Repositioning Canagliflozin for Mitigation of Aluminium Chloride-Induced Alzheimer’s Disease: Involvement of TXNIP/NLRP3 Inflammasome Axis, Mitochondrial Dysfunction, and SIRT1/HMGB1 Signalling
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Drugs and Chemicals
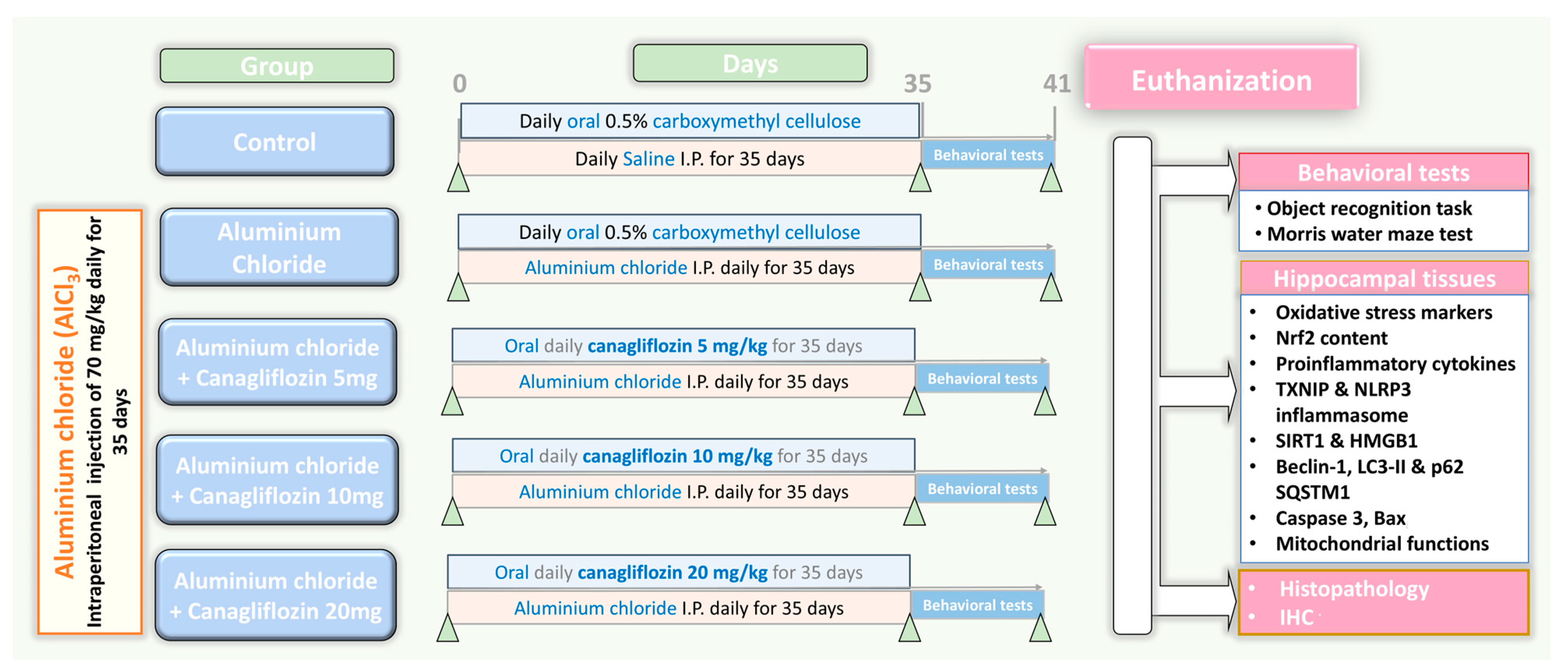
2.3. The Experimental Design
2.4. Determination of the Behavioural Changes in the Studied Animals
2.4.1. Morris Water Maze Test
2.4.2. Object Recognition Test
2.5. Assessment of the Impact of Different Doses of Canagliflozin on Aluminium Chloride-Induced Changes in Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Content and the Redox State of the Hippocampal Tissues
2.6. Determination of the Hippocampal Tissue Tumour Necrosis Factor Alpha (TNF-α), Interleukin 1 Beta (IL-1β), and IL-6
2.7. Assessment of the Hippocampal Tissue Levels of Thioredoxin-Interacting Protein (TXNIP), Nuclear Factor Kappa B (NF-κB) p65, and Nucleotide-Binding Domain-like Receptor Family, Pyrin Domain-Containing 3 (NLRP3) Inflammasome
2.8. Quantification of the Hippocampal Tissue Levels of High-Mobility Group Box 1 (HMGB1) and Sirtuin-1 (SIRT1)
2.9. Determination of the Hippocampal Tissue Levels of the Autophagy Markers
2.10. Assessment of the Hippocampal Tissue Levels of Caspase 3 and Bax
2.11. Determination of the Mitochondrial Functions in the Hippocampal Tissues
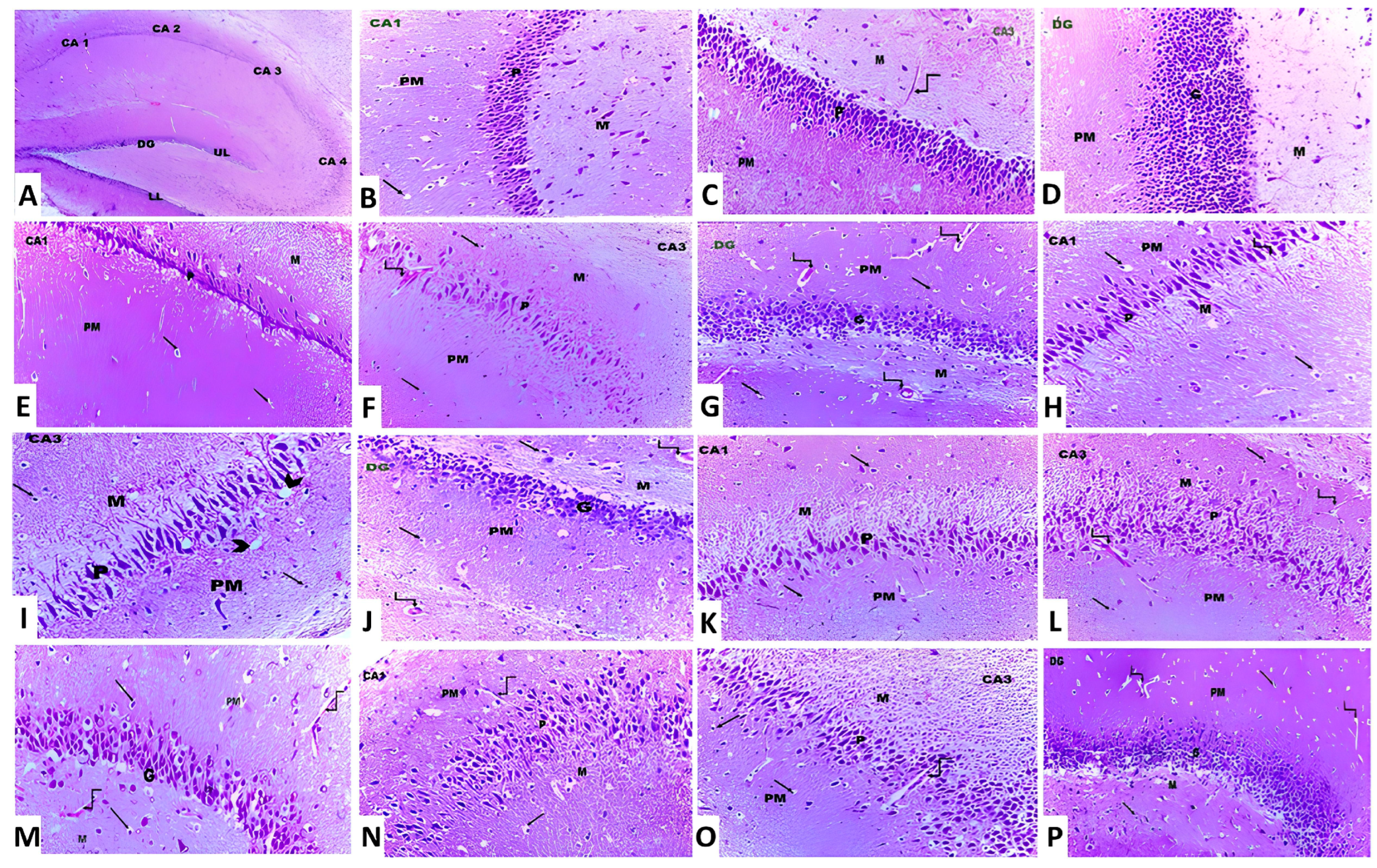
2.12. Microscopic Evaluation of the Pathological Changes in the Hippocampal Tissues
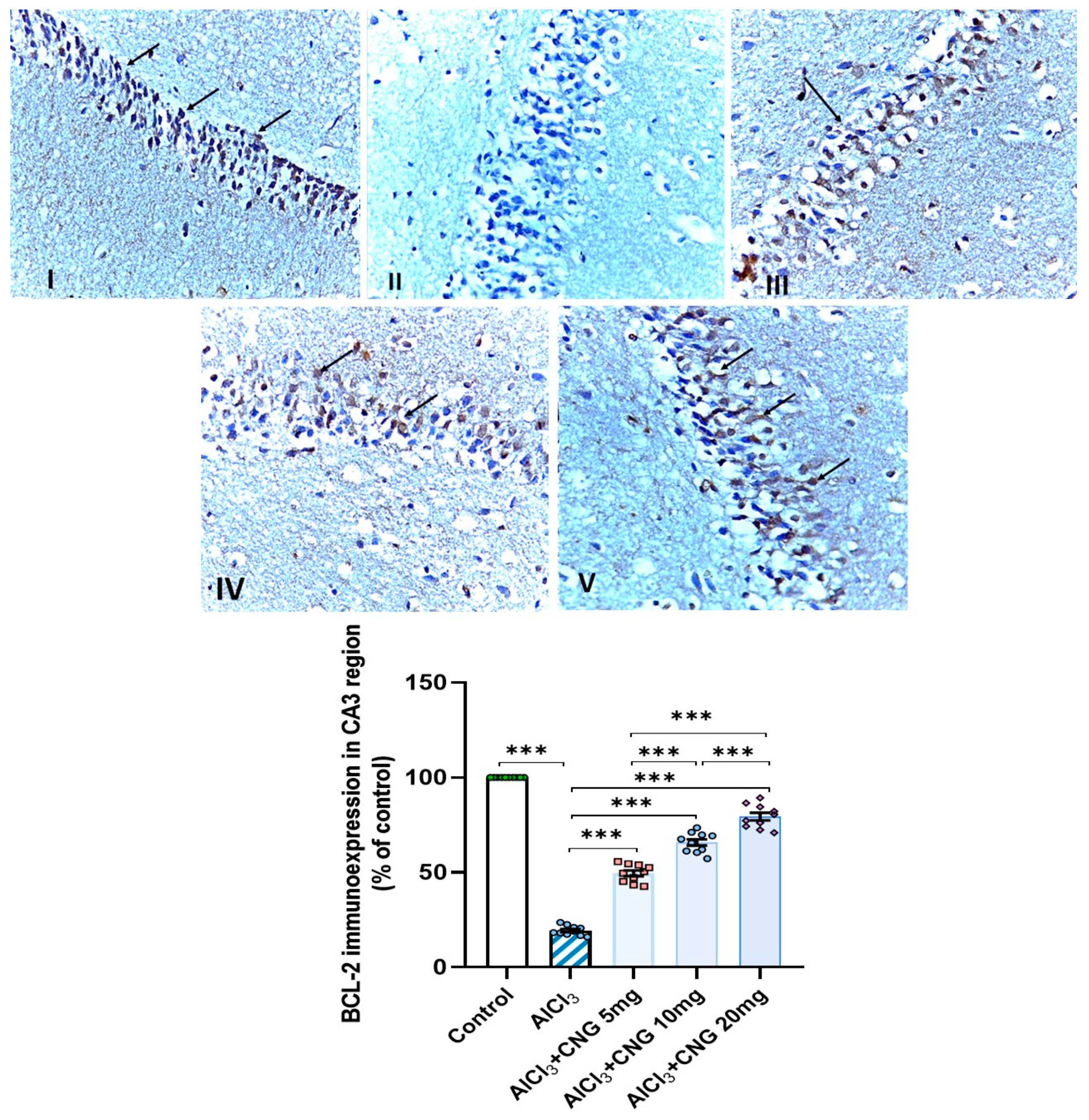
2.13. Evaluation of the Extent of the Immunohistochemical Positive Expression of B-Cell Lymphoma-2 (BCL-2) Protein in the Hippocampal Tissues
2.14. Data Evaluation
3. Results
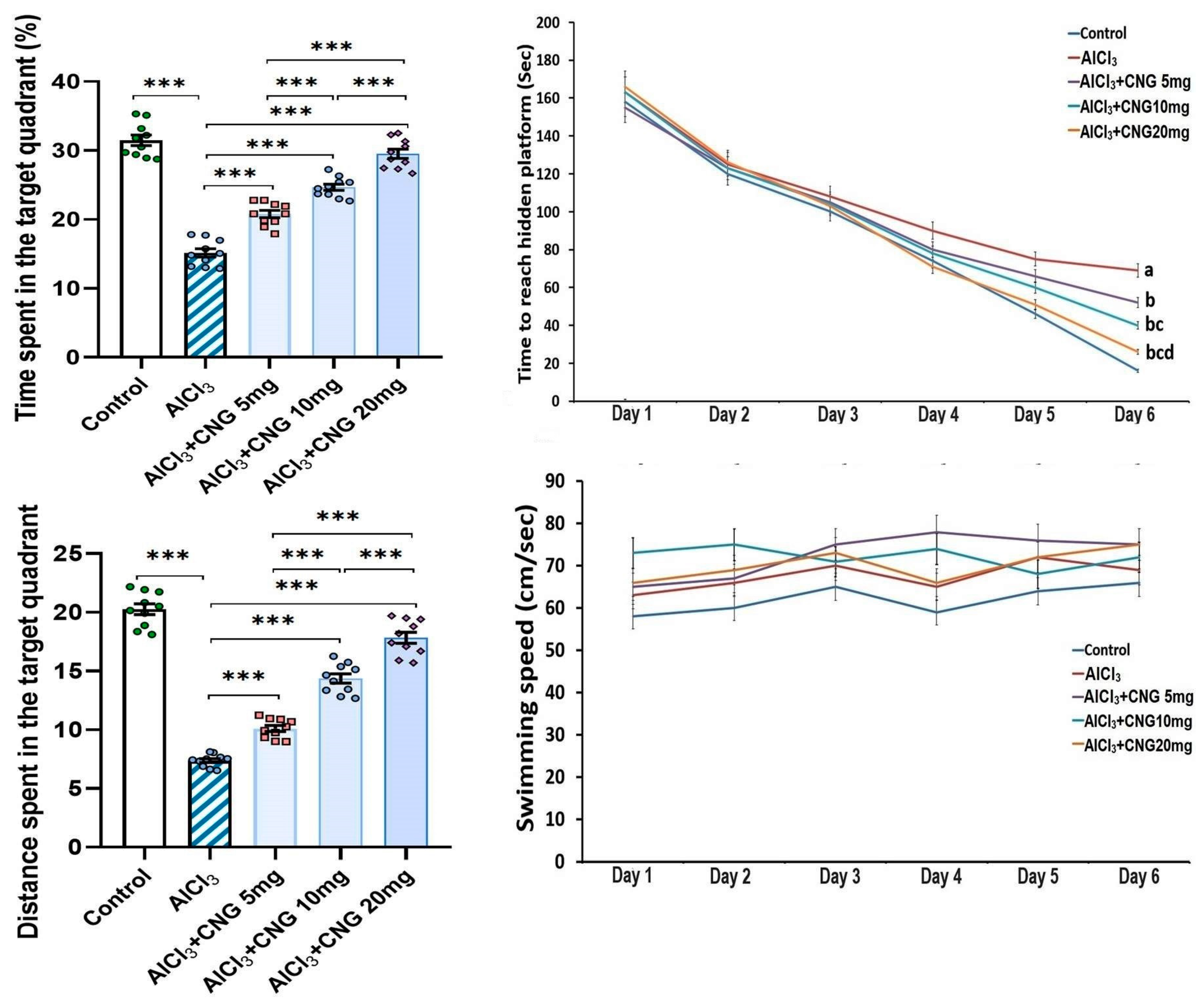
3.1. Canagliflozin Dose-Dependently Mitigated the Behavioural Changes Induced by Aluminium Chloride Injection
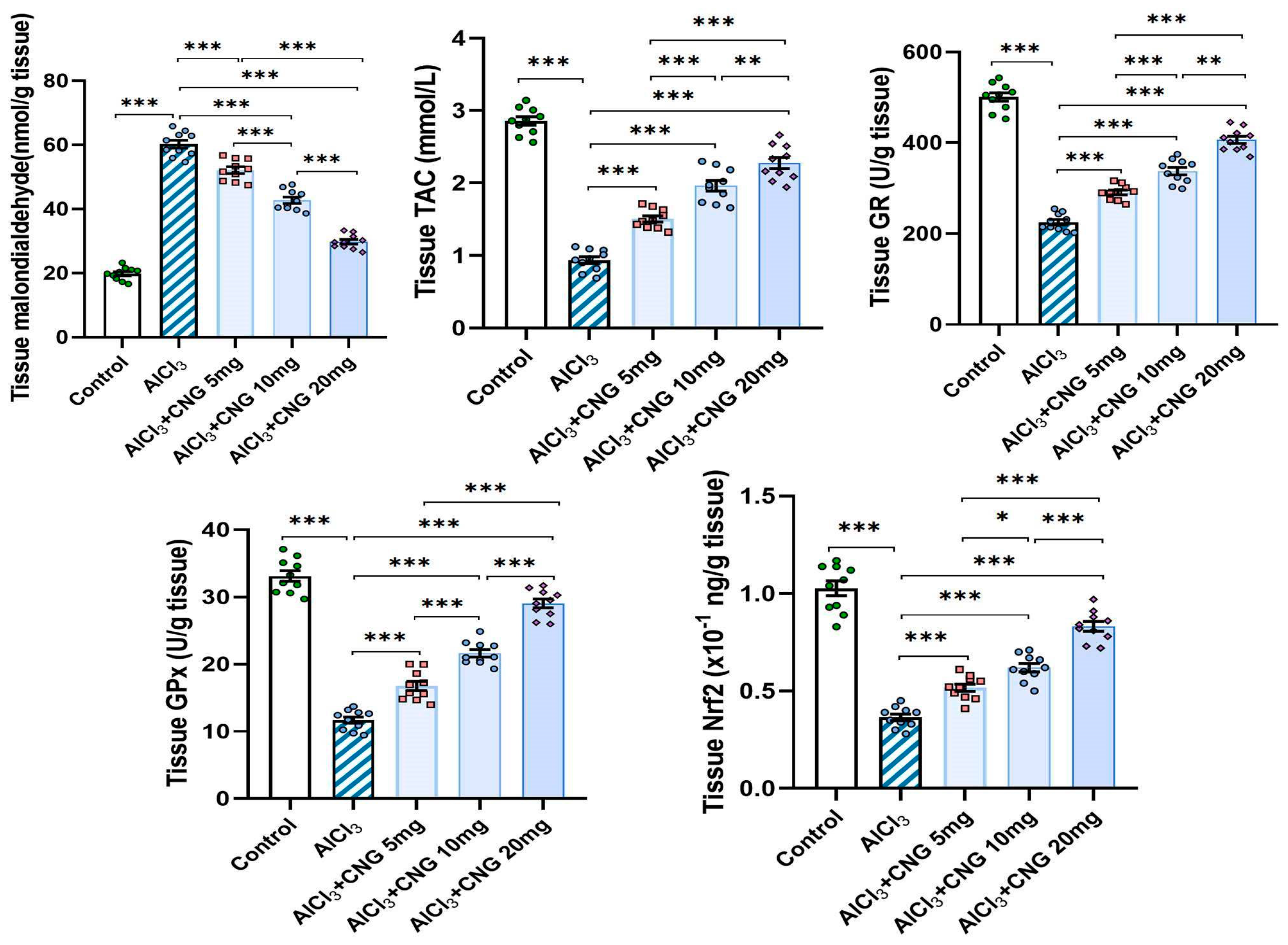
3.2. Canagliflozin Dose-Dependently Abrogated the Effect of Aluminium Chloride Injection on the Redox State and Nrf2 Content of the Hippocampal Tissue Specimens
3.3. Canagliflozin Dose-Dependently Counteracted the Effect of Aluminium Chloride Administration on TXNIP/NF-κB/NLRP3 Inflammasome Signalling and the Inflammatory Cascade in the Hippocampal Tissue Specimens
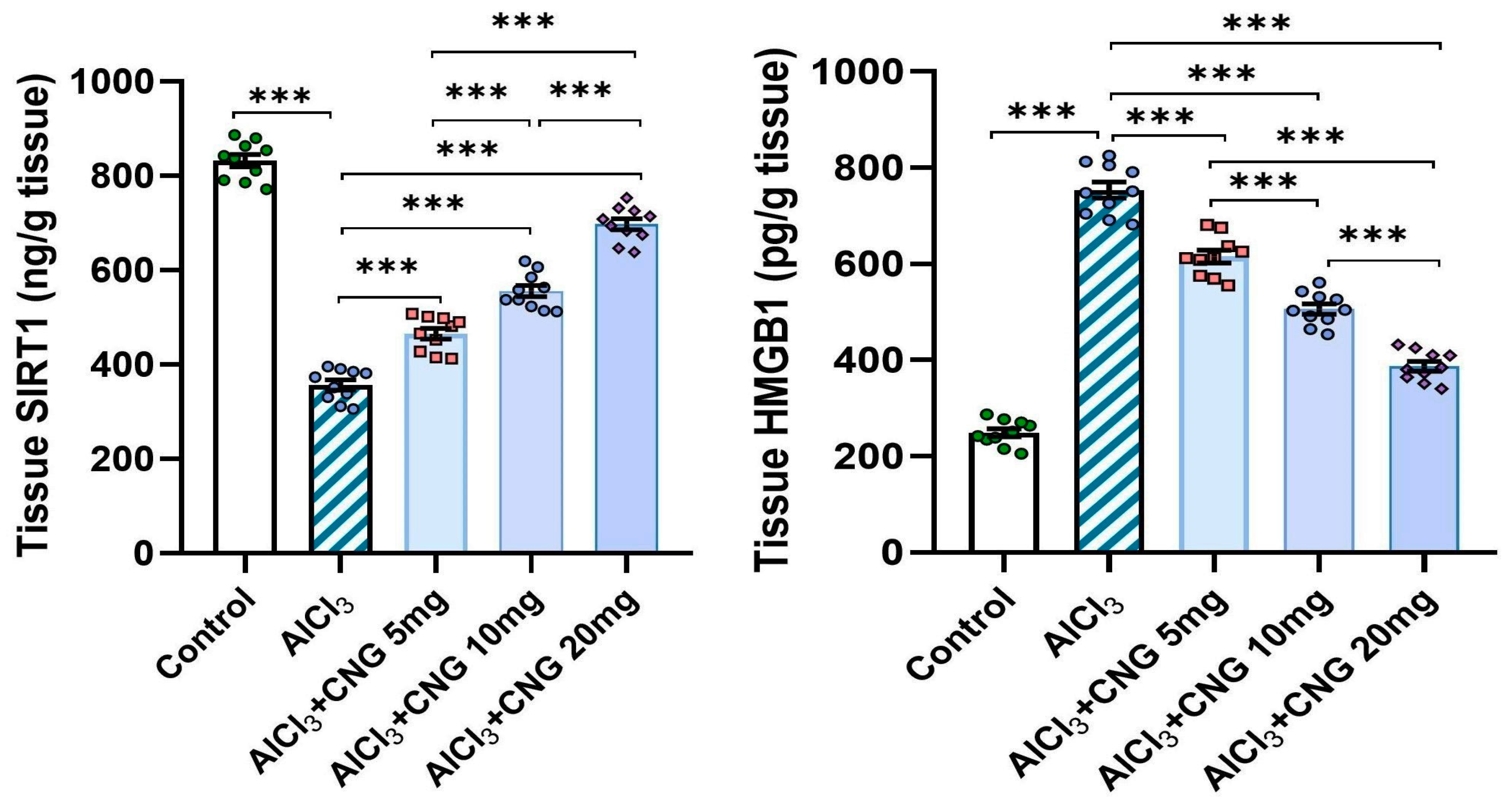
3.4. Canagliflozin Dose-Dependently Mitigated the Effect of Aluminium Chloride Administration on SIRT1/HMGB1 Signalling in the Hippocampal Tissue Specimens
3.5. Canagliflozin Dose-Dependently Augmented Autophagy in the Hippocampal Tissue Specimens of Animals Treated with Aluminium Chloride
3.6. Canagliflozin Produced a Dose-Dependent Decline in the Hippocampal Tissue Levels of Caspase 3 and Bax in Aluminium Chloride-Treated Animals
3.7. Canagliflozin Dose-Dependently Ameliorated the Perturbations of the Mitochondrial Functions Induced by Aluminium Chloride in the Hippocampal Tissues
3.8. Canagliflozin Dose-Dependently Mitigated the Histopathological Changes in the Hippocampal Tissues Created as a Result of Aluminium Chloride Administration
3.9. Canagliflozin Dose-Dependently Augmented BCL-2 Immuno-Expression in the Hippocampal Tissues Induced by Aluminium Chloride
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M.; Arab, H.H.; Atef, A.; Estfanous, R.S. Omarigliptin/galangin combination mitigates lipopolysaccharide-induced neuroinflammation in rats: Involvement of glucagon-like peptide-1, toll-like receptor-4, apoptosis and Akt/GSK-3β signaling. Life Sci. 2022, 295, 120396. [Google Scholar] [CrossRef]
- Meftah, S.; Gan, J. Alzheimer’s disease as a synaptopathy: Evidence for dysfunction of synapses during disease progression. Front. Synaptic Neurosci. 2023, 15, 1129036. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; Silvester, B.; Duff, K. Neural Mechanisms of Motor Dysfunction in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. Rep. 2022, 6, 307–344. [Google Scholar] [CrossRef] [PubMed]
- Elmaaboud, M.A.A.; Estfanous, R.S.; Atef, A.; Kabel, A.M.; Alnemari, K.A.; Naguib, T.M.; Alsufyani, S.E.; Darwish, H.W.; Arab, H.H. Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer’s Disease in Rats. Pharmaceuticals 2023, 16, 1370. [Google Scholar] [CrossRef]
- Liu, Y.; Si, Z.-Z.; Zou, C.-J.; Mei, X.; Li, X.-F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.-X.; Wu, L. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar] [CrossRef]
- Eshraghi, M.; Adlimoghaddam, A.; Mahmoodzadeh, A.; Sharifzad, F.; Yasavoli-Sharahi, H.; Lorzadeh, S.; Albensi, B.C.; Ghavami, S. Alzheimer’s Disease Pathogenesis: Role of Autophagy and Mitophagy Focusing in Microglia. Int. J. Mol. Sci. 2021, 22, 3330. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Zhang, Y. Animal models of Alzheimer’s disease: Applications, evaluation, and perspectives. Zool. Res. 2022, 43, 1026–1040. [Google Scholar] [CrossRef]
- Akhtar, A.; Gupta, S.M.; Dwivedi, S.; Kumar, D.; Shaikh, M.F.; Negi, A. Preclinical Models for Alzheimer’s Disease: Past, Present, and Future Approaches. ACS Omega 2022, 7, 47504–47517. [Google Scholar] [CrossRef]
- Rapaka, D.; Adiukwu, P.C.; Bitra, V.R. Experimentally induced animal models for cognitive dysfunction and Alzheimer’s disease. MethodsX 2022, 9, 101933. [Google Scholar] [CrossRef] [PubMed]
- Chiroma, S.M.; Moklas, M.A.M.; Taib, C.N.M.; Baharuldin, M.T.H.; Amon, Z. d-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed. Pharmacother. 2018, 103, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Zeb, Z.; Sharif, A.; Akhtar, B.; Shahnaz. 3-Acetyl coumarin alleviate neuroinflammatory responses and oxidative stress in aluminum chloride-induced Alzheimer’s disease rat model. Inflammopharmacology 2024, 32, 1371–1386. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Jiang, Y.; Gluhcheva, Y.G.; Tizabi, Y.; Lobinski, R.; Tinkov, A.A. Molecular mechanisms of aluminum neurotoxicity: Update on adverse effects and therapeutic strategies. Adv. Neurotoxicol. 2021, 5, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Jakher, H.; Chang, T.I.; Tan, M.; Mahaffey, K.W. Canagliflozin review—Safety and efficacy profile in patients with T2DM. Diabetes, Metab. Syndr. Obes. Targets Ther. 2019, 12, 209–215. [Google Scholar] [CrossRef]
- Tharmaraja, T.; Ho, J.S.; Sia, C.-H.; Lim, N.-A.; Chong, Y.F.; Lim, A.Y.; Rathakrishnan, R.R.; Yeo, L.L.; Sharma, V.K.; Tan, B.Y. Sodium-glucose cotransporter 2 inhibitors and neurological disorders: A scoping review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221086996. [Google Scholar] [CrossRef]
- Nakhal, M.M.; Jayaprakash, P.; Aburuz, S.; Sadek, B.; Akour, A. Canagliflozin Ameliorates Oxidative Stress and Autistic-like Features in Valproic-Acid-Induced Autism in Rats: Comparison with Aripiprazole Action. Pharmaceuticals 2023, 16, 769. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Kocer, C.; Sayour, A.A.; Kraft, P.; Benker, M.I.; Abulizi, S.; Georgevici, A.-I.; Brlecic, P.; Radovits, T.; Loganathan, S.; et al. The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2021, 22, 7774. [Google Scholar] [CrossRef]
- Hamdan, A.M.E.; Alharthi, F.H.J.; Alanazi, A.H.; El-Emam, S.Z.; Zaghlool, S.S.; Metwally, K.; Albalawi, S.A.; Abdu, Y.S.; Mansour, R.E.-S.; Salem, H.A.; et al. Neuroprotective Effects of Phytochemicals against Aluminum Chloride-Induced Alzheimer’s Disease through ApoE4/LRP1, Wnt3/β-Catenin/GSK3β, and TLR4/NLRP3 Pathways with Physical and Mental Activities in a Rat Model. Pharmaceuticals 2022, 15, 1008. [Google Scholar] [CrossRef]
- Dong, S.-T.; Niu, H.-M.; Wu, Y.; Jiang, J.-L.; Li, Y.; Jiang, K.-Y.; Wang, X.; Zhang, M.-F.; Han, M.-F.; Meng, S.-N. Plasma Pharmacokinetic Determination of Canagliflozin and Its Metabolites in a Type 2 Diabetic Rat Model by UPLC-MS/MS. Molecules 2018, 23, 1229. [Google Scholar] [CrossRef]
- Tian, H.; Ding, N.; Guo, M.; Wang, S.; Wang, Z.; Liu, H.; Yang, J.; Li, Y.; Ren, J.; Jiang, J.; et al. Analysis of Learning and Memory Ability in an Alzheimer’s Disease Mouse Model using the Morris Water Maze. J. Vis. Exp. 2019, 29, e60055. [Google Scholar] [CrossRef]
- Ramirez, M.J.; Bengoetxea, X.; Rodriguez-Perdigon, M. Object recognition test for studying cognitive impairments in animal models of Alzheimer s disease. Front. Biosci. 2015, 7, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.A.; Meigs, R.A. Mitochondria from human term placenta. I. Isolation and assay conditions for oxidative phosphorylation. Biochim. Biophys. Acta (BBA) Bioenerg. 1975, 376, 426–435. [Google Scholar] [CrossRef]
- Maity, P.; Bindu, S.; Dey, S.; Goyal, M.; Alam, A.; Pal, C.; Mitra, K.; Bandyopadhyay, U. Indomethacin, a non-steroidal anti-inflammatory drug, develops gastropathy by inducing reactive oxygen species-mediated mitochondrial pathology and associated apoptosis in gastric mucosa: A novel role of mitochondrial aconitase oxidation. J. Biol. Chem. 2009, 284, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Teipel, S.; Gustafson, D.; Ossenkoppele, R.; Hansson, O.; Babiloni, C.; Wagner, M.; Riedel-Heller, S.G.; Kilimann, I.; Tang, Y. Alzheimer Disease: Standard of Diagnosis, Treatment, Care, and Prevention. J. Nucl. Med. 2022, 63, 981–985. [Google Scholar] [CrossRef]
- Hoogmartens, J.; Cacace, R.; Van Broeckhoven, C. Insight into the genetic etiology of Alzheimer’s disease: A comprehensive review of the role of rare variants. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2021, 13, e12155. [Google Scholar] [CrossRef]
- Xie, J.; Van Hoecke, L.; Vandenbroucke, R.E. The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology. Front. Immunol. 2022, 12, 796867. [Google Scholar] [CrossRef]
- Luo, L.; Yan, T.; Yang, L.; Zhao, M. Aluminum chloride and D-galactose induced a zebrafish model of Alzheimer’s disease with cognitive deficits and aging. Comput. Struct. Biotechnol. J. 2024, 23, 2230–2239. [Google Scholar] [CrossRef]
- Tomljenovic, L. Aluminum and Alzheimer’s disease: After a century of controversy, is there a plausible link? J. Alzheimer’s Dis. 2011, 23, 567–598. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Santamaria, A.; Rocha, J.B.T.; Mansouri, B.; Tizabi, Y.; Madeddu, R.; Lu, R.; Lee, E.; Tinkov, A.A. Epigenetic Mechanisms of Aluminum-Induced Neurotoxicity and Alzheimer’s Disease: A Focus on Non-Coding RNAs. Neurochem. Res. 2024, 49, 2988–3005. [Google Scholar] [CrossRef]
- Mold, M.J.; O’farrell, A.; Morris, B.; Exley, C. Aluminum and Tau in Neurofibrillary Tangles in Familial Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 283–294. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Tharakan, M.; Culberson, J.; Reddy, A.P.; Reddy, P.H. Role of Nrf2 in aging, Alzheimer’s and other neurodegenerative diseases. Ageing Res. Rev. 2022, 82, 101756. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, G.A.; Metaxas, A.; Kourti, M. Natural antioxidants that act against Alzheimer’s disease through modulation of the NRF2 pathway: A focus on their molecular mechanisms of action. Front. Endocrinol. 2023, 14, 1217730. [Google Scholar] [CrossRef]
- Tejo, F.V.; Quintanilla, R.A. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Spaeth-Cook, D.; Burch, M.; Belton, R.; Demoret, B.; Grosenbacher, N.; David, J.; Stets, C.; Cohen, D.; Shakya, R.; Hays, J.L.; et al. Loss of TXNIP enhances peritoneal metastasis and can be abrogated by dual TORC1/2 inhibition. Oncotarget 2018, 9, 35676–35686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ding, Z.-X.; Huang, W.; Luo, J.; Ye, S.; Hu, S.-L.; Zhou, P.; Cai, B. Chrysophanol exerts a protective effect against Aβ25-35-induced Alzheimer’s disease model through regulating the ROS/TXNIP/NLRP3 pathway. Inflammopharmacology 2023, 31, 1511–1527. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, F.; Qu, K.; Liu, C.; Zhang, J. TXNIP: A Double-Edged Sword in Disease and Therapeutic Outlook. Oxid. Med. Cell. Longev. 2022, 2022, 7805115. [Google Scholar] [CrossRef]
- Sbai, O.; Djelloul, M.; Auletta, A.; Ieraci, A.; Vascotto, C.; Perrone, L. RAGE-TXNIP axis drives inflammation in Alzheimer’s by targeting Aβ to mitochondria in microglia. Cell Death Dis. 2022, 13, 302. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, G.; Shao, N.; Qin, Y.; Chen, Q.; Wang, Y.; Zhou, P.; Cai, B. Thioredoxin-interacting protein (TXNIP) as a target for Alzheimer’s disease: Flavonoids and phenols. Inflammopharmacology 2021, 29, 1317–1329. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 845185. [Google Scholar] [CrossRef]
- Tsubaki, H.; Tooyama, I.; Walker, D.G. Thioredoxin-Interacting Protein (TXNIP) with Focus on Brain and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9357. [Google Scholar] [CrossRef] [PubMed]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef] [PubMed]
- Althagafy, H.S.; Ali, F.E.; Hassanein, E.H.; Mohammedsaleh, Z.M.; El-Sayed, M.I.K.; Atwa, A.M.; Sayed, A.M.; Soubh, A.A. Canagliflozin ameliorates ulcerative colitis via regulation of TLR4/MAPK/NF-κB and Nrf2/PPAR-γ/SIRT1 signaling pathways. Eur. J. Pharmacol. 2023, 960, 176166. [Google Scholar] [CrossRef] [PubMed]
- Campeau, M.-A.; Leask, R.L. Empagliflozin mitigates endothelial inflammation and attenuates endoplasmic reticulum stress signaling caused by sustained glycocalyx disruption. Sci. Rep. 2022, 12, 12681. [Google Scholar] [CrossRef]
- Sabaa, M.; Sharawy, M.H.; El-Sherbiny, M.; Said, E.; Salem, H.A.; Ibrahim, T.M. Canagliflozin interrupts mTOR-mediated inflammatory signaling and attenuates DMBA-induced mammary cell carcinoma in rats. Biomed. Pharmacother. 2022, 155, 113675. [Google Scholar] [CrossRef]
- Elesawy, R.O.; El-Deeb, O.S.; Eltokhy, A.K.; Arakeep, H.M.; Ali, D.A.; Elkholy, S.S.; Kabel, A.M. Postnatal baicalin ameliorates behavioral and neurochemical alterations in valproic acid-induced rodent model of autism: The possible implication of sirtuin-1/mitofusin-2/ Bcl-2 pathway. Biomed. Pharmacother. 2022, 150, 112960. [Google Scholar] [CrossRef]
- Mehramiz, M.; Porter, T.; Laws, S.M.; Rainey-Smith, S.R. Sleep, Sirtuin 1 and Alzheimer’s disease: A review. Aging Brain 2022, 2, 100050. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lyu, X.; Zhang, X.; Zhang, F.; Chen, Y.; Li, G. Astaxanthin attenuates cognitive deficits in Alzheimer’s disease models by reducing oxidative stress via the SIRT1/PGC-1α signaling pathway. Cell Biosci. 2023, 13, 173. [Google Scholar] [CrossRef]
- Maiese, K. Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural Regen. Res. 2021, 16, 448–455. [Google Scholar] [CrossRef]
- Yan, J.; Luo, A.; Gao, J.; Tang, X.; Zhao, Y.; Zhou, B.; Zhou, Z.; Li, S. The role of SIRT1 in neuroinflammation and cognitive dysfunction in aged rats after anesthesia and surgery. Am. J. Transl. Res. 2019, 11, 1555–1568. [Google Scholar]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Fikry, E.M.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Al-Hossaini, A.M.; Saad, M.A.; Al-Shorbagy, M.Y.; Eid, A.H. Stimulation of Autophagy by Dapagliflozin Mitigates Cadmium-Induced Testicular Dysfunction in Rats: The Role of AMPK/mTOR and SIRT1/Nrf2/HO-1 Pathways. Pharmaceuticals 2023, 16, 1006. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, M.; Ahmed, M.; Surapaneni, K.M.; Veeraraghavan, V.P.; Arulselvan, P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J. Biol. Sci. 2021, 28, 4232–4239. [Google Scholar] [CrossRef] [PubMed]
- Memudu, A.E.; Adanike, R.P. Alpha lipoic acid reverses scopolamine-induced spatial memory loss and pyramidal cell neurodegeneration in the prefrontal cortex of Wistar rats. IBRO Neurosci. Rep. 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Li, R.-L.; Wang, L.-Y.; Duan, H.-X.; Zhang, Q.; Guo, X.; Wu, C.; Peng, W. Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases. Front. Pharmacol. 2022, 13, 937289. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Liu, D.; Jiang, H.; Cai, C.; Li, G.; Yu, G. Canagliflozin Prevents Lipid Accumulation, Mitochondrial Dysfunction, and Gut Microbiota Dysbiosis in Mice with Diabetic Cardiovascular Disease. Front. Pharmacol. 2022, 13, 839640. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Zhuravlev, A.D.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 5371. [Google Scholar] [CrossRef]
- Jhuo, S.-J.; Lin, Y.-H.; Liu, I.-H.; Lin, T.-H.; Wu, B.-N.; Lee, K.-T.; Lai, W.-T. Sodium Glucose Cotransporter 2 (SGLT2) Inhibitor Ameliorate Metabolic Disorder and Obesity Induced Cardiomyocyte Injury and Mitochondrial Remodeling. Int. J. Mol. Sci. 2023, 24, 6842. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Zhang, J.; Zhang, X.; Yang, G. Molecular Mechanism of Autophagy: Its Role in the Therapy of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 720–739. [Google Scholar] [CrossRef]
- Raj, K.; Gupta, G.D.; Singh, S. Spermine protects aluminium chloride and iron-induced neurotoxicity in rat model of Alzheimer’s disease via attenuation of tau phosphorylation, Amyloid-β (1–42) and NF-κB pathway. Inflammopharmacology 2021, 29, 1777–1793. [Google Scholar] [CrossRef]
- Weng, M.-H.; Chen, S.-Y.; Li, Z.-Y.; Yen, G.-C. Camellia oil alleviates the progression of Alzheimer’s disease in aluminum chloride-treated rats. Free Radic. Biol. Med. 2020, 152, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.H.; Desouky, M.A.; Zidan, A.; Bassem, M.; Qasem, A.; Farouk, M.; AlDeab, H.; Fouad, M.; Hany, C.; Basem, N.; et al. Neuromodulatory effect of vardenafil on aluminium chloride/d-galactose induced Alzheimer’s disease in rats: Emphasis on amyloid-beta, p-tau, PI3K/Akt/p53 pathway, endoplasmic reticulum stress, and cellular senescence. Inflammopharmacology 2023, 31, 2653–2673. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-Y.; Lin, C.-H.; Fang, J.-Y. Natural compounds and aging: Between autophagy and inflammasome. BioMed Res. Int. 2014, 2014, 297293. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sun, P.; Zhang, X.; Lin, G.; Xin, Q.; Niu, Y.; Chen, Y.; Xu, N.; Zhang, Y.; Xie, W. Canagliflozin Modulates Hypoxia-Induced Metastasis, Angiogenesis and Glycolysis by Decreasing HIF-1α Protein Synthesis via AKT/mTOR Pathway. Int. J. Mol. Sci. 2021, 22, 13336. [Google Scholar] [CrossRef]
- Du, S.; Shi, H.; Xiong, L.; Wang, P.; Shi, Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front. Endocrinol. 2022, 13, 1011669. [Google Scholar] [CrossRef]
- Zhuo, M.; Hawley, C.E.; Paik, J.M.; Bessette, L.G.; Wexler, D.J.; Kim, D.H.; Tong, A.Y.; Kim, S.C.; Patorno, E. Association of Sodium-Glucose Cotransporter–2 Inhibitors with Fracture Risk in Older Adults with Type 2 Diabetes. JAMA Netw. Open 2021, 4, e2130762. [Google Scholar] [CrossRef]
- Sloan, G.; Kakoudaki, T.; Ranjan, N. Prolonged diabetic ketoacidosis associated with canagliflozin. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 17-0177. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elariny, H.A.; Kabel, A.M.; Selim, H.M.R.M.; Helal, A.I.; Abdelrahman, D.; Borg, H.M.; Elkady, M.A.; Dawood, L.M.; El-Badawy, M.F.; Almalawi, H.F.A.; et al. Repositioning Canagliflozin for Mitigation of Aluminium Chloride-Induced Alzheimer’s Disease: Involvement of TXNIP/NLRP3 Inflammasome Axis, Mitochondrial Dysfunction, and SIRT1/HMGB1 Signalling. Medicina 2024, 60, 1805. https://doi.org/10.3390/medicina60111805
Elariny HA, Kabel AM, Selim HMRM, Helal AI, Abdelrahman D, Borg HM, Elkady MA, Dawood LM, El-Badawy MF, Almalawi HFA, et al. Repositioning Canagliflozin for Mitigation of Aluminium Chloride-Induced Alzheimer’s Disease: Involvement of TXNIP/NLRP3 Inflammasome Axis, Mitochondrial Dysfunction, and SIRT1/HMGB1 Signalling. Medicina. 2024; 60(11):1805. https://doi.org/10.3390/medicina60111805
Chicago/Turabian StyleElariny, Hemat A., Ahmed M. Kabel, Heba Mohammed Refat M. Selim, Azza I. Helal, Doaa Abdelrahman, Hany M. Borg, Mennatallah A. Elkady, Lamees M. Dawood, Mohamed F. El-Badawy, Haifa Faisal A. Almalawi, and et al. 2024. "Repositioning Canagliflozin for Mitigation of Aluminium Chloride-Induced Alzheimer’s Disease: Involvement of TXNIP/NLRP3 Inflammasome Axis, Mitochondrial Dysfunction, and SIRT1/HMGB1 Signalling" Medicina 60, no. 11: 1805. https://doi.org/10.3390/medicina60111805
APA StyleElariny, H. A., Kabel, A. M., Selim, H. M. R. M., Helal, A. I., Abdelrahman, D., Borg, H. M., Elkady, M. A., Dawood, L. M., El-Badawy, M. F., Almalawi, H. F. A., Arafa, E.-S. A., Alsufyani, S. E., & Arab, H. H. (2024). Repositioning Canagliflozin for Mitigation of Aluminium Chloride-Induced Alzheimer’s Disease: Involvement of TXNIP/NLRP3 Inflammasome Axis, Mitochondrial Dysfunction, and SIRT1/HMGB1 Signalling. Medicina, 60(11), 1805. https://doi.org/10.3390/medicina60111805






