The Potential of Silver Diamine Fluoride in Non-Operative Management of Dental Caries in Primary Teeth: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Focus Question
2.3. Information Sources and Search
2.4. Selection of Studies
2.5. Inclusion and Exclusion Criteria
2.6. Data Extraction
2.7. Risk of Bias Assessment
2.8. Statistical Analysis
3. Results
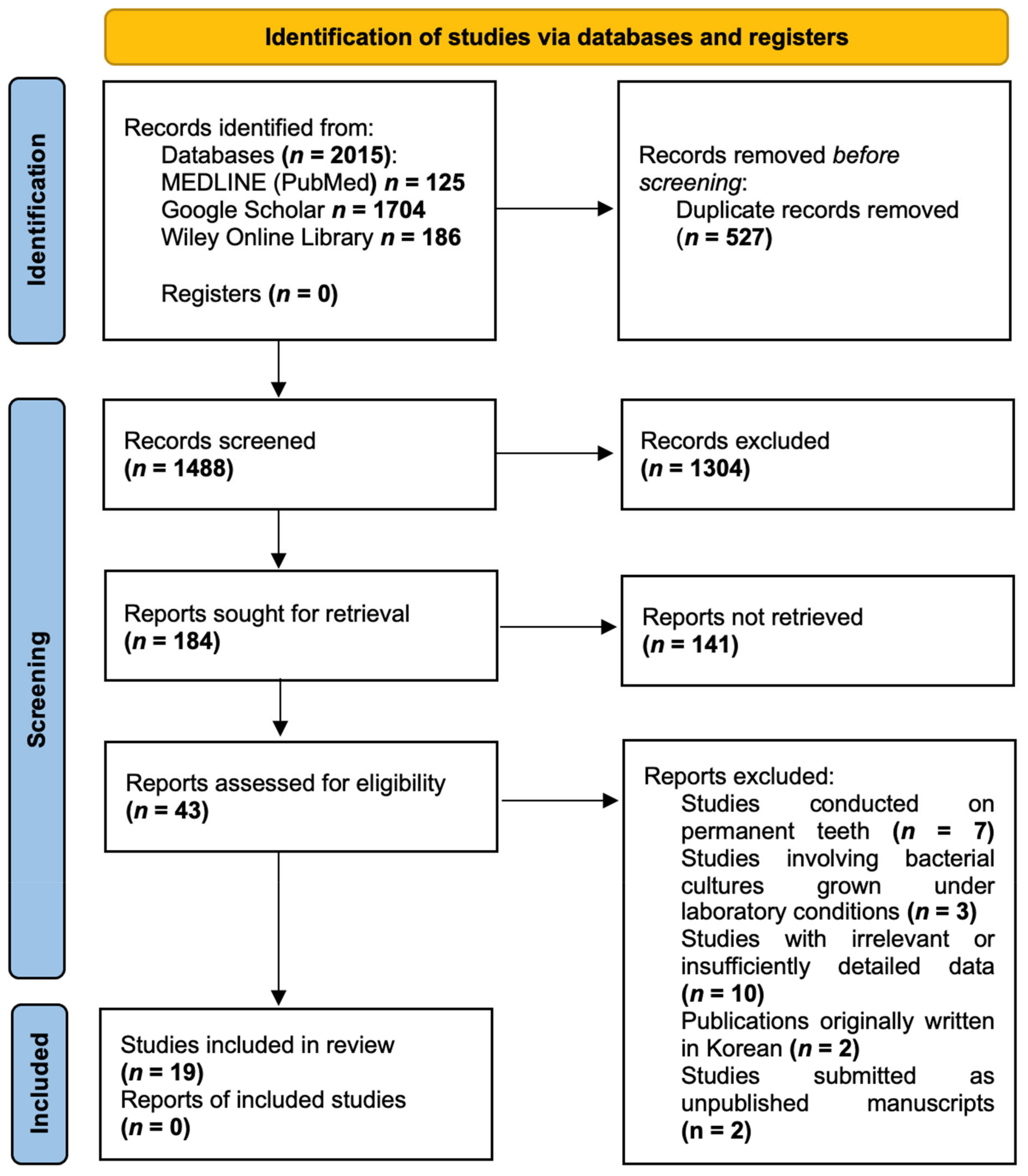
3.1. Study Selection
3.2. Quality Assessment
3.3. Characteristics of Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Santo, K.; Wong, G.; Sohn, W.; Spallek, H.; Chow, C.; Irving, M. Mobile Apps for Dental Caries Prevention: Systematic Search and Quality Evaluation. JMIR MHealth UHealth 2021, 9, e19958. [Google Scholar] [CrossRef] [PubMed]
- Kazeminia, M.; Abdi, A.; Shohaimi, S.; Jalali, R.; Vaisi-Raygani, A.; Salari, N.; Mohammadi, M. Dental caries in primary and permanent teeth in children’s worldwide, 1995 to 2019: A systematic review and meta-analysis. Head Face Med. 2020, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Hsu, K.L.; Melo, M.A.; Dhar, V.; Tinanoff, N. Mapping Evidence on Early Childhood Caries Prevalence: Complexity of Worldwide Data Reporting. Int. J. Clin. Pediatr. Dent. 2021, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2022, 101, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zi, H.; Zeng, X. Changes in the global burden of untreated dental caries from 1990 to 2019: A systematic analysis for the Global Burden of Disease study. Heliyon 2022, 8, e10714. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Uribe, S.E.; Innes, N.; Maldupa, I. The global prevalence of early childhood caries: A systematic review with meta-analysis using the WHO diagnostic criteria. Int. J. Paediatr. Dent. 2021, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Gudipaneni, R.K.; Patil, S.R.; Assiry, A.A.; Karobari, M.I.; Bandela, V.; Metta, K.K.; Almuhanna, R. Association of oral hygiene practices with the outcome of untreated dental caries and its clinical consequences in pre- and primary school children: A cross-sectional study in a northern province of Saudi Arabia. Clin. Exp. Dent. Res. 2021, 7, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.S.; Martins-Júnior, P.A.; Paiva, S.M.; Klein, D.; Torres, F.M.; Giacomin, A.; Gonçalves, B.M.; Konrath, A.C.; Bolan, M.; Cardoso, M. Prevalence of self-reported dental pain and associated factors among eight- to ten-year-old Brazilian schoolchildren. PLoS ONE 2019, 14, e0214990. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Faria, P.; Viana, K.A.; Raggio, D.P.; Hosey, M.T.; Costa, L.R. Recommended procedures for the management of early childhood caries lesions–a scoping review by the Children Experiencing Dental Anxiety: Collaboration on Research and Education (CEDACORE). BMC Oral Health 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Bridge, G.; Martel, A.-S.; Lomazzi, M. Silver Diamine Fluoride: Transforming Community Dental Caries Program. Int. Dent. J. 2021, 71, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Haworth, S.; Dudding, T.; Waylen, A.; Thomas, S.J.; Timpson, N.J. Ten years on: Is dental general anaesthesia in childhood a risk factor for caries and anxiety? Br. Dent. J. 2017, 222, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Cabalén, M.B.; Molina, G.F.; Bono, A.; Burrow, M.F. Nonrestorative Caries Treatment: A Systematic Review Update. Int. Dent. J. 2022, 72, 746–764. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Contractor, I.A.; Ms, G.; Md, I. Silver Diamine Fluoride: Extending the spectrum of Preventive Dentistry, a literature review. Pediatr. Dent. J. 2021, 31, 17–24. [Google Scholar] [CrossRef]
- Gao, S.S.; Chen, K.J.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Preventing early childhood caries with silver diamine fluoride: Study protocol for a randomised clinical trial. Trials 2020, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mabangkhru, S.; Duangthip, D.; Chu, C.H.; Phonghanyudh, A.; Jirarattanasopha, V. A randomized clinical trial to arrest dentin caries in young children using silver diamine fluoride. J. Dent. 2020, 99, 103375. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhao, I.S.; Hiraishi, N.; Duangthip, D.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. Clinical Trials of Silver Diamine Fluoride in Arresting Caries among Children: A Systematic Review. JDR Clin. Transl. Res. 2016, 1, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment–a systematic review. BMC Oral. Health 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.C.; Worthington, H.V.; Walsh, T.; Clarkson, J.E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013, 2014, CD002279. [Google Scholar] [CrossRef] [PubMed]
- Chibinski, A.C.; Wambier, L.M.; Feltrin, J.; Loguercio, A.D.; Wambier, D.S.; Reis, A. Silver Diamine Fluoride Has Efficacy in Controlling Caries Progression in Primary Teeth: A Systematic Review and Meta-Analysis. Caries Res. 2017, 51, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Mayanagi, G.; Azumi, M.; Otani, H.; Fukushima, A.; Sasaki, K.; Takahashi, N. Sodium fluoride and silver diamine fluoride-coated tooth surfaces inhibit bacterial acid production at the bacteria/tooth interface. J. Dent. 2019, 84, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chibinski, A.C.R. The Use of Silver Diamine Fluoride in Pediatric Dentistry; Bilbilova, E.Z., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Crystal, Y.O. Silver diamine fluoride (SDF): Its role in caries management. Dent. Update 2019, 46, 1016–1022. [Google Scholar] [CrossRef]
- Zheng, F.M.; Yan, I.G.; Duangthip, D.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Silver diamine fluoride therapy for dental care. Jpn. Dent. Sci. Rev. 2022, 58, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.A.; Heima, M. Prevention of Dental Caries by Silver Diamine Fluoride. Compend. Contin. Educ. Dent. 2019, 40, 158–163. [Google Scholar] [PubMed]
- Li, Y.; Liu, Y.; Psoter, W.J.; Nguyen, O.M.; Bromage, T.G.; Walters, M.A.; Hu, B.; Rabieh, S.; Kumararaja, F.C. Assessment of the Silver Penetration and Distribution in Carious Lesions of Deciduous Teeth Treated with Silver Diamine Fluoride. Caries Res. 2019, 53, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Randomized Trial of Silver Nitrate with Sodium Fluoride for Caries Arrest. JDR Clin. Trans. Res. 2019, 4, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2022, 131, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Garrastazu, M.D.; Mathias-Santamaria, I.F.; Rocha, R.S.; Diniz, M.B.; Caneppele, T.M.F.; Bresciani, E. Three-Month Effect of Silver Diamine Fluoride (SDF) in Salivary Levels of Streptococcus Mutans in Children. An. Exploratory Trial. Oral Health Prev. Dent. 2020, 18, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Chhattani, B.; Kulkarni, P.; Agrawal, N.; Mali, S.; Kumar, A.; Thakur, N.S. Comparative evaluation of antimicrobial efficacy of silver diamine fluoride, chlorhexidine varnish with conventional fluoride varnish as a caries arresting agent. An in vivo sodium dodecyl sulfate-polyacrylamide gel electrophoresis study. J. Indian. Soc. Pedod. Prev. Dent. 2021, 39, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Yan, Z.; Duangthip, D.; Niu, J.Y.; Yu, O.Y.; You, M.; Lo, E.C.; Chu, C.H. Effect of silver diamine fluoride on plaque microbiome in children. J. Dent. 2020, 102, 103479. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.; Kaikure, M.; Sowmya, B. Comparison of Anti-Bacterial Efficacy of Silver Diamine Fluoride and Sodium Fluoride in pre-School Children-An In vivo Study. Int. J. Innov. Sci. Res. Technol. 2021, 6, 8. [Google Scholar]
- Sulyanto, R.M.; Kang, M.; Srirangapatanam, S.; Berger, M.; Candamo, F.; Wang, Y.; Dickson, J.; Ng, M.; Ho, S. Biomineralization of dental tissues treated with silver diamine fluoride. J. Dent. Res. 2021, 100, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Sulyanto, R.M.; Beall, C.J.; Berger, M.B.; Goodell, C.P.; Koo, S.; Candamo, F.; Dickson, J.R.; Kang, M.; Ho, S.P.; Ng, M.W.; et al. Silver diamine fluoride alters microbial communities in subsurface dentin. JADA Found. Sci. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Thakur, S.; Sojan, M.; Singhal, P.; Chauhan, D. A Comparative Study to Evaluate the Effectiveness of Silver Diamine Fluoride at Different Time Durations of Application in Treating Carious Primary Teeth: A Randomized Trial. Int. J. Clin. Pediatr. Dent. 2022, 15, S147. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.; El-Tekeya, M.M.; Talat, D.A.; Nagui, D.A. In-vitro remineralizing effect of silver diamine fluoride on dentin caries in primary teeth. Alex. Dent. J. 2022, 47, 170–177. [Google Scholar] [CrossRef]
- Hassan, M.; Bakhurji, E.; AlSheikh, R. Application of Er, Cr: YSGG laser versus photopolymerization after silver diamine fluoride in primary teeth. Sci. Rep. 2021, 11, 20780. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Liu, J.; Zhang, D.; Yang, Z.L.; Feng, Y.P.; Wang, M. Effect of silver diammine fluoride on micro-ecology of plaque from extensive caries of deciduous teeth–in vitro study. BMC Oral Health 2020, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Punhagui, M.-F.; Jussiani, E.-I.; Andrello, A.-C.; Favaro, J.-C.; Guiraldo, R.-D.; Lopes, M.-B.; Berger, S.-B. Effect of application time and concentration of silver diamine fluoride on the enamel remineralization. J. Clin. Exp. Dent. 2021, 13, e653–e658. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, M.B.; D’Alessandro, M.G.; Freitas Moraes, K.A.; de Medeiros Urquiza, S.P.; Pereira Moro, B.L.; Kerber Tedesco, T.; Pettorossi Imparato, J.C. Silver Diamine Fluoride versus Bioactive Giomer Light-Curing Varnish: An In Vitro Study on Caries Arrest. Pesqui. Bras. Em Odontopediatria E Clínica Integr. 2021, 21, e0230. [Google Scholar] [CrossRef]
- Sai, V.P.; Pranitha, K.; Sankar, A.J.S.; Sridhar, M.; Sankar, K.S.; Ramgopal, A.K. Determining the Efficacy of Three Potential Remineralizing Agents on Artificial Carious Lesions. J. Oral Health Community Dent. 2020, 14, 1–5. [Google Scholar] [CrossRef]
- Toopchi, S.; Bakhurji, E.; Loo, C.Y.; Hassan, M. Effect of light curing on silver diamine fluoride in primary incisors: A microscopic ex vivo study. Pediatr. Dent. 2021, 43, 44–49. [Google Scholar] [PubMed]
- Yilmaz, N.; Mert, O.; Zeynep, Ö. Remineralization of primary molar dentine with silver diamine fluoride and sodium fluoride: An in vitro study. Cumhur. Dent. J. 2020, 23, 340–347. [Google Scholar] [CrossRef]
- Abdil-Nafaa, S.A.; Qasim, A.A. The Effect of Silver Diamine Fluoride and Fluoride Varnish on Microhardness of Primary Teeth Enamel (An In Vitro Study). Al-Rafidain Dent. J. 2020, 20, 283–295. [Google Scholar] [CrossRef]
- Hussein, F.; Hashem, S.N.; Elsayed, S.R. The synergetic effect of silver diamine fluoride with potassium iodide and grape seed extract on dentin remineralization. Al-Azhar Dent. J. Girls 2021, 8, 71–80. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farahmand Far, M.H. Effect of fluoridated varnish and silver diamine fluoride on enamel demineralization resistance in primary dentition. J. Indian. Soc. Pedod. Prev. Dent. 2018, 36, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, B.B.; Punhagui, M.F.; Hoeppner, M.G.; Almeida RSC de Juliani, F.A.; Guiraldo, R.D.; Berger, S.B. In vitro evaluation of the remineralizing potential and antimicrobial activity of a cariostatic agent with silver nanoparticles. Braz. Dent. J. 2017, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021, 231, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Amarquaye, G.; Arrow, P.; Bansal, K.; Bedi, R.; Campus, G.; Chen, K.J.; Chibinski, A.C.R.; Chinzorig, T.; Crystal, Y.O.; et al. Global Oral Health Policies and Guidelines: Using Silver Diamine Fluoride for Caries Control. Front. Oral Health. 2021, 2, 685557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, I.S.; Gao, S.S.; Hiraishi, N.; Burrow, M.F.; Duangthip, D.; Mei, M.L.; Lo, E.C.; Chu, C.H. Mechanisms of silver diamine fluoride on arresting caries: A literature review. Int. Dent. J. 2018, 68, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Weatherell, J.A. Kinetics of fluoride in the oral fluids. J. Dent. Res. 1990, 69, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.; Hannas, A.R.; Kato, M.T. Saliva and dental erosion. J. Appl. Oral Sci. 2012, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Driessens, F.C. Mineral aspects of dentistry. Monogr. Oral. Sci. 1982, 10, 1–215. [Google Scholar] [PubMed]
- ten Cate, J.M.; Duijsters, P.P. Influence of fluoride in solution on tooth demineralization. I. Chem. Data. Caries Res. 1983, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Greenwall-Cohen, J.; Greenwall, L.; Barry, S. Silver diamine fluoride–an overview of the literature and current clinical techniques. Br. Dent. J. 2020, 228, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Nishikawara, F.; Nomura, Y.; Imai, S.; Senda, A.; Hanada, N. Evaluation of cariogenic bacteria. Eur. J. Dent. 2007, 1, 31–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Maishi, N.; Akahori, E.; Hasebe, A.; Takeda, R.; Matsuda, A.Y.; Hida, Y.; Nam, J.M.; Onodera, Y.; Kitagawa, Y.; et al. The oral bacterium Streptococcus mutans promotes tumor metastasis by inducing vascular inflammation. Cancer Sci. 2022, 113, 3980–3994. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Chen, Y.Y.; Chen, W.C.; Chen, M.F. Streptococcus mutans promotes tumor progression in oral squamous cell carcinoma. J. Cancer. 2022, 13, 3358–3367. [Google Scholar] [CrossRef] [PubMed]
- Abed, K.; Paciorek, M.; Bursa, D. Potential infection foci in the oral cavity and their impact on the formation of central nervous system abscesses: A literature review. Medicine 2023, 102, e35898. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.T.; Huang, X.; Ellepola, K.; Liao, S.; Li, Y. Lactobacilli and human dental caries: More than mechanical retention. Microbiology 2022, 168, 001196. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. Caries-arresting effects of silver diamine fluoride and sodium fluoride on dentine caries lesions. J. Dent. 2018, 78, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Got, S.-R.; Yin, I.X.; Lo, E.C.-M.; Chu, C.-H. A Concise Review of Silver Diamine Fluoride on Oral Biofilm. Appl. Sci. 2021, 11, 3232. [Google Scholar] [CrossRef]
- Ahmed, A.; Dachang, W.; Lei, Z.; Jianjun, L.; Juanjuan, Q.; Yi, X. Effect of Lactobacillus species on Streptococcus mutans biofilm formation. Pak. J. Pharm. Sci. 2014, 27, 1523–1528. [Google Scholar] [PubMed]
- Zhu, B.; Li, J.Y.; Hao, Y.Q.; Zhou, X.D. Establishment and evaluation of the in vitro dynamic biofilm model. Shanghai J. Stomatol. 2010, 19, 60–65. [Google Scholar]
- Teughels, W.; Van Essche, M.; Sliepen, I.; Quirynen, M. Probiotics and oral healthcare. Periodontology 2000, 48, 111–147. [Google Scholar] [CrossRef] [PubMed]
- Claudia, F.; Catalina, A.; Francisca, L.; Fernanda, R. Role of probiotics as bacteriotherapy in dentistry: A literature review. J. Pharm. Bioalled Sci. 2018, 14, S34–S38. [Google Scholar] [CrossRef]
- Cura, F.; Palmieri, A.; Girardi, A.; Martinelli, M.; Scapoli, L.; Carinci, F. Lab-Test(®) 4: Dental caries and bacteriological analysis. Dent. Res. J. 2012, 9 (Suppl. S2), S139–S141. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez YBaena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. Biomed. Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed]
- Mungur, A.; Chen, H.; Shahid, S.; Baysan, A. A systematic review on the effect of silver diamine fluoride for management of dental caries in permanent teeth. Clin. Exp. Dent. Res. 2023, 9, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lo, E.; Li, C. Effect of silver and fluoride ions on enamel demineralization: A quantitative study using micro-computed tomography. Aust. Dent. J. 2012, 57, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Yao, X.; Ganguly, A.; Xu, C.; Dusevich, V.; Walker, M.P.; Wang, Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J. Dent. 2010, 38, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Li, Q.L.; Chu, C.H.; Yiu, C.K.; Lo, E.C. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 2012, 28, 903–908. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Policy on the use of silver diamine fluoride for pediatric dental patients. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2022; pp. 72–75. [Google Scholar]
- World Health Organization. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2021 (Including the 22nd WHO Model List of Essential Medicines and the 8th WHO Model List of Essential Medicines for Children); World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Saad, A.A.; Kamel, M.A.; Hamza, N.K.; Niazi, H.A. The Effect of Silver Diamine Fluoride on Surface Characterization of Demineralized Dentin. Ain Shams Dent. J. 2020, 19, 21–28. [Google Scholar] [CrossRef]
- Soekanto, S.A.; Rosithahakiki, N.; Suniarti, D.F.; Sahlan, M. Comparison of the potency of several fluoride-based varnishes as an anticariogenic on calcium, phosphate, and fluoride ion levels. Int. J. Appl. Pharm. 2018, 9, 55–59. [Google Scholar] [CrossRef]
- Soekanto, S.A.; Fadillah, F.; Nuraisiya, P.; Gultom, F.; Sarwono, A.T. The potential of several fluoride-based varnishes as remineralization agents: Morphological studies, dentin surface hardness, and crystallinity tests. Int. J. Appl. Pharm. 2018, 9, 60. [Google Scholar] [CrossRef]
- Yu, O.Y.; Mei, M.L.; Zhao, I.S.; Li, Q.-L.; Lo, E.C.-M.; Chu, C.-H. Remineralisation of enamel with silver diamine fluoride and sodium fluoride. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, e344–e352. [Google Scholar] [CrossRef] [PubMed]
- Satyanegara, A.; Darwita, R.R.; Setiawati, F.; Adiatman, M.; Muhammad, R. An in vitro study of caries arresting effect of propolis fluoride and silver diamine fluoride on dentine carious lesions. J. Int. Dent. Med. Res. 2017, 10, 751–756. [Google Scholar]
- Srisomboon, S.; Kettratad, M.; Stray, A.; Pakawanit, P.; Rojviriya, C.; Patntirapong, S.; Panpisut, P. Effects of Silver Diamine Nitrate and Silver Diamine Fluoride on Dentin Remineralization and Cytotoxicity to Dental Pulp Cells: An In Vitro Study. J. Funct. Biomater. 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Karched, M.; Ali, D.; Ngo, H. In vivo antimicrobial activity of silver diammine fluoride on carious lesions in dentin. J. Oral. Sci. 2019, 61, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Favaro, J.C.; Detomini, T.R.; Maia, L.P.; Poli, R.C.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Anticaries Agent Based on Silver Nanoparticles and Fluoride: Characterization and Biological and Remineralizing Effects—An In Vitro Study. Int. J. Dent. 2022, 2022, 9483589. [Google Scholar] [CrossRef] [PubMed]
- Moca, A.E.; Pelea, D.C.; Negruţiu, B.M.; Burta, O.L.; Vaida, L.L.; Haliţchi, G. In-vitro assessment of silver diamine fluoride’s effect on a dental plaque specimen. Int. J. Med. Dent. 2020, 24, 375. [Google Scholar]
- Chanratchakool, N.; Chavengvorakul, W.; Taweesedt, S.; Trairatvorakul, C.; Thanyasrisung, P. Effect of Light on the Antibacterial Property of Silver Diamine Fluoride. Srinakharinwirot Univ. Dent. J. 2022, 15, 86–92. [Google Scholar]
- Romão, D.A.; Fernández, C.E.; de Melo Santos, L. Commercial Silver Diamine Fluoride (SDF) Products on Caries Lesion Progression in Primary Enamel: An In Vitro Study. Oral. Health Prev. Dent. 2020, 18, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Kunte, S.; Gholap, C.; Deo, P.; Jagtap, C.; Desai, S.; Lakade, L. Evaluation of the Histology of Carious Human Primary Incisor after Treatment with 38% Silver Diamine Fluoride. Int. J. Sci. Study 2021, 9, 121–124. [Google Scholar]
- Abdil-Nafaa, S.A.; Qasim, A.A. The Effect of Silver Diamine Fluoride and Fluoride Varnish on Roughness of Primary Teeth Enamel (An In Vitro Study). Al-Rafidain Dent. J. 2020, 20, 296–307. [Google Scholar] [CrossRef]
- Thomas, C.S.; Sharma, D.S.; Sheet, D.; Mukhopadhyay, A.; Sharma, S. Cross-sectional visual comparison of remineralization efficacy of various agents on early smooth surface caries of primary teeth with swept source optical coherence tomography. J. Oral. Biol. Craniofacial Res. 2021, 11, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, P.; Horst, J.A.; Ludwig, S.; Rothen, M.; Chaffee, B.W.; Lyalina, S.; Pollard, K.S.; DeRisi, J.L.; Mancl, L. Topical silver diamine fluoride for dental caries arrest in preschool children: A randomized controlled trial and microbiological analysis of caries associated microbes and resistance gene expression. J. Dent. 2018, 68, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jabin, Z.; Nasim, I.; Vishnu Priya, V.; Agarwal, N. Quantitative Analysis and Effect of SDF, APF, NaF on Demineralized Human Primary Enamel Using SEM, XRD, and FTIR. Int. J. Clin. Pediatr. Dent. 2021, 14, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ruff, R.R.; Paul, B.; Sierra, M.A.; Xu, F.; Li, X.; Crystal, Y.O.; Saxena, D. Predicting Treatment Nonresponse in Hispanic/Latino Children Receiving Silver Diamine Fluoride for Caries Arrest: A Pilot Study Using Machine Learning. Front. Oral. Health 2021, 2, 695759. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Sierra, M.A.; Xu, F.; Crystal, Y.O.; Li, X.; Saxena, D.; Ruff, R.R. Microbial population shift and metabolic characterization of silver diamine fluoride treatment failure on dental caries. PLoS ONE 2021, 16, e0242396. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, É.T.; Silva, M.V.; de Campos, T.A.; Martins, V.d.P.; Bezzerra, A.C.B. Antimicrobial effects of silver diamine fluoride: An in vivo study. Am. J. Dent. 2021, 34, 49–53. [Google Scholar] [PubMed]
- Lee, K.; Ahn, J.; Kim, J.S.; Han, M.; Lee, J.; Shin, J. Effect of Sodium Fluoride Varnish and Potassium Iodide on Remineralization Efficacy of Silver Diamine Fluoride. J. Korean Acad. Pediatr. Dent. 2021, 48, 467–475. [Google Scholar] [CrossRef]
- Seo, M.; Song, J.-S.; Shin, T.J.; Hyun, H.-K.; Kim, J.-W.; Jang, K.-T.; Lee, S.-H.; Kim, Y.-J. The Effect of Silver Diamine Fluoride on Salivary Biofilm. J. Korean Acad. Pediatr. Dent. 2020, 47, 406–415. [Google Scholar] [CrossRef]
- Pornprasertsuk-Damrongsri, S.; Karnowakul, J.; Punyanirun, K.; Jirakran, K.; Thanyasrisung, P.; Techatharatip, O.; Pornprasertsuk-Damrongsri, S.; Trairatvorakul, C. Enhanced effectiveness of silver diamine fluoride application with light curing on natural dentin carious lesions: An in-vitro study. Odontology 2023, 111, 439–450. [Google Scholar]
- Seto, J.; Horst, J.A.; Parkinson, D.Y.; Frachella, J.C.; DeRisi, J.L. Silver microwires from treating tooth decay with silver diamine fluoride. biorXiv 2017. [Google Scholar] [CrossRef]

| Framework Item | Description |
|---|---|
| Population | Caries-affected (in vivo, in vitro) or intact (in vitro) primary teeth extracted for orthodontic reasons |
| Intervention | Application of varying concentrations of SDF to caries lesions. In in vitro studies involving intact teeth, artificial caries lesions were created by subjecting the teeth to demineralising and remineralising solutions with varying pH levels |
| Comparison | The potential of SDF is assessed either without control or in comparison with placebo or other prophylactic agents with antibacterial or remineralising properties, such as NaF, silver nitrate, chlorhexidine digluconate (CHX), etc. The influence of different application methods of SDF on clinical outcomes is also compared |
| Outcome | The outcomes of interest include the diversity and quantity of oral cavity microflora, changes in mineral density and mineral content of hard tissues, as well as alterations in microhardness of demineralised tissues (enamel or dentine) |
| Author, Year | Abdellatif et al., 2022 [39] | Abdil-Nafaa and Qasim, 2020 [47] | Hassan et al., 2021 [40] | Hussein et al., 2021 [48] | Liu et al., 2020 [41] | Mohammadi and Farahmand Far, 2018 [49] | Punhagui et al., 2021 [42] | Reis et al., 2021 [43] | Sai et al., 2020 [44] | Scarpelli et al., 2017 [50] | Toopchi et al., 2021 [45] | Yılmaz et al., 2020 [46] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criteria | ||||||||||||
| Clearly stated aims/objectives | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 |
| Detailed explanation of sample size calculation | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 2 |
| Detailed explanation of sampling technique | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Details of comparison group | 2 | 2 | 2 | 2 | not applicable | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Detailed explanation of methodology | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Operator details | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Randomization | 2 | 1 | 1 | 1 | not applicable | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| Method of measurement of outcome | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Outcome assessor details | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Blinding | 0 | 0 | 0 | 0 | not applicable | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Statistical analysis | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Presentation of results | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 |
| Author, Year | Country | Study Design | Sample Size | Concentration of SDF | Time of Sampling | Quantitative Changes in Oral Microflora ± SD | ||
|---|---|---|---|---|---|---|---|---|
| Chhattani et al., 2021 [33] | India | In vivo | 90 3–9-year-old children | 38% | Baseline | Blood agar: 400.83 CFU | Lactobacillus agar: 150.43 CFU | Mitis salivarius agar: 192.2 CFU |
| After a 21-day period | Blood agar: 66.3 ± 73.91 CFU | Lactobacillus agar: 22.1 ± 31.85 CFU | Mitis salivarius agar: 43.83 ± 52.52 CFU | |||||
| Baseline | Biofilm formation | |||||||
| Lactobacillus agar: 0.026 ± 0.005 | Mitis salivarius agar: 0.024 ± 0.004 | |||||||
| After a 21-day period | Lactobacillus agar: 0 | Mitis salivarius agar: 0.003 ± 0.008 | ||||||
| Garrastazu et al., 2020 [32] | Brazil | Exploratory trial | 90 6–10-year-old children | 30% | Baseline | 8.79 × 107 + 4.62 × 108 | ||
| After 24 h | 3.92 × 106 + 5.92 × 106 | |||||||
| After 30 days | 3.06 × 104 + 3.09 × 104 | |||||||
| After 90 days | 2.94 × 106 + 5.97 × 106 | |||||||
| Shetty et al., 2021 [35] | India | In vivo | 22 3–6-year-old children | 38% | Baseline | 5.21 ± 0.88 × 106 CFU/mL | ||
| After 3 days | 1.88 ± 0.58 × 106 CFU/mL | |||||||
| After 6 months | 0.88 ± 0.53 × 106 CFU/mL | |||||||
| 3 days after the reapplication at 6 months | 0.3 ± 0.23 × 106 CFU/mL | |||||||
| Sulyanto et al., 2022 [37] | USA | Case–control study | 13 1–4-year-old children | 38% | After 8–12 weeks | Percentage of dead microbes in SDF-treated plaque: 56% Percentage of dead microbes in SDF-untreated plaque: 67% | ||
| Author, Year | Country | Study Design | Sample Size | SDF, % | Outcome | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al., 2020 [41] | China | In vitro | 5 6–12-year-old children | 38% | The most prevalent microorganisms observed in the samples | ||||||||||||||
| Saliva | Plaque from intact teeth | Plaque from carious teeth | Plaque from SDF-treated teeth after a 24 h period | Plaque from SDF-treated teeth after a 1-week period | |||||||||||||||
| Streptococcus | Streptococcus | Pseudomonas | Pseudomonas | Pseudomonas | |||||||||||||||
| Neisseria | Neisseria | Oisenella | Streptococcus | Oisenella | |||||||||||||||
| Haemophilus | Leptotrichia | Bifidobacterium | Oisenella | Bifidobacterium | |||||||||||||||
| Veillonella | Actinomyces | Streptococcus | Veillonella | Fusobacterium | |||||||||||||||
| Leptotrichia | Veillonella | Prevotella | Bifidobacterium | Pseudoramibacter | |||||||||||||||
| Mei et al., 2020 [34] | Hong Kong | In vivo | 14 5-year-old children | 38% | The most prevalent microorganisms observed in the plaque samples from arrested caries lesions | ||||||||||||||
| Pre-SDF treatment | 2 weeks post-SDF treatment | 12 weeks post-SDF treatment | |||||||||||||||||
| Neisseria sp. | Neisseria sp. | Neisseria sp. | |||||||||||||||||
| Leptotrichia sp. | Veillonella sp. | Leptotrichia sp. | |||||||||||||||||
| Veillonella sp. | Corynebacterium sp. | Veillonella sp. | |||||||||||||||||
| Corynebacterium sp. | Lauptropia mirabilis | Porphyromonas sp. | |||||||||||||||||
| Capnocytophaga sp. | Capnocytophaga sp. | Lauptropia mirabilis | |||||||||||||||||
| The most prevalent microorganisms observed in the plaque samples from active caries lesions | |||||||||||||||||||
| Pre-SDF treatment | 2 weeks post-SDF treatment | 12 weeks post-SDF treatment | |||||||||||||||||
| Neisseria sp. | Rothia sp. | Veillonella sp. | |||||||||||||||||
| Leptotrichia sp. | Veillonella sp. | Neisseria sp. | |||||||||||||||||
| Veillonella sp. | Streptococcus mutans | Leptotrichia sp. | |||||||||||||||||
| Corynebacterium sp. | Lactobacillus sp. | Rothia sp. | |||||||||||||||||
| Lauptropia mirabilis | Corynebacterium sp. | Corynebacterium sp. | |||||||||||||||||
| Sulyanto et al., 2022 [37] | USA | Case–control study | 29 1–4-year-old children | 38% | Mean abundance of microbial species on intact enamel and carious surface biofilm | ||||||||||||||
| Intact enamel | Pre-SDF treatment | Post-SDF treatment | |||||||||||||||||
| Streptococcus mitis: 10.2% | Veillonella atypica: 15.9% | Rothia dentocariosa: 16.8% | |||||||||||||||||
| Veillonella atypica: 9.3% | Rothia dentocariosa: 14.6% | Veillonella atypica: 14.2% | |||||||||||||||||
| Haemophilus parainfluenzae: 7.8% | Streptococcus mutans: 10% | Streptococcus mitis: 8.7% | |||||||||||||||||
| Rothia dentocariosa: 7.3% | Streptococcus mitis: 6.4% | Streptococcus mutans: 6.5% | |||||||||||||||||
| Neisseria flava: 5.8% | Prevotella histicola: 3.3% | Rothia aeria: 3.2% | |||||||||||||||||
| Mean abundance of species in subsurface carious dentine | |||||||||||||||||||
| Post-SDF treatment | No SDF treatment | ||||||||||||||||||
| Lactobacillus casei rhamnosus: 10.1% | Streptococcus mutans: 28.7% | ||||||||||||||||||
| Veillonella atypica: 9.3% | Veillonella atypica: 9.9% | ||||||||||||||||||
| Actinomyces viscosus: 8.7% | Parascardovia denticolens: 6.2% | ||||||||||||||||||
| Streptococcus mutans: 8.2% | Scardovia wiggsiae: 5.4% | ||||||||||||||||||
| Parascardovia denticolens: 6.5% | Actinomyces IP073: 4.6% | ||||||||||||||||||
| Author, Year | Country | Study Design | Sample Size | Concentration of SDF | Assessment of Microhardness | Assessment Time | Microhardness Values ± SD |
|---|---|---|---|---|---|---|---|
| Abdil-Nafaa and Qasim, 2020 [47] | Iraq | In vitro | 150 anterior primary teeth | 30% | Vickers microhardness tester (OTTO Wolpert–WERKE GMBH, Ludwigshafen, Germany), load of 5 g, time of 15 s | Baseline | 204.764 ± 9.36 kgf/mm2 |
| After SDF application and demineralisation cycle | 189.882 ± 8.897 kgf/mm2 | ||||||
| Mohammadi and Farahmand Far, 2018 [49] | Iran | In vitro | 45 anterior primary teeth | 38% | Microhardness tester Shimadzu HMV-2000 (Shimadzu Corporation, Kyoto, Japan), load of 25 g, time of 5 s | Baseline | 252 kgf/mm2 |
| After demineralisation cycle | 155 kgf/mm2 | ||||||
| Reis et al., 2021 [43] | Brazil | In vitro | 36 primary teeth | 30% | HVS-1000 microhardness tester (Pantec, São Paulo, SP, Brazil), load of 5 g, time of 5 s | After demineralisation cycle | 36.1 ± 9.95 kgf/mm2 |
| 30 days post-SDF application | 39.3 ± 7.31 kgf/mm2 | ||||||
| Sai et al., 2020 [44] | India | In vitro | 30 anterior primary teeth | 38% | Digital Micro Vickers hardness tester, load of 200 g, time of 20 s | Baseline | 300.58 ± 27.58 kgf/mm2 |
| After demineralisation cycle | 244.76 ± 25.28 kgf/mm2 | ||||||
| 2 weeks post-SDF application | 394.25 ± 47.66 kgf/mm2 | ||||||
| Scarpelli et al., 2017 [50] | Brazil | In vitro | 100 primary molars | 38% | Knoop-type penetrator (HMV-G; Shimadzu, Tokyo, Japan), load of 25 g, time of 5 s | 8 days post-SDF application | 28.55 ± 11.75% |
| Author, Year | Country | Study Design | Sample Size | Concentration of SDF | Technique for Assessment | Outcome | |
|---|---|---|---|---|---|---|---|
| Abdellatif et al., 2022 [39] | Egypt | In vitro | 40 anterior primary teeth | 38% | Energy dispersive X-ray spectroscopy | Ca content in dentine ± SD | |
| Baseline | 24.86 ± 1.55% | ||||||
| After demineralisation cycle | 18.54 ± 2.34% | ||||||
| After remineralisation cycle | 28.69 ± 2.26% | ||||||
| P content in dentine ± SD | |||||||
| Baseline | 12.92 ± 0.91% | ||||||
| After demineralisation cycle | 10.7 ± 1.27% | ||||||
| After remineralisation cycle | 13.44 ± 1.62% | ||||||
| Ca/P ratio in dentine ± SD | |||||||
| Baseline | 1.92 ± 0.05% | ||||||
| After demineralisation cycle | 1.73 ± 0.1% | ||||||
| After remineralisation cycle | 2.14 ± 0.16% | ||||||
| Sulyanto et al., 2021 [36] | USA | In vivo | 11 primary teeth | 38% | X-ray fluorescence, energy-dispersive X-ray spectroscopy, microcomputed tomography | SDF penetration depth in SDF-minutes group | ~0.5 ± 0.02 mm |
| SDF penetration depth SDF-weeks group | ~0.6 ± 0.05 mm | ||||||
| The number of dentinal tubules occluded with Ag ions in SDF-minutes group | 6% | ||||||
| The number of dentinal tubules occluded with Ag ions in SDF-weeks group | 20% | ||||||
| The highest counts of Zn ions | Carious dentine, around the pulp chamber | ||||||
| The lowest counts of Zn ions | Sound dentine, inside the pulp chamber | ||||||
| Yılmaz et al., 2020 [46] | Turkey | In vitro | 54 primary molars | 38% | Micro-computed tomography | Mineral density value ± SD | |
| Baseline | 1.376 ± 0.07 gHApcm−3 | ||||||
| After demineralisation cycle | 0.961 ± 0.221 gHApcm−3 | ||||||
| After remineralisation cycle | 1.623 ± 0.171 gHApcm−3 | ||||||
| Author, Year | Country | Study Design | Sample Size | Outcome Measures | Follow-Up | SDF Application Technique | Results | |
|---|---|---|---|---|---|---|---|---|
| Hassan et al., 2021 [40] | Saudi Arabia | In vitro | 30 primary molars | Superficial microhardness ± SD | After 1 month | 38% SDF 30 s + Er, Cr:YSGG laser 10 s | 891.24 ± 37.33 kgf/mm2 | |
| 38% SDF 30 s + 40 s light curing | 266.65 ± 90.81 kgf/mm2 | |||||||
| 38% SDF 30 s | 117.91 ± 19.19 kgf/mm2 | |||||||
| Hussein et al., 2021 [48] | Egypt | In vitro | 60 primary second molars | Percentage change in dentine microhardness ± SD | After 1 week | 30% SDF 3 min | 60.96 ± 9.89% | |
| 6.5% grape seed extract 10 min + 30% SDF 3 min | 85.03 ± 5.52% | |||||||
| Punhagui et al., 2021 [42] | Brazil | In vitro | 45 primary molars | Percentage of surface remineralisation ± SD | 48 h after SDF application | 30% SDF 1 min | 30.82 ± 13.60% | |
| 30% SDF 3 min | 34.49 ± 13.67% | |||||||
| 38% SDF 1 min | 31.47 ± 18.77% | |||||||
| 38% SDF 3 min | 28.54 ± 8.59% | |||||||
| Thakur et al., 2022 [38] | India | Randomised controlled trial | 176 primary molars 16 primary incisors | Percentage of arrested caries lesions | After 3 weeks | 38% SDF 30 s | 73.33% | |
| 38% SDF 1 min | 72.29% | |||||||
| 38% SDF 2 min | 86.92% | |||||||
| After 3 months | 38% SDF 30 s | 74.31% | ||||||
| 38% SDF 1 min | 75.86% | |||||||
| 38% SDF 2 min | 82.45% | |||||||
| After 6 months | 38% SDF 30 s | 79.15% | ||||||
| 38% SDF 1 min | 77.29% | |||||||
| 38% SDF 2 min | 75.96% | |||||||
| Toopchi et al., 2021 [45] | Saudi Arabia | Ex vivo | 16 primary incisors | SDF penetration depth ± SD | After 1 month | 38% SDF 1 min | 130 ± 50 μm | |
| 38% SDF 1 min + 40 s light curing | 60 ± 10 μm | |||||||
| Superficial microhardness ± SD | 38% SDF 1 min | 558.07 ± 119.08 kgf/mm2 | ||||||
| 38% SDF 1 min + 40 s light curing | 702.26 ± 144.6 kgf/mm2 | |||||||
| Silver ion precipitation ± SD | 38% SDF 1 min | Infected dentine | 9.28 ± 3.53% | |||||
| Affected dentine | 5.22 ± 5.07% | |||||||
| Sound dentine | 1.56 ± 1.96% | |||||||
| 38% SDF 1 min + 40 s light curing | Infected dentine | 24.61 ± 14.74% | ||||||
| Affected dentine | 4.51 ± 4.15% | |||||||
| Sound dentine | 1.19 ± 1.28% | |||||||
| Fluoride ion precipitation ± SD | 38% SDF 1 min | Infected dentine | 0.231 ± 0.19% | |||||
| Affected dentine | 0.289 ± 0.31% | |||||||
| Sound dentine | 0.34 ± 0.37% | |||||||
| 38% SDF 1 min + 40 s curing | Infected dentine | 0.259 ± 0.63% | ||||||
| Affected dentine | 0.38 ± 0.42% | |||||||
| Sound dentine | 0.452 ± 0.51% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalnikovaitė, K.; Narbutaitė, J.; Andruškevičienė, V.; Bendoraitienė, E.A.; Razmienė, J. The Potential of Silver Diamine Fluoride in Non-Operative Management of Dental Caries in Primary Teeth: A Systematic Review. Medicina 2024, 60, 1738. https://doi.org/10.3390/medicina60111738
Rogalnikovaitė K, Narbutaitė J, Andruškevičienė V, Bendoraitienė EA, Razmienė J. The Potential of Silver Diamine Fluoride in Non-Operative Management of Dental Caries in Primary Teeth: A Systematic Review. Medicina. 2024; 60(11):1738. https://doi.org/10.3390/medicina60111738
Chicago/Turabian StyleRogalnikovaitė, Kornelija, Julija Narbutaitė, Vilija Andruškevičienė, Eglė Aida Bendoraitienė, and Jaunė Razmienė. 2024. "The Potential of Silver Diamine Fluoride in Non-Operative Management of Dental Caries in Primary Teeth: A Systematic Review" Medicina 60, no. 11: 1738. https://doi.org/10.3390/medicina60111738
APA StyleRogalnikovaitė, K., Narbutaitė, J., Andruškevičienė, V., Bendoraitienė, E. A., & Razmienė, J. (2024). The Potential of Silver Diamine Fluoride in Non-Operative Management of Dental Caries in Primary Teeth: A Systematic Review. Medicina, 60(11), 1738. https://doi.org/10.3390/medicina60111738







