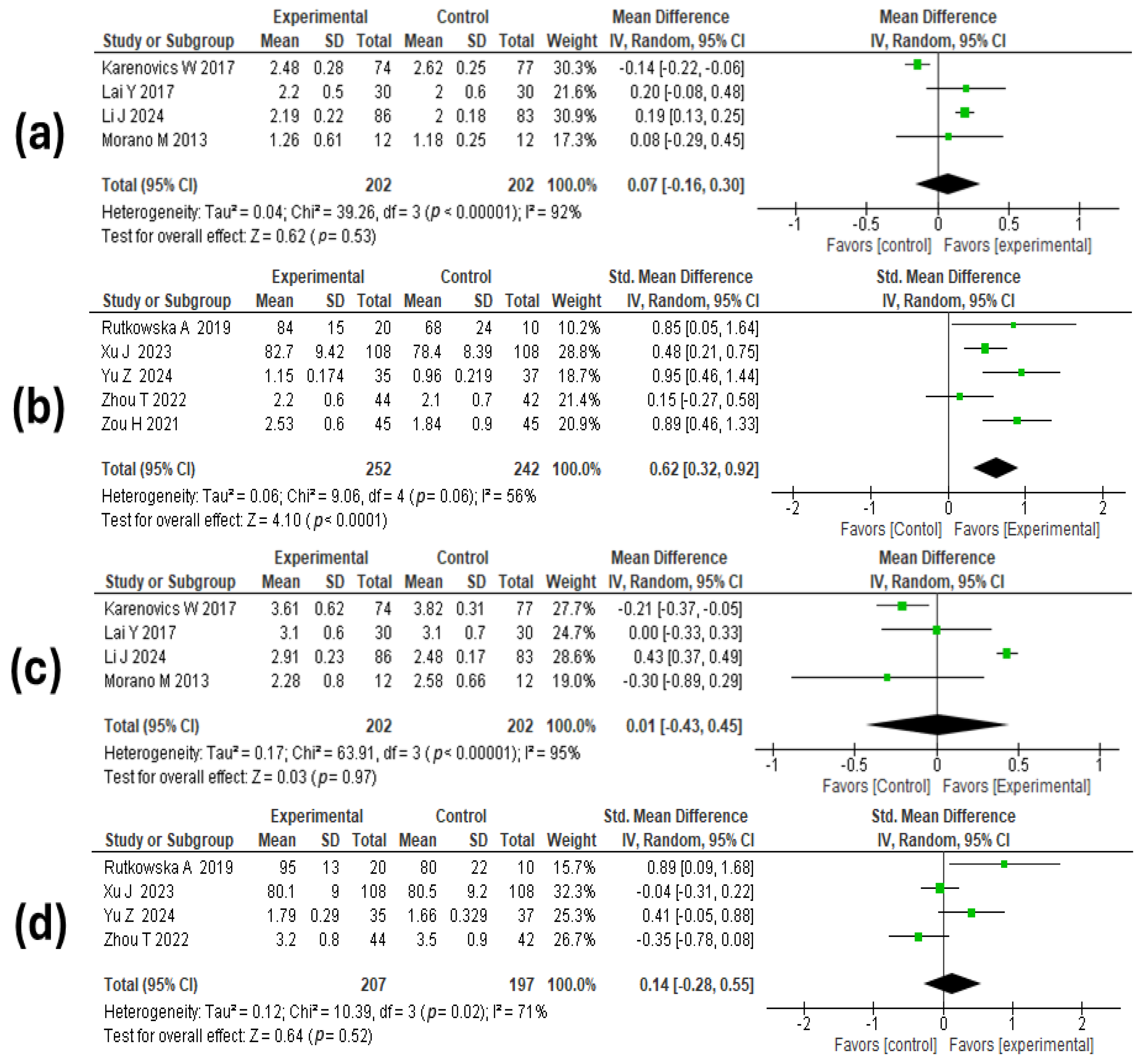
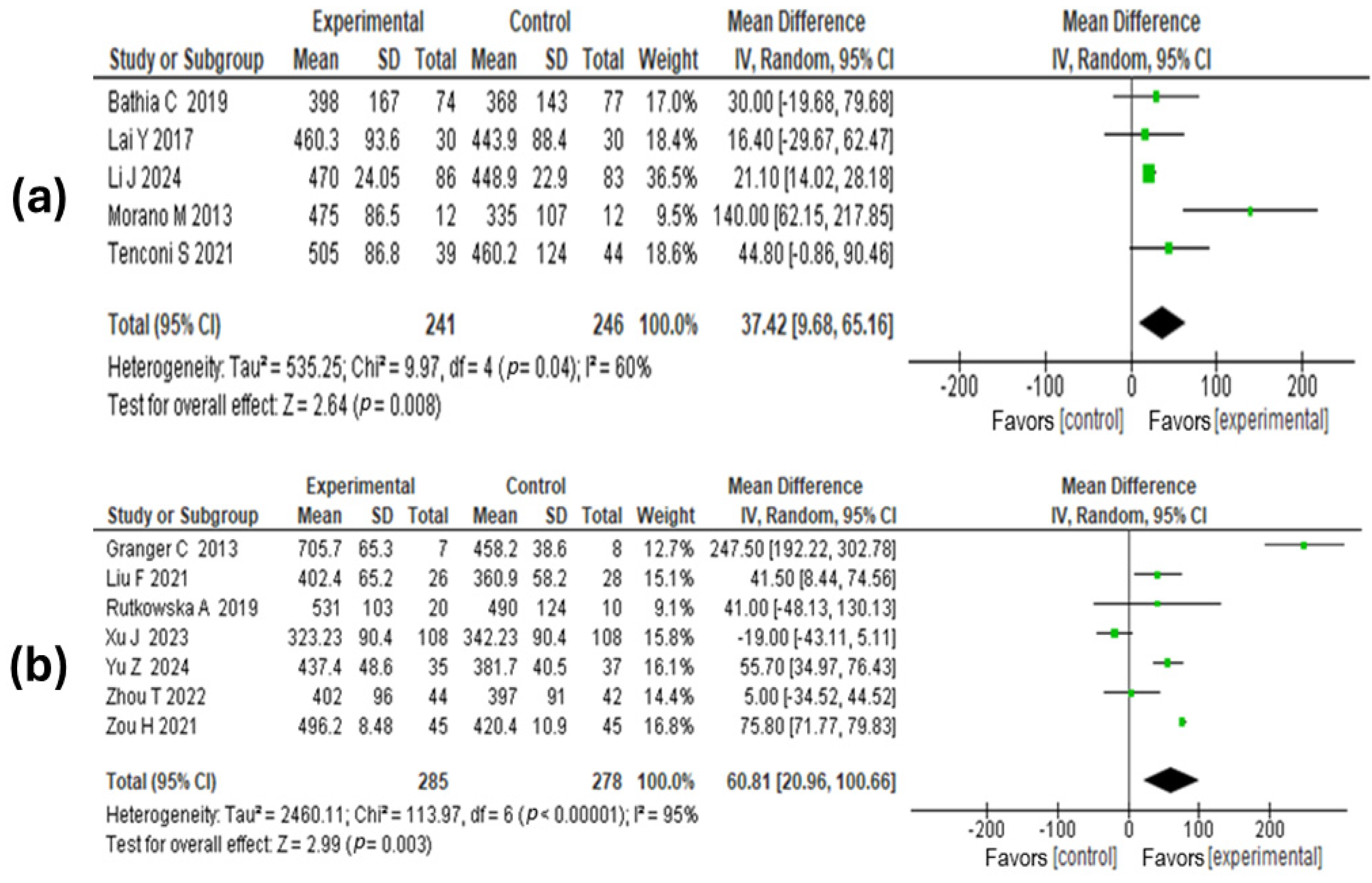
3.6. Meta-Analysis Results
3.6.1. Assessment of the Quality of the Evidence
3.6.2. Lung Function
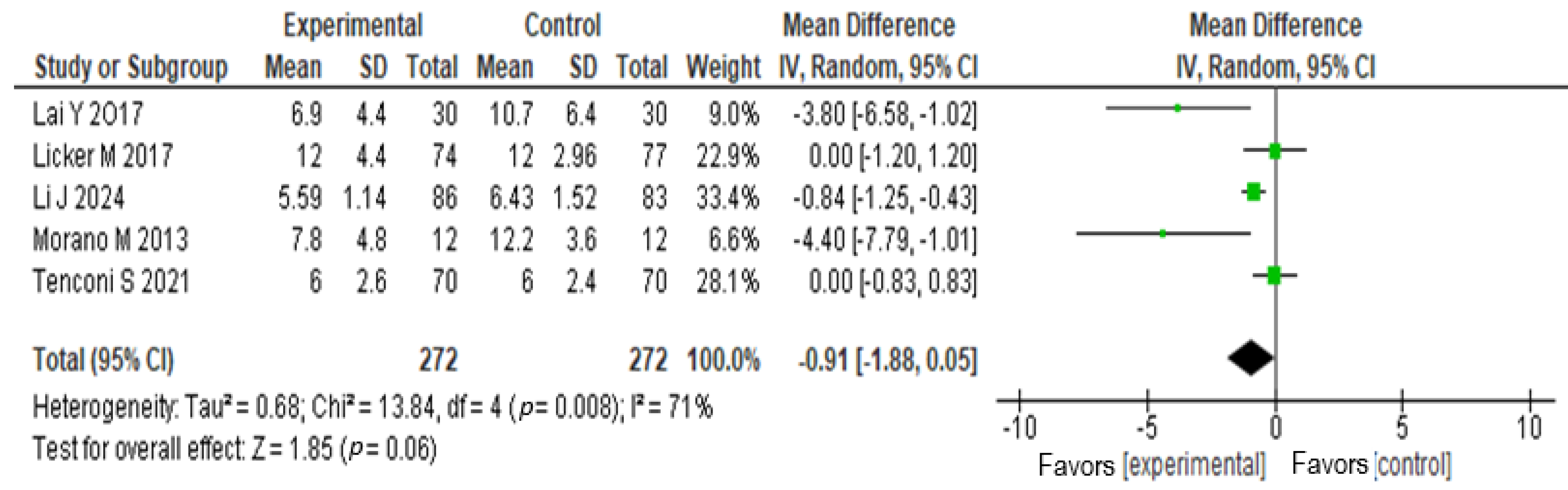
3.6.3. Walking Distance
3.6.4. Hospital Stay
4. Discussion
- The lack of direct supervision and specialized resources at home can limit the effectiveness of interventions.
- The absence of a controlled and supervised environment may increase the risk of errors in technique, as patients might not receive the necessary guidance to perform exercises correctly.
4.1. Limitations
4.2. Challenges in Applying the Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCLC | Non-Small-Cell Lung Cancer |
| SCLC | Small Cell Lung Cancer |
| SF-36 | Short Form 36 Health Survey |
| SF-12 | Short Form 12 Health Survey |
| FACT-L | Functional Assessment of Cancer Therapy—Lung |
| QLQ-C30 | Quality of Life Questionnaire—Core 30 |
| RCT | Randomized Controlled Trial |
| COPD | Chronic Obstructive Pulmonary Disease |
| LC | Lung Cancer |
| PR | Pulmonary Rehabilitation |
| FEV1 | Forced Expiratory Volume in 1 s |
| FVC | Forced Vital Capacity |
References
- Kauczor, H.-U.; Baird, A.-M.; Blum, T.G.; Bonomo, L.; Bostantzoglou, C.; Burghuber, O.; Čepická, B.; Comanescu, A.; Couraud, S.; Devaraj, A.; et al. ESR/ERS Statement Paper on Lung Cancer Screening. Eur. Radiol. 2020, 30, 3277–3294. [Google Scholar] [CrossRef] [PubMed]
- Shue, Y.T.; Lim, J.S.; Sage, J. Tumor Heterogeneity in Small Cell Lung Cancer Defined and Investigated in Pre-Clinical Mouse Models. Transl. Lung Cancer Res. 2018, 7, 21–31. [Google Scholar] [CrossRef]
- Tanoue, L.T.; Tanner, N.T.; Gould, M.K.; Silvestri, G.A. Lung Cancer Screening. Am. J. Respir. Crit. Care Med. 2015, 191, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Asamura, H.; Travis, W.D.; Rusch, V.W. Lung Cancer—Major Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 138–155. [Google Scholar] [CrossRef]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.-T.; Heidi, A. A Review of Nanoparticle Photosensitizer Drug Delivery Uptake Systems for Photodynamic Treatment of Lung Cancer. Photodiagnosis Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya Thandra, K.; Barsouk, A.; Saginala, K.; Sukumar Aluru, J.; Barsouk, A. Epidemiology of Lung Cancer. Contemp. Oncol./Współczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung Cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Restrepo, J.C.; Martínez Guevara, D.; Pareja López, A.; Montenegro Palacios, J.F.; Liscano, Y. Identification and Application of Emerging Biomarkers in Treatment of Non-Small-Cell Lung Cancer: Systematic Review. Cancers 2024, 16, 2338. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.L. Lung Cancer: Epidemiology and Screening. Surg. Clin. N. Am. 2022, 102, 335–344. [Google Scholar] [CrossRef]
- Cardona, A.F.; Mejía, S.A.; Viola, L.; Chamorro, D.F.; Rojas, L.; Ruíz-Patiño, A.; Serna, A.; Martínez, S.; Muñoz, Á.; Rodríguez, J.; et al. Lung Cancer in Colombia. J. Thorac. Oncol. 2022, 17, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Raso, M.G.; Bota-Rabassedas, N.; Wistuba, I.I. Pathology and Classification of SCLC. Cancers 2021, 13, 820. [Google Scholar] [CrossRef]
- Chen, H.J.; Poran, A.; Unni, A.M.; Huang, S.X.; Elemento, O.; Snoeck, H.-W.; Varmus, H. Generation of Pulmonary Neuroendocrine Cells and SCLC-like Tumors from Human Embryonic Stem Cells. J. Exp. Med. 2019, 216, 674–687. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-Small-Cell Lung Cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Blechter, B.; Wong, J.Y.Y.; Chien, L.-H.; Shiraishi, K.; Shu, X.-O.; Cai, Q.; Zheng, W.; Ji, B.-T.; Hu, W.; Rahman, M.L.; et al. Age at Lung Cancer Diagnosis in Females versus Males Who Never Smoke by Race and Ethnicity. Br. J. Cancer 2024, 130, 1286–1294. [Google Scholar] [CrossRef]
- Cho, J.; Choi, S.M.; Lee, J.; Lee, C.-H.; Lee, S.-M.; Kim, D.-W.; Yim, J.-J.; Kim, Y.T.; Yoo, C.-G.; Kim, Y.W.; et al. Proportion and Clinical Features of Never-Smokers with Non-Small Cell Lung Cancer. Chin. J. Cancer 2017, 36, 20. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical Features and Outcome of Patients With Non–Small-Cell Lung Cancer Who Harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Apple, J.; DerSarkissian, M.; Shah, A.; Chang, R.; Chen, Y.; He, X.; Chun, J. Economic Burden of Early-Stage Non-Small-Cell Lung Cancer: An Assessment of Healthcare Resource Utilization and Medical Costs. J. Comp. Eff. Res. 2023, 12, e230107. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The Global Burden of Lung Cancer: Current Status and Future Trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef]
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.-M. The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives—State of the Art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef]
- Fantin, A.; Castaldo, N.; Palou, M.S.; Viterale, G.; Crisafulli, E.; Sartori, G.; Patrucco, F.; Vailati, P.; Morana, G.; Mei, F.; et al. Beyond Diagnosis: A Narrative Review of the Evolving Therapeutic Role of Medical Thoracoscopy in the Management of Pleural Diseases. J. Thorac. Dis. 2024, 16, 2177–2195. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Stiles, B.M.; Denlinger, C.E.; Antippa, P.; Daniel, T.M. Pulmonary Segmentectomy: Results and Complications. Ann. Thorac. Surg. 2003, 76, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Rotman, J.A.; Plodkowski, A.J.; Hayes, S.A.; De Groot, P.M.; Shepard, J.-A.O.; Munden, R.F.; Ginsberg, M.S. Postoperative Complications after Thoracic Surgery for Lung Cancer. Clin. Imaging 2015, 39, 735–749. [Google Scholar] [CrossRef]
- Raza, A.; Alzetani, A. Principles of Posterolateral Thoracotomy and Pneumonectomy. Surgery 2017, 35, 274–280. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Yang, M.; Hlaing, S.S.; Chen, M.; Gu, P.; Meng, Y.; Yang, H. Radiotherapy Improves the Outcomes of Immunotherapy with Sintilimab in Non-Small-Cell Lung Cancer: A Real-World Analysis. Front. Immunol. 2022, 13, 991431. [Google Scholar] [CrossRef]
- Abidi, Y.; Fekete, M.; Farkas, Á.; Horváth, A.; Varga, J.T. Effectiveness and Quality of Life in Lung Cancer, Pre-, Post- and Perioperative Rehabilitation—A Review. Physiol. Int. 2023, 110, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Rice, S.J.; Belani, C.P. Pulmonary Rehabilitation in Lung Cancer. PMR 2016, 8, 990–996. [Google Scholar] [CrossRef]
- Burnett, C.; Bestall, J.C.; Burke, S.; Morgan, E.; Murray, R.L.; Greenwood-Wilson, S.; Williams, G.F.; Franks, K.N. Prehabilitation and Rehabilitation for Patients with Lung Cancer: A Review of Where We Are Today. Clin. Oncol. 2022, 34, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Perez, H.; Nana-Sinkam, P. Integrating Pulmonary Rehabilitation into the Multidisciplinary Management of Lung Cancer: A Review. Respir. Med. 2015, 109, 437–442. [Google Scholar] [CrossRef]
- Li, X.-H.; Zhu, J.-L.; Hong, C.; Zeng, L.; Deng, L.-M.; Jin, L.-Y. Effects of Systematic Rehabilitation Programs on Quality of Life in Patients Undergoing Lung Resection. Mol. Clin. Oncol. 2013, 1, 200–208. [Google Scholar] [CrossRef]
- Mao, X.; Ni, Y.; Niu, Y.; Jiang, L. The Clinical Value of Pulmonary Rehabilitation in Reducing Postoperative Complications and Mortality of Lung Cancer Resection: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 685485. [Google Scholar] [CrossRef]
- Wang, J.; Deng, N.; Qi, F.; Li, Q.; Jin, X.; Hu, H. The Effectiveness of Postoperative Rehabilitation Interventions That Include Breathing Exercises to Prevent Pulmonary Atelectasis in Lung Cancer Resection Patients: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2023, 23, 276. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Yoon, U.; Vogt, P.M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and Publication Bias. J. Cranio-Maxillofac. Surg. 2011, 39, 91–92. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- O’Connor, A.M.; Anderson, K.M.; Goodell, C.K.; Sargeant, J.M. Conducting Systematic Reviews of Intervention Questions I: Writing the Review Protocol, Formulating the Question and Searching the Literature. Zoonoses Public Health 2014, 61, 28–38. [Google Scholar] [CrossRef]
- Chmura Kraemer, H.; Periyakoil, V.S.; Noda, A. Kappa Coefficients in Medical Research. Stat. Med. 2002, 21, 2109–2129. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Jadad, A.R.; Nichol, G.; Penman, M.; Tugwell, P.; Walsh, S. Assessing the Quality of Randomized Controlled Trials: An Annotated Bibliography of Scales and Checklists. Control. Clin. Trials 1995, 16, 62–73. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, J. Effect of Lung Rehabilitation Training Combined with Nutritional Intervention on Patients after Thoracoscopic Resection of Lung Cancer. Oncol. Lett. 2024, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.; Pimenta, S.; Garcia, A.L.; Nogueira, T.; Silva, S.; Dos Santos, C.L.; Martins, M.V.; Canha, A.; Oliveiros, B.; Martins, R.A.; et al. Effect of Preoperative Home-Based Exercise Training on Quality of Life After Lung Cancer Surgery: A Multicenter Randomized Controlled Trial. Ann. Surg. Oncol. 2024, 31, 847–859. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Cao, H.-P.; Liu, X.; Yang, Z.; Yin, Y.-Y.; Ma, R.-C.; Xie, J. Effect of Breathing Exercises in Patients with Non-Small Cell Lung Cancer Receiving Surgical Treatment: A Randomized Controlled Trial. Eur. J. Integr. Med. 2020, 38, 101175. [Google Scholar] [CrossRef]
- Bhatia, C.; Kayser, B. Preoperative High-Intensity Interval Training Is Effective and Safe in Deconditioned Patients with Lung Cancer: A Randomized Clinical Trial. J. Rehabil. Med. 2019, 51, 712–718. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Yang, M.; Su, J.; Liu, J.; Che, G. Seven-Day Intensive Preoperative Rehabilitation for Elderly Patients with Lung Cancer: A Randomized Controlled Trial. J. Surg. Res. 2017, 209, 30–36. [Google Scholar] [CrossRef]
- Licker, M.; Karenovics, W.; Diaper, J.; Frésard, I.; Triponez, F.; Ellenberger, C.; Schorer, R.; Kayser, B.; Bridevaux, P.-O. Short-Term Preoperative High-Intensity Interval Training in Patients Awaiting Lung Cancer Surgery: A Randomized Controlled Trial. J. Thorac. Oncol. 2017, 12, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Morano, M.T.; Araújo, A.S.; Nascimento, F.B.; Da Silva, G.F.; Mesquita, R.; Pinto, J.S.; De Moraes Filho, M.O.; Pereira, E.D. Preoperative Pulmonary Rehabilitation Versus Chest Physical Therapy in Patients Undergoing Lung Cancer Resection: A Pilot Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2013, 94, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sebio García, R.; Yáñez-Brage, M.I.; Giménez Moolhuyzen, E.; Salorio Riobo, M.; Lista Paz, A.; Borro Mate, J.M. Preoperative Exercise Training Prevents Functional Decline after Lung Resection Surgery: A Randomized, Single-Blind Controlled Trial. Clin. Rehabil. 2017, 31, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Karenovics, W.; Licker, M.; Ellenberger, C.; Christodoulou, M.; Diaper, J.; Bhatia, C.; Robert, J.; Bridevaux, P.-O.; Triponez, F. Short-Term Preoperative Exercise Therapy Does Not Improve Long-Term Outcome after Lung Cancer Surgery: A Randomized Controlled Study. Eur. J. Cardio-Thorac. Surg. 2017, 52, 47–54. [Google Scholar] [CrossRef]
- Tenconi, S.; Mainini, C.; Rapicetta, C.; Braglia, L.; Galeone, C.; Cavuto, S.; Merlo, D.F.; Costi, S.; Paci, M.; Piro, R.; et al. Rehabilitation for Lung Cancer Patients Undergoing Surgery: Results of the PUREAIR Randomized Trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 1002–1011. [Google Scholar] [CrossRef]
- Yu, Z.; Xie, G.; Qin, C.; He, H.; Wei, Q. Effect of Postoperative Exercise Training on Physical Function and Quality of Life of Lung Cancer Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. Medicine 2024, 103, e37285. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Zeng, J.; Zhou, Y.; Li, Q.; Bai, Z.; Zhang, Y.; Xiao, J. Effect of Baduanjin Qigong on Postoperative Pulmonary Rehabilitation in Patients with Non-Small Cell Lung Cancer: A Randomized Controlled Trial. Support. Care Cancer 2024, 32, 73. [Google Scholar] [CrossRef]
- Ferreira, V.; Minnella, E.M.; Awasthi, R.; Gamsa, A.; Ferri, L.; Mulder, D.; Sirois, C.; Spicer, J.; Schmid, S.; Carli, F. Multimodal Prehabilitation for Lung Cancer Surgery: A Randomized Controlled Trial. Ann. Thorac. Surg. 2021, 112, 1600–1608. [Google Scholar] [CrossRef]
- Zhou, T.; Sun, C. Effect of Physical Manipulation Pulmonary Rehabilitation on Lung Cancer Patients after Thoracoscopic Lobectomy. Thorac. Cancer 2022, 13, 308–315. [Google Scholar] [CrossRef]
- Zou, H.; Qin, Y.; Gong, F.; Liu, J.; Zhang, J.; Zhang, L. ABCDEF Pulmonary Rehabilitation Program Can Improve the Mid-Term Lung Function of Lung Cancer Patients after Thoracoscopic Surgery: A Randomized Controlled Study. Geriatr. Nurs. 2022, 44, 76–83. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, T.; Pei, L.; Zhang, Y.; Xu, L.; Cui, Y.; Liang, N.; Li, S.; Chen, W.; Huang, Y. Two-Week Multimodal Prehabilitation Program Improves Perioperative Functional Capability in Patients Undergoing Thoracoscopic Lobectomy for Lung Cancer: A Randomized Controlled Trial. Anesth. Analg. 2020, 131, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Kuo, N.-Y.; Fang, T.-P.; Chen, J.-O.; Lu, H.-I.; Lin, H.-L. A Six-Week Inspiratory Muscle Training and Aerobic Exercise Improves Respiratory Muscle Strength and Exercise Capacity in Lung Cancer Patients after Video-Assisted Thoracoscopic Surgery: A Randomized Controlled Trial. Clin. Rehabil. 2021, 35, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Messaggi-Sartor, M.; Marco, E.; Martínez-Téllez, E.; Rodriguez-Fuster, A.; Palomares, C.; Chiarella, S.; Muniesa, J.M.; Orozco-Levi, M.; Barreiro, E.; Güell, M.R. Combined Aerobic Exercise and High-Intensity Respiratory Muscle Training in Patients Surgically Treated for Non-Small Cell Lung Cancer: A Pilot Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, A.; Jastrzebski, D.; Rutkowski, S.; Żebrowska, A.; Stanula, A.; Szczegielniak, J.; Ziora, D.; Casaburi, R. Exercise Training in Patients With Non–Small Cell Lung Cancer During In-Hospital Chemotherapy Treatment: A RANDOMIZED CONTROLLED TRIAL. J. Cardiopulm. Rehabil. Prev. 2019, 39, 127–133. [Google Scholar] [CrossRef]
- Brocki, B.C.; Andreasen, J.J.; Langer, D.; Souza, D.S.R.; Westerdahl, E. Postoperative Inspiratory Muscle Training in Addition to Breathing Exercises and Early Mobilization Improves Oxygenation in High-Risk Patients after Lung Cancer Surgery: A Randomized Controlled Trial. Eur. J. Cardiothorac. Surg. 2016, 49, 1483–1491. [Google Scholar] [CrossRef]
- Edvardsen, E.; Skjonsberg, O.H.; Holme, I.; Nordsletten, L.; Borchsenius, F.; Anderssen, S.A. High-Intensity Training Following Lung Cancer Surgery: A Randomised Controlled Trial. Thorax 2015, 70, 244–250. [Google Scholar] [CrossRef]
- Arbane, G.; Douiri, A.; Hart, N.; Hopkinson, N.S.; Singh, S.; Speed, C.; Valladares, B.; Garrod, R. Effect of Postoperative Physical Training on Activity after Curative Surgery for Non-Small Cell Lung Cancer: A Multicentre Randomised Controlled Trial. Physiotherapy 2014, 100, 100–107. [Google Scholar] [CrossRef]
- Stigt, J.A.; Uil, S.M.; Van Riesen, S.J.H.; Simons, F.J.N.A.; Denekamp, M.; Shahin, G.M.; Groen, H.J.M. A Randomized Controlled Trial of Postthoracotomy Pulmonary Rehabilitation in Patients with Resectable Lung Cancer. J. Thorac. Oncol. 2013, 8, 214–221. [Google Scholar] [CrossRef]
- Granger, C.L.; Chao, C.; McDonald, C.F.; Berney, S.; Denehy, L. Safety and Feasibility of an Exercise Intervention for Patients Following Lung Resection: A Pilot Randomized Controlled Trial. Integr. Cancer Ther. 2013, 12, 213–224. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Brintnall, R.A.; Cooper, J. Merging Technology and Clinical Research for Optimized Post-Surgical Rehabilitation of Lung Cancer Patients. Ann. Transl. Med. 2016, 4, 28. [Google Scholar] [CrossRef]
- De Sousa Pinto, J.M.; Martín-Nogueras, A.M.; Calvo-Arenillas, J.I.; Ramos-González, J. Clinical Benefits of Home-Based Pulmonary Rehabilitation in Patients With Chronic Obstructive Pulmonary Disease. J. Cardiopulm. Rehabil. Prev. 2014, 34, 355–359. [Google Scholar] [CrossRef]
- Borghetti, P.; Branz, J.; Volpi, G.; Pancera, S.; Buraschi, R.; Bianchi, L.N.C.; Bonù, M.L.; Greco, D.; Facheris, G.; Tomasi, C.; et al. Home-Based Pulmonary Rehabilitation in Patients Undergoing (Chemo)Radiation Therapy for Unresectable Lung Cancer: A Prospective Explorative Study. Radiol. Med. 2022, 127, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Olivier, C.; Grosbois, J.-M.; Cortot, A.B.; Peres, S.; Heron, C.; Delourme, J.; Gierczynski, M.; Hoorelbeke, A.; Scherpereel, A.; Le Rouzic, O. Real-Life Feasibility of Home-Based Pulmonary Rehabilitation in Chemotherapy-Treated Patients with Thoracic Cancers: A Pilot Study. BMC Cancer 2018, 18, 178. [Google Scholar] [CrossRef]
- Saito, T.; Ono, R.; Tanaka, Y.; Tatebayashi, D.; Okumura, M.; Makiura, D.; Inoue, J.; Fujikawa, T.; Kondo, S.; Inoue, T.; et al. The Effect of Home-Based Preoperative Pulmonary Rehabilitation before Lung Resection: A Retrospective Cohort Study. Lung Cancer 2021, 162, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Coats, V.; Maltais, F.; Simard, S.; Fréchette, É.; Tremblay, L.; Ribeiro, F.; Saey, D. Feasibility and Effectiveness of a Home-Based Exercise Training Program Before Lung Resection Surgery. Can. Respir. J. 2013, 20, e10–e16. [Google Scholar] [CrossRef]
- Rispoli, M.; Salvi, R.; Cennamo, A.; Di Natale, D.; Natale, G.; Meoli, I.; Gioia, M.R.; Esposito, M.; Nespoli, M.R.; De Finis, M.; et al. Effectiveness of Home-Based Preoperative Pulmonary Rehabilitation in COPD Patients Undergoing Lung Cancer Resection. Tumori J. 2020, 106, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Edbrooke, L.; Denehy, L.; Granger, C.L.; Kapp, S.; Aranda, S. Home-Based Rehabilitation in Inoperable Non-Small Cell Lung Cancer—The Patient Experience. Support. Care Cancer 2020, 28, 99–112. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Cheville, A.; Smith, S.; Barksdale, T.; Asher, A. Cancer Rehabilitation. In Braddom’s Physical Medicine and Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 568–593.e7. ISBN 978-0-323-62539-5. [Google Scholar]
- Codima, A.; Das Neves Silva, W.; De Souza Borges, A.P.; De Castro, G. Exercise Prescription for Symptoms and Quality of Life Improvements in Lung Cancer Patients: A Systematic Review. Support. Care Cancer 2021, 29, 445–457. [Google Scholar] [CrossRef]
- Rodriguez-Larrad, A.; Lascurain-Aguirrebena, I.; Abecia-Inchaurregui, L.C.; Seco, J. Perioperative Physiotherapy in Patients Undergoing Lung Cancer Resection. Interact. CardioVas. Thorac. Surg. 2014, 19, 269–281. [Google Scholar] [CrossRef]
- Singh, G.K.; Jemal, A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J. Environ. Public Health 2017, 2017, 2819372. [Google Scholar] [CrossRef] [PubMed]
- Gravier, F.-E.; Smondack, P.; Prieur, G.; Medrinal, C.; Combret, Y.; Muir, J.-F.; Baste, J.-M.; Cuvelier, A.; Boujibar, F.; Bonnevie, T. Effects of Exercise Training in People with Non-Small Cell Lung Cancer before Lung Resection: A Systematic Review and Meta-Analysis. Thorax 2022, 77, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-C.; Shun, S.-C.; Liao, W.-Y.; Yu, C.-J.; Yang, P.-C.; Lai, Y.-H. Quality of Life and Related Factors in Patients With Newly Diagnosed Advanced Lung Cancer: A Longitudinal Study. Oncol. Nurs. Forum 2014, 41, E44–E55. [Google Scholar] [CrossRef] [PubMed]
- Marschner, N.; Zacharias, S.; Lordick, F.; Hegewisch-Becker, S.; Martens, U.; Welt, A.; Hagen, V.; Gleiber, W.; Bohnet, S.; Kruggel, L.; et al. Association of Disease Progression With Health-Related Quality of Life Among Adults With Breast, Lung, Pancreatic, and Colorectal Cancer. JAMA Netw. Open 2020, 3, e200643. [Google Scholar] [CrossRef]
- Cella, D.F.; Bonomi, A.E.; Lloyd, S.R.; Tulsky, D.S.; Kaplan, E.; Bonomi, P. Reliability and Validity of the Functional Assessment of Cancer Therapy—Lung (FACT-L) Quality of Life Instrument. Lung Cancer 1995, 12, 199–220. [Google Scholar] [CrossRef]
- Yun, Y.H.; Park, Y.S.; Lee, E.S.; Bang, S.-M.; Heo, D.S.; Park, S.Y.; You, C.H.; West, K. Validation of the Korean Version of the EORTC QLQ-C30. Qual. Life Res. 2004, 13, 863–868. [Google Scholar] [CrossRef]
- Jenkinson, C.; Layte, R.; Jenkinson, D.; Lawrence, K.; Petersen, S.; Paice, C.; Stradling, J. A Shorter Form Health Survey: Can the SF-12 Replicate Results from the SF-36 in Longitudinal Studies? J. Public Health 1997, 19, 179–186. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Ho, M.-H.; Chau, P.H.; Cheung, D.S.T.; Lin, C.-C. Factors Related to Functional Capacity Deterioration in Surgical Lung Cancer Patients: A Systematic Review. Cancer Nurs. 2023. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, R.; Liao, X.; Huang, X.; Zhao, C.; Chen, M. The Efficacy of Pulmonary Rehabilitation Exercise Training on Complications and Mortality after Lung Cancer Resection: A Systematic Review and Meta-Analysis. Transl. Cancer Res. TCR 2022, 11, 1321–1329. [Google Scholar] [CrossRef]
- Sebio Garcia, R.; Yáñez Brage, M.I.; Giménez Moolhuyzen, E.; Granger, C.L.; Denehy, L. Functional and Postoperative Outcomes after Preoperative Exercise Training in Patients with Lung Cancer: A Systematic Review and Meta-Analysis. Interact. CardioVas. Thorac. Surg. 2016, 23, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, J.; Jekunen, A.; Sihvo, E.; Johansson, M.; Andersén, H. Effect of Adherence to Treatment Guidelines on Overall Survival in Elderly Non-Small-Cell Lung Cancer Patients. Lung Cancer 2022, 171, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cavalheri, V.; Granger, C. Preoperative Exercise Training for Patients with Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2017, 6, CD012020. [Google Scholar] [CrossRef] [PubMed]
- Divisi, D.; Di Francesco, C.; Di Leonardo, G.; Crisci, R. Preoperative Pulmonary Rehabilitation in Patients with Lung Cancer and Chronic Obstructive Pulmonary Disease. Eur. J. Cardio-Thorac. Surg. 2013, 43, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Hatakeyama, K.; Konno, H.; Matsunaga, T.; Shimada, Y.; Minamiya, Y. Impact of Pulmonary Rehabilitation on Postoperative Complications in Patients with Lung Cancer and Chronic Obstructive Pulmonary Disease. Thorac. Cancer 2017, 8, 451–460. [Google Scholar] [CrossRef]
- Schmidt-Hansen, M.; Page, R.; Hasler, E. The Effect of Preoperative Smoking Cessation or Preoperative Pulmonary Rehabilitation on Outcomes After Lung Cancer Surgery: A Systematic Review. Clin. Lung Cancer 2013, 14, 96–102. [Google Scholar] [CrossRef]
- Janssen, S.M.J.; Abbink, J.J.; Lindeboom, R.; Vliet Vlieland, T.P.M. Outcomes of Pulmonary Rehabilitation After Treatment for Non-Small Cell Lung Cancer Stages I to IIIa: AN OBSERVATIONAL STUDY. J. Cardiopulm. Rehabil. Prev. 2017, 37, 65–71. [Google Scholar] [CrossRef]
| Author, Year | Country | Disease | Patients I/C | Sex (% Male) | Age (Years) | Time of Enrollment | Program Duration | Evaluated Outcomes |
|---|---|---|---|---|---|---|---|---|
| Li J et al., 2024 [46] | China | NSCLC | I: 86 C: 83 | 40% | 57.7 | Pre-surgical | 2 weeks | Health-related quality of life, 6 min walking distance, hospital stay, and lung function. |
| Machado P et al., 2023 [47] | Portugal | NSCLC | I: 20 C: 21 | 68.30% | 68.1 | Pre-surgical | NS | Health-related quality of life, 6 min walking distance, dyspnea improvement, and adverse events. |
| Wang Y et al., 2020 [48] | China | NSCLC, SCLC | I: 31 C: 34 | 34% | 57.2 | Pre-surgical | NS | 6 min walking distance and dyspnea improvement. |
| Bhatia C et al., 2019 [49] | Switzerland | NSCLC | I: 74 C: 77 | 60% | 64 | Pre-surgical | 2–3 weeks | Lung function and 6 min walking distance. |
| Lai Y et al., 2017 [50] | China | NSCLC | I: 30 C: 30 | 57% | 72 | Pre-surgical | 1 week | Lung function, quality of life, 6 min walking distance, adverse events, and hospital stay. |
| Licker M et al., 2017 [51] | Switzerland | NSCLC | I: 74 C: 77 | 60% | 64 | Pre-surgical | NS | 6 min walking distance, mortality, and hospital stay. |
| Morano M et al., 2013 [52] | Brazil | NSCLC | I: 12 C: 12 | 37.50% | 66.4 | Pre-surgical | 4 weeks | Lung function, 6 min walking distance, and hospital stay. |
| Sebio G et al., 2017 [53] | Spain | NSCLC | I: 10 C: 12 | 90% | 70 | Pre-surgical | NS | Health-related quality of life. |
| Karenovics W et al., 2017 [54] | Switzerland | NSCLC | I: 74 C: 77 | 60% | 64 | Pre-surgical | NS | Lung function and health-related quality of life. |
| Tenconi S et al., 2021 [55] | Italy | NSCLC | I: 70 C: 70 | 61.40% | 66.8 | Pre-surgical | NS | 6 min walking distance, dyspnea improvement, adverse events, and hospital stay. |
| Author, Year | Country | Disease | Patients I/C | Sex (% Male) | Age (Years) | Time of Enrollment | Program Duration | Evaluated Outcomes |
|---|---|---|---|---|---|---|---|---|
| Yu Z et al., 2024 [56] | China | NSCLC, COPD | I: 44 C: 40 | 68% | 69 | Post-surgical | 2 weeks | Lung function, health-related quality of life, and 6 min walking distance. |
| Xu J et al., 2023 [57] | China | NSCLC | I: 108 C:108 | NS | NS | Post-surgical | 12 weeks | Lung function, 6 min walking distance, and dyspnea improvement. |
| Ferreira V et al., 2021 [58] | Canada | NSCLC | I: 43 C: 52 | 53.60% | 66.9 | Post-surgical | 8 weeks | Health-related quality of life, 6 min walking distance, and hospital stay. |
| Zhou T et al., 2022 [59] | China | NSCLC | I: 44 C: 42 | 57% | 61.7 | Post-surgical | 2 weeks | Lung function, 6 min walking distance, and hospital stay. |
| Zou H et al., 2021 [60] | China | NSCLC | I: 45 C: 45 | 49% | 58.4 | Post-surgical | 8 weeks | Lung function, 6 min walking distance, dyspnea improvement, and hospital stay. |
| Liu Z et al., 2020 [61] | China | NSCLC | I: 37 C: 36 | 31.50% | 56.2 | Post-surgical | 2 weeks | Lung function, 6 min walking distance, mortality, and hospital stay. |
| Liu F et al., 2021 [62] | Taiwan | NSCLC, SCLC | I: 32 C: 31 | 41% | 65.2 | Post-surgical | 6 weeks | 6 min walking distance. |
| Messaggi M et al., 2019 [63] | Spain | NSCLC | I: 16 C:21 | 70.30% | 64.6 | Post-surgical | 8 weeks | Health-related quality of life. |
| Rutkowska A et al., 2019 [64]. | Poland | NSCLC | I: 20 C: 10 | 90% | 60.2 | Post-surgical | 6 weeks | Lung function, 6 min walking distance, and dyspnea improvement. |
| Brocki B et al., 2016 [65] | Denmark | NSCLC, SCLC | I: 34 C: 30 | 57.50% | 70 | Post-surgical | 2 weeks | Lung function and 6 min walking distance. |
| Edvardsen E et al., 2015 [66] | Norway | NSCLC | I: 30 C: 31 | 46% | 65.1 | Post-surgical | 20 weeks | Health-related quality of life. |
| Arbane G et al., 2014 [67] | United Kingdom | NSCLC | I: 64 C:67 | 55% | 68 | Post-surgical | NS | Health-related quality of life. |
| Stigt JA et al., 2013 [68] | Netherlands | NSCLC | I: 23 C: 26 | 82% | 63.4 | Post-surgical | 12 weeks | Lung function, health-related quality of life, and 6 min walking distance. |
| Granger C et al., 2013 [69] | Australia | NSCLC, SCLC | I: 7 C: 8 | 53% | 65.5 | Post-surgical | 8 weeks | Health-related quality of life, 6 min walking distance, and adverse events. |
| Author, Year | Primary Setting | Activities | Number of Sessions | Results |
|---|---|---|---|---|
| Li J et al., 2024 [46] | Health center | Respiratory training, walking, and individualized nutritional support. | NS | PR combined with nutritional support promotes better recovery and higher quality of life after surgery. |
| Machado P et al., 2023 [47] | Home | Educational session, aerobic exercises, resistance training, and telephone supervision. | 17 | PR can effectively prevent the decline in quality of life after lung cancer surgery. |
| Wang Y et al., 2020 [48] | Health center | Abdominal respiratory training, pursed-lip breathing, and incentive spirometry exercises. | NS | Pre-surgical respiratory exercise can relieve dyspnea, improve inspiratory capacity, and reduce dyspnea level. |
| Bhatia C et al., 2019 [49] | Health center | High-intensity interval training. | 8 | High-intensity training is safe in the preoperative period and increases cardiorespiratory fitness. |
| Lai Y et al., 2017 [50] | Health center | Resistance training and inspiratory muscle training. | 7 | The program led to a positive effect on peak expiratory flow and 6 min walking distance. |
| Licker M et al., 2017 [51] | Health center | High-intensity interval training. | NS | The intervention was safe and brought short-term positive effects for patients awaiting lung cancer surgery. |
| Morano M et al., 2013 [52] | Health center | Upper limb strength training, resistance training, and inspiratory muscle training. | 20 | Pre-surgical pulmonary rehabilitation improves functional capacity and reduces postoperative respiratory morbidity. |
| Sebio G et al., 2017 [53] | Health center and home | Resistance training, aerobic training, and respiratory exercises. | NS | A pre-surgical pulmonary rehabilitation program appears to improve patients’ preoperative condition and may prevent functional decline after surgery. |
| Karenovics W et al., 2017 [54] | Health center | High-intensity interval training. | NS | Short-term high-intensity preoperative rehabilitation does not improve lung function 1 year after lung cancer resection. |
| Tenconi S et al., 2021 [55] | Health center and home | Aerobic training, resistance training, inspiratory muscle training, and education. | 39 | Rehabilitation was associated with greater exercise tolerance. No differences were found in quality of life. |
| Author, Year | Setting | Activities | Number of Sessions | Results |
|---|---|---|---|---|
| Yu Z et al., 2024 [56] | Health center | Early mobilization, coughing technique, and aerobic training. | 24 | A short-term post-surgical exercise training program can facilitate the recovery of functional capacity in lung cancer patients. |
| Xu J et al., 2023 [57] | Health center and home | Respiratory exercises plus education. | 48 | The intervention, like conventional care, improved postoperative lung function and the quality of life of the studied patients. |
| Ferreira V et al., 2021 [58] | Home | Aerobic exercises, resistance training, and psychological and nutritional support. | 24 | Rehabilitation initiated 4 weeks before surgery is as effective in recovering functional capacity as postoperative rehabilitation. |
| Zhou T et al., 2022 [59] | Health center | Aerobic training, strengthening of intercostal muscles and abdominal muscles, and education. | 14 | Rehabilitation could improve early lung function in lung cancer patients after a thoracoscopic lobectomy and reduce the length of hospital stay. |
| Zou H et al., 2021 [60] | Health center | Respiratory and lower limb training, positive expiratory pressure breathing exercises, cycling, dancing, and education. | NS | Pulmonary rehabilitation could effectively improve lung function, exercise tolerance, and reduce postoperative hospital stay in lung cancer patients. |
| Liu Z et al., 2020 [61] | Home | Aerobic and resistance exercises, respiratory training, and nutritional and psychological counseling. | NS | The intervention was associated with clinically relevant improvements in perioperative functional capacity in lung cancer patients. |
| Liu F et al., 2021 [62] | Health center | Inspiratory muscle training and aerobic exercises. | 30 | Patients who underwent the intervention showed significant improvements starting from two weeks. |
| Messaggi M et al., 2021 [63] | Health center | Continuous aerobic training and inspiratory and expiratory muscle training. | 24 | The intervention improved exercise capacity but had no impact on quality of life. |
| Rutkowska A et al., 2019 [64] | Health center | Respiratory training and aerobic, resistance, and relaxation exercises. | 30 | The training program was associated with a greater walking distance. Additionally, an improvement in lung function was observed. |
| Brocki B et al., 2016 [65] | Health center and home | Inspiratory muscle training plus respiratory exercises. | NS | The intervention did not have a significant impact on the evaluated outcomes; however, it improved lung oxygenation. |
| Edvardsen E et al., 2015 [66] | Health center | High-intensity strength and resistance training. Inspiratory muscle training. | 60 | The intervention was associated with improved quality of life in lung cancer patients. |
| Arbane G et al., 2014 [67] | Health center | Daily mobilization, strength training, and aerobic training. | NS | There were no differences in walking distance or quality of life between the intervention and control groups. |
| Stigt JA et al., 2013 [68] | Health center | Muscle training and aerobic exercises. | 24 | Rehabilitation did not result in better quality of life in the studied lung cancer patients. |
| Granger C et al., 2013 [69] | Health center | Aerobic and resistance training. | 16 | The intervention was associated with positive trends in the 6 min walk test and some domains of quality of life. |
| Author | The Study Is Randomized | The Intervention Is Double-Blind | Study Withdrawals Are Accounted for and Described | The Randomization Procedure Is Adequate | Selection Criteria | Score |
|---|---|---|---|---|---|---|
| Karenovics et al., 2017 [54] | 1 | 0 | 1 | 1 | 1 | 4 |
| Lai et al., 2017 [50] | 1 | 0 | 1 | 0 | 1 | 3 |
| Li et al., 2024 [46] | 1 | 0 | 1 | 1 | 1 | 4 |
| Morano et al., 2013 [52] | 1 | 0 | 1 | 1 | 1 | 4 |
| Xu et al., 2023 [57] | 1 | 0 | 1 | 1 | 1 | 4 |
| Yu et al., 2024 [56] | 1 | 0 | 1 | 1 | 1 | 4 |
| Zhou et al., 2020 [59] | 1 | 0 | 1 | 1 | 1 | 4 |
| Zou et al., 2021 [60] | 1 | 0 | 1 | 1 | 1 | 4 |
| Rutkowska et al., 2019 [64] | 1 | 0 | 1 | 1 | 1 | 4 |
| Bhatia et al., 2019 [49] | 1 | 0 | 1 | 1 | 1 | 4 |
| Tenconi et al., 2021 [55] | 1 | 0 | 1 | 0 | 1 | 3 |
| Granger et al., 2013 [69] | 1 | 1 | 1 | 1 | 1 | 5 |
| Liu et al., 2020 [61] | 1 | 0 | 1 | 1 | 1 | 4 |
| Licker et al., 2017 [51] | 1 | 0 | 1 | 1 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Mosquera, F.E.; Murillo, S.R.; Naranjo Rojas, A.; Perlaza, C.L.; Castro Osorio, D.; Liscano, Y. Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis. Medicina 2024, 60, 1725. https://doi.org/10.3390/medicina60111725
Cruz Mosquera FE, Murillo SR, Naranjo Rojas A, Perlaza CL, Castro Osorio D, Liscano Y. Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis. Medicina. 2024; 60(11):1725. https://doi.org/10.3390/medicina60111725
Chicago/Turabian StyleCruz Mosquera, Freiser Eceomo, Saray Rios Murillo, Anisbed Naranjo Rojas, Claudia Lorena Perlaza, Diana Castro Osorio, and Yamil Liscano. 2024. "Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis" Medicina 60, no. 11: 1725. https://doi.org/10.3390/medicina60111725
APA StyleCruz Mosquera, F. E., Murillo, S. R., Naranjo Rojas, A., Perlaza, C. L., Castro Osorio, D., & Liscano, Y. (2024). Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis. Medicina, 60(11), 1725. https://doi.org/10.3390/medicina60111725






