Rosiridin Protects Against Aluminum Chloride-Induced Memory Impairment via Modulation of BDNF/NFκB/PI3K/Akt Pathway in Rats
Abstract
1. Introduction
2. Methodology
2.1. Chemicals
2.2. Experimental Animals
2.3. Study Plan
- Control—each rat received a regular diet without any treatment;
- AlCl3 (100 mg/kg) + rosiridin (10 mg/kg p.o.);
- AlCl3 (100 mg/kg) + rosiridin (20 mg/kg p.o.).
2.4. Parameters (Behavioral)
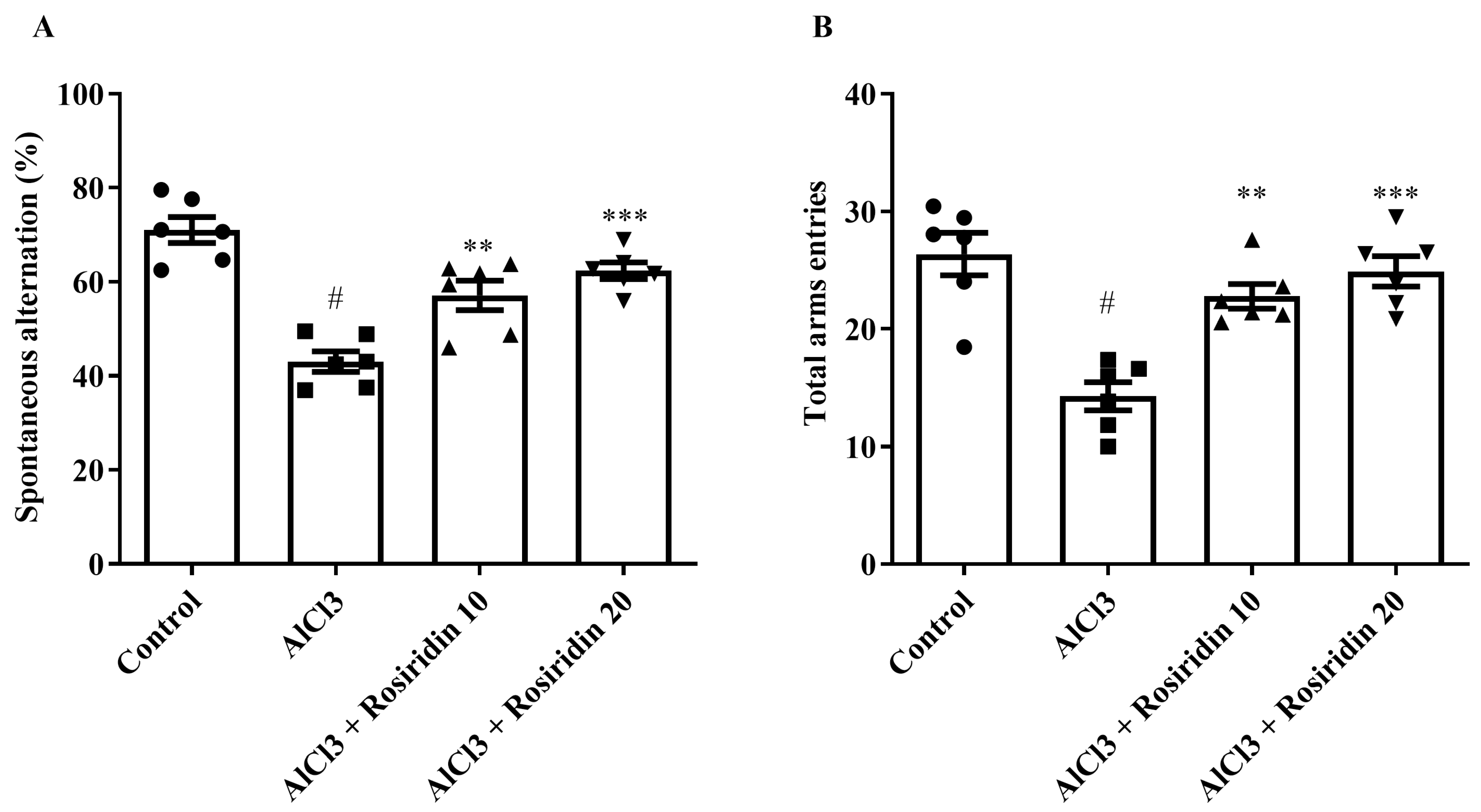
2.4.1. Y-Maze Test
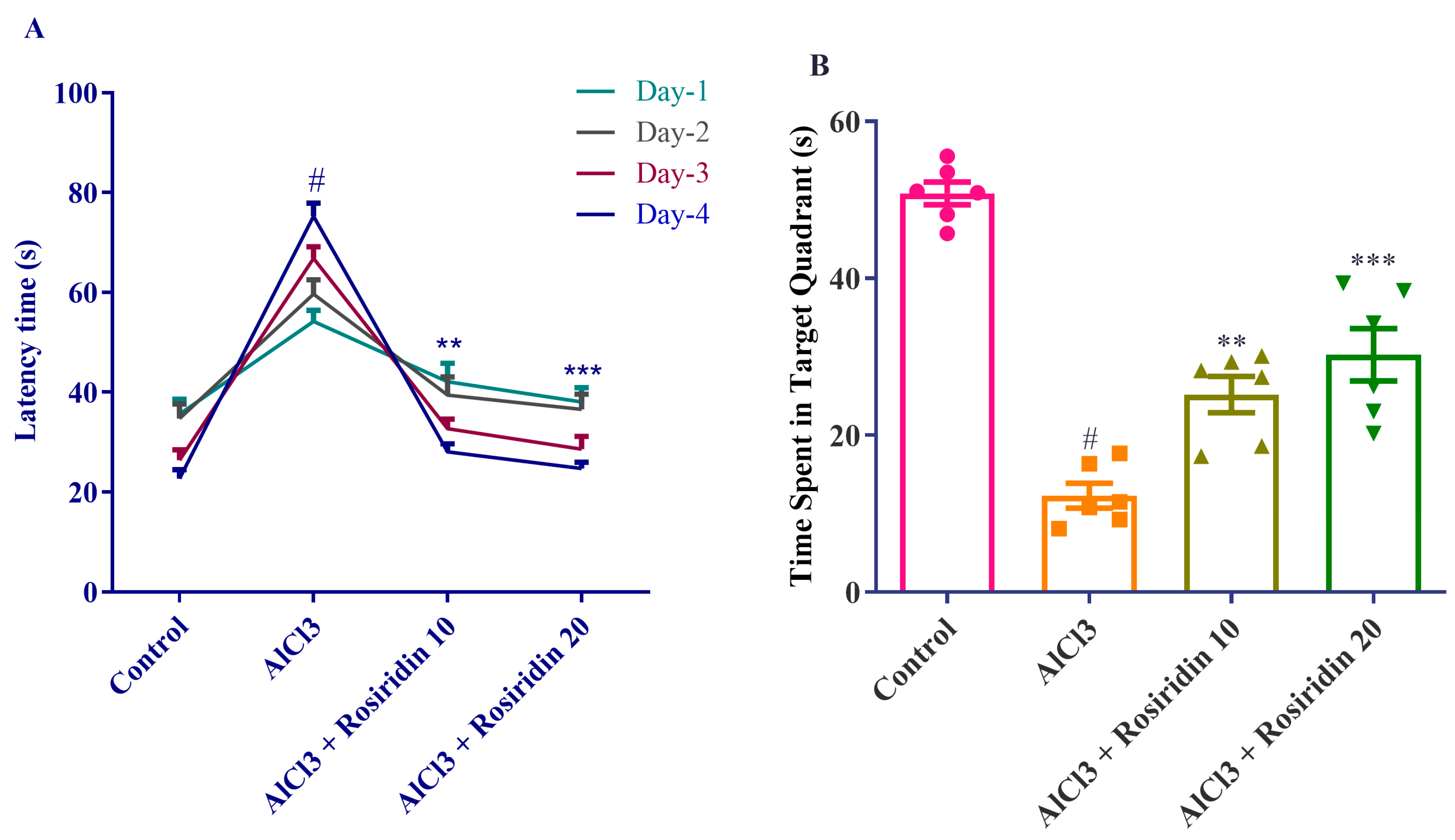
2.4.2. Morris Water Maze (MWM) Test
2.4.3. Open Field Test
2.5. Biochemical Analysis
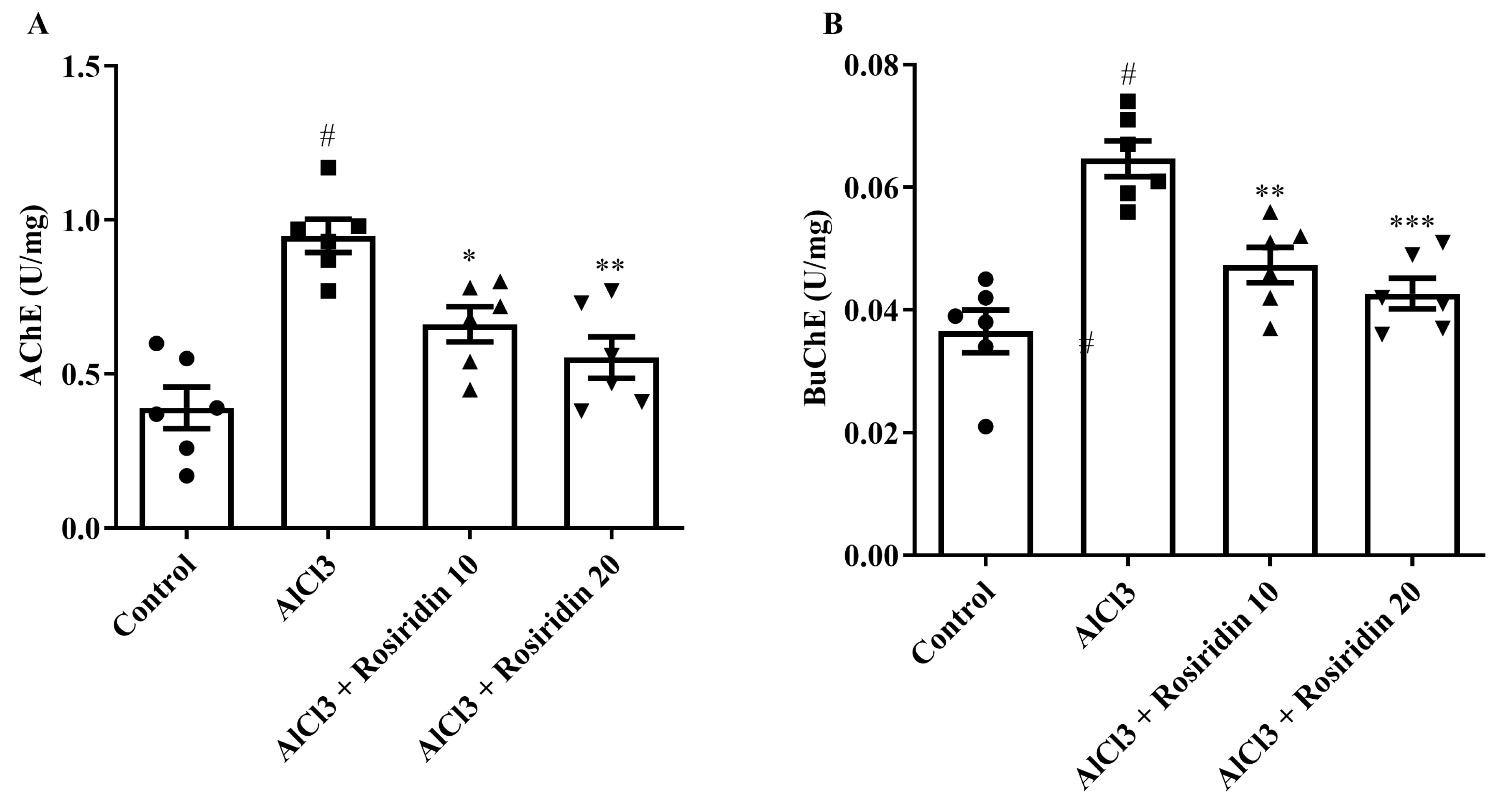
2.5.1. AChE and BuChE
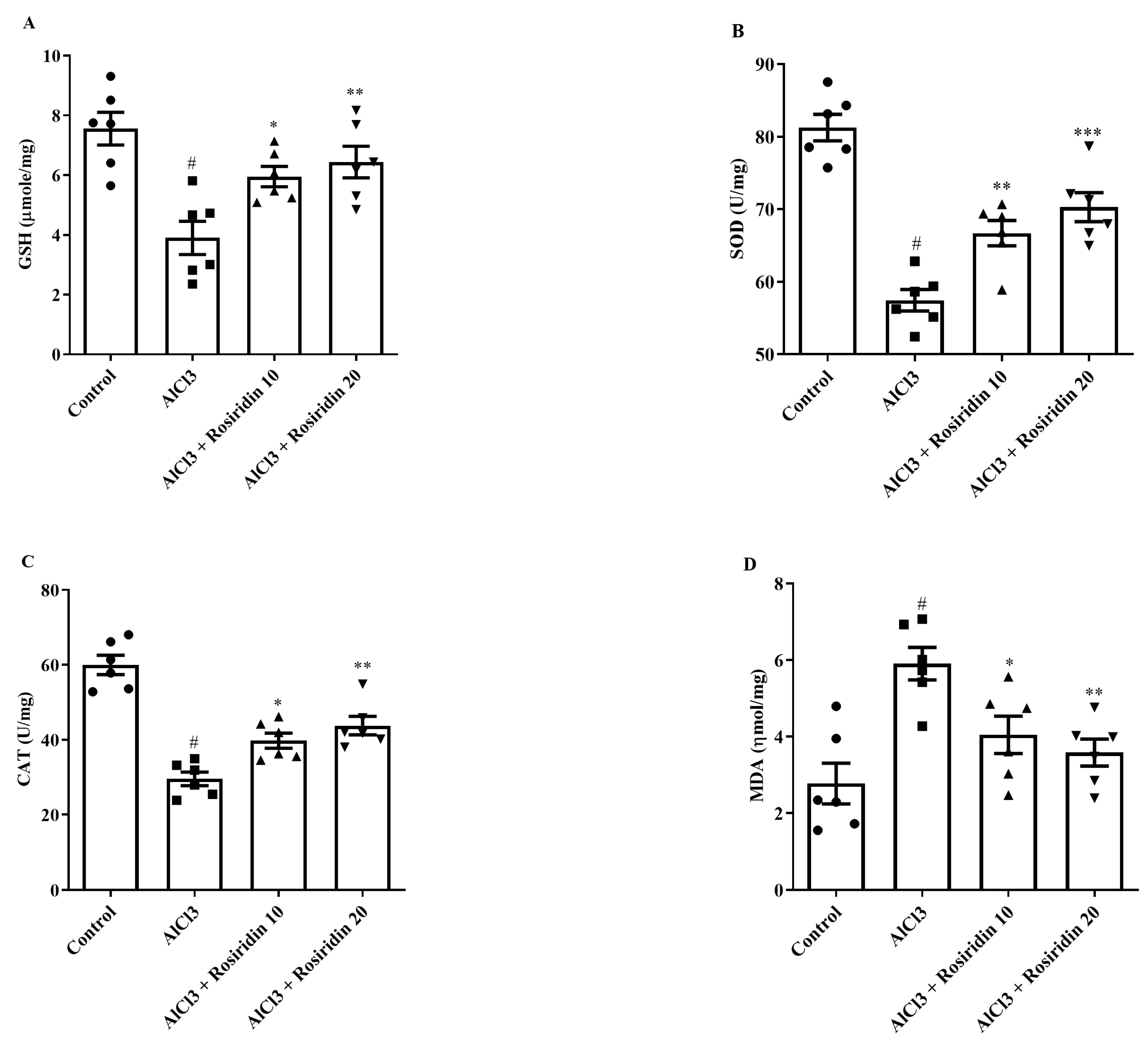
2.5.2. Estimation of MDA, GSH, SOD, and CAT
2.5.3. Determination of Neurotransmitters
2.5.4. Nitric Oxide (NO)
2.5.5. Determination of Inflammatory Mediators
2.5.6. Estimation of BDNF, NFκB, and PI3K/pAkt
2.6. Statistical Assessment
3. Results
3.1. Behavioral Parameters
3.1.1. Y-Maze Test
3.1.2. MWM Test
3.1.3. Open Field Test
3.2. Biochemical Analysis
3.2.1. AChE and BuChE Determination
3.2.2. Estimation of Antioxidant Parameters
3.2.3. Estimation of Dopamine (DA), Serotonin (5-HT), and Ach
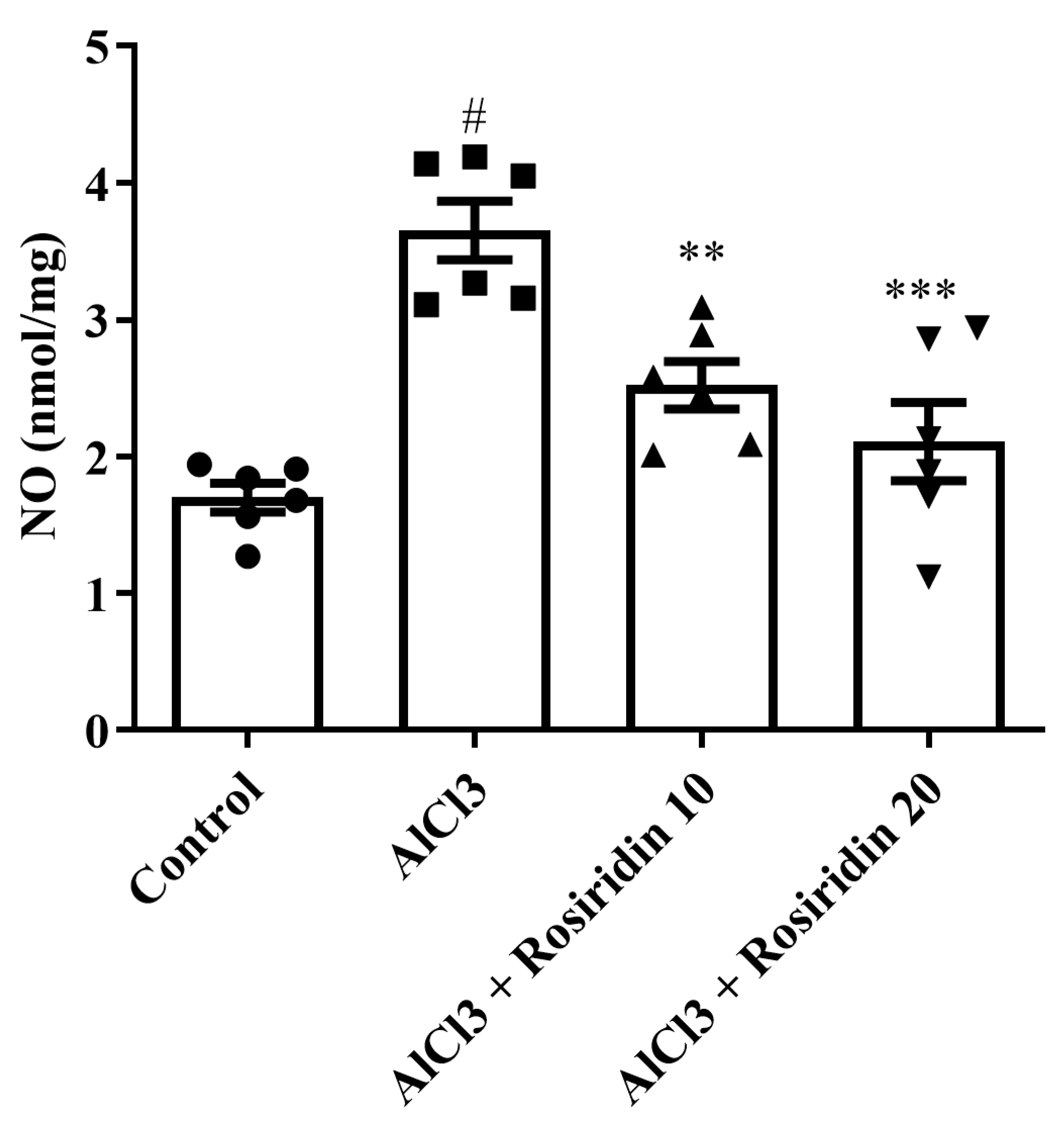
3.2.4. NO Content
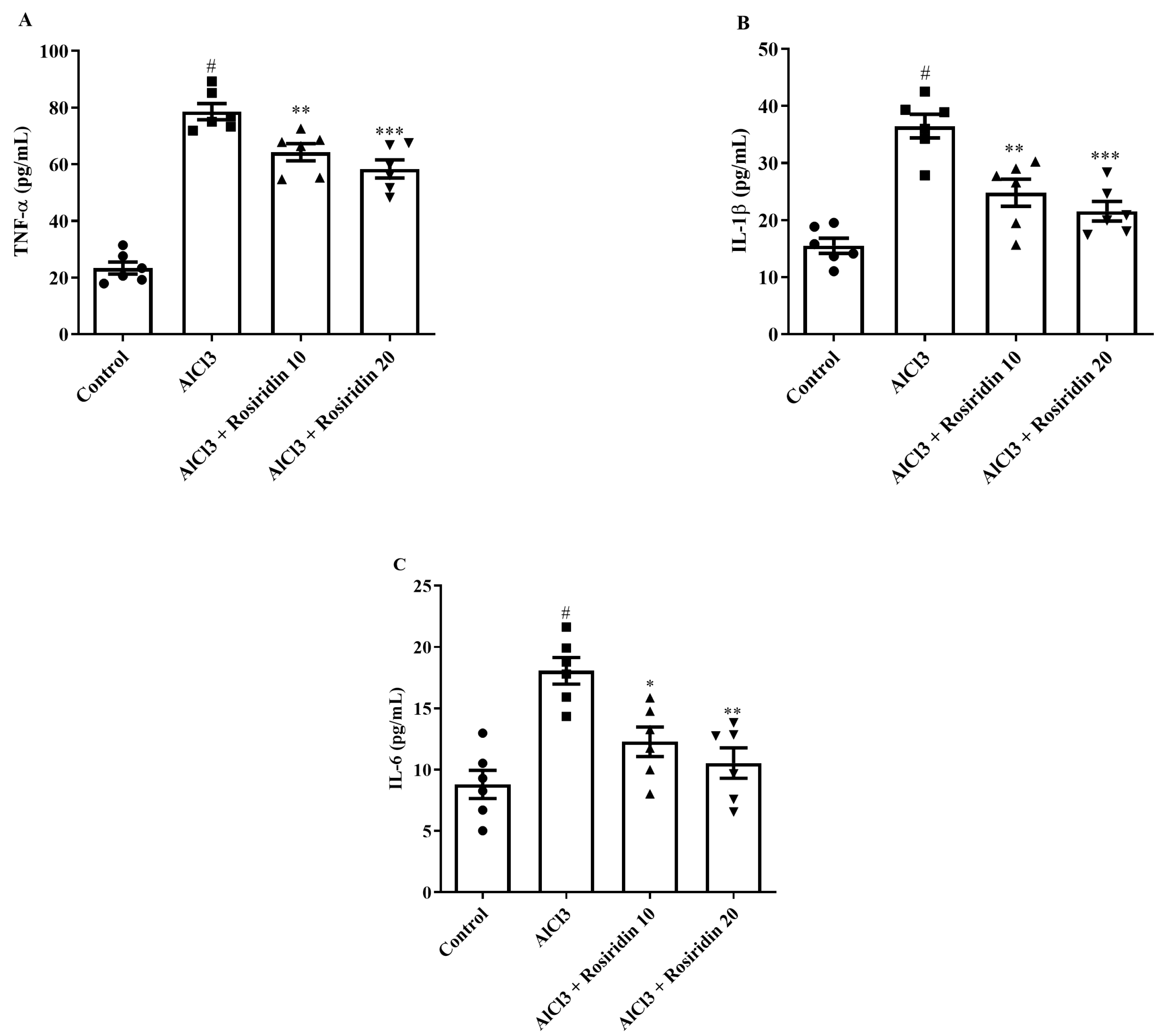
3.2.5. Estimation of Neuroinflammatory Mediators
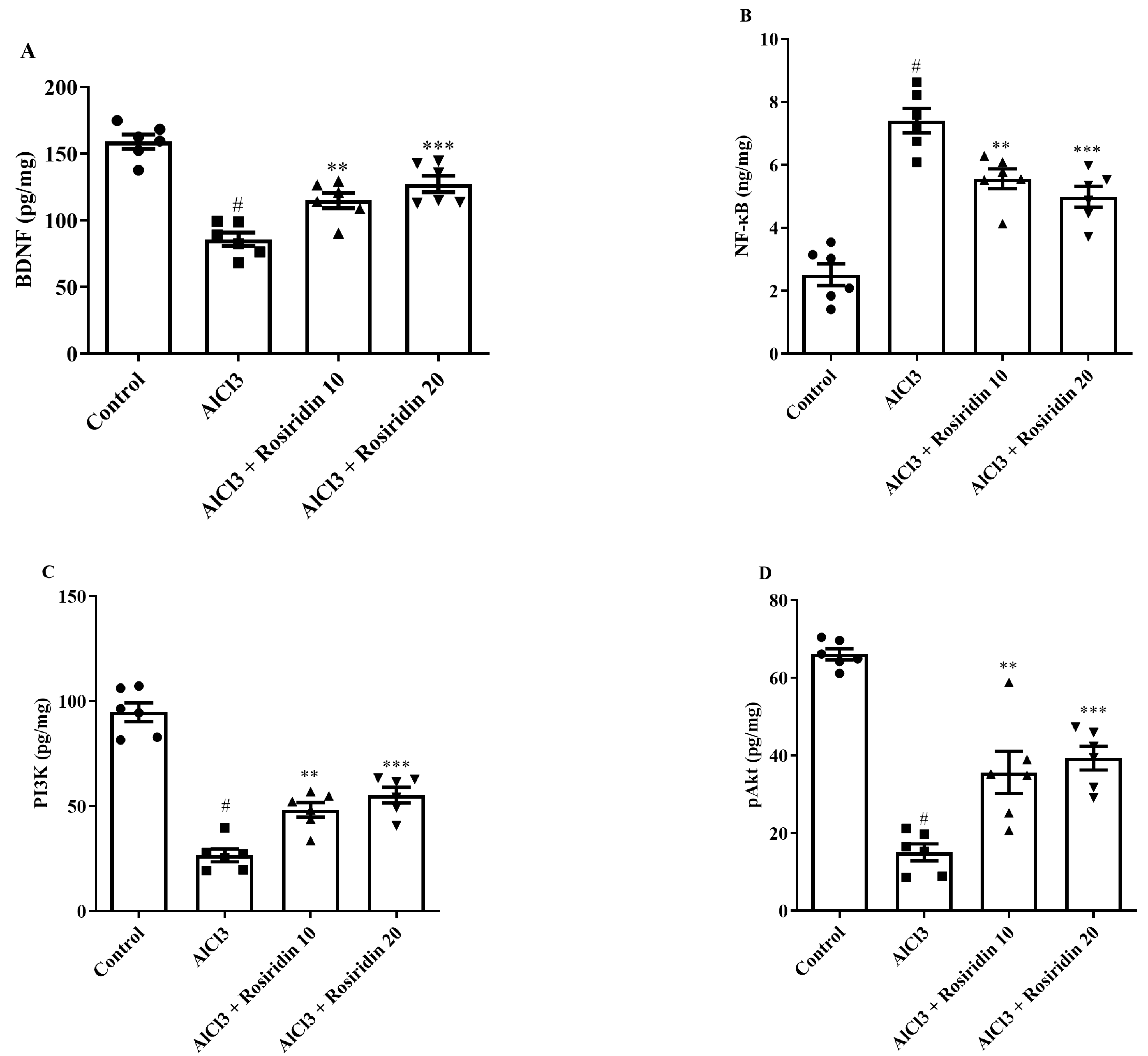
3.2.6. Effect of Rosiridin on the BDNF, PI3K, pAkt, and NF-κB Levels
4. Discussion
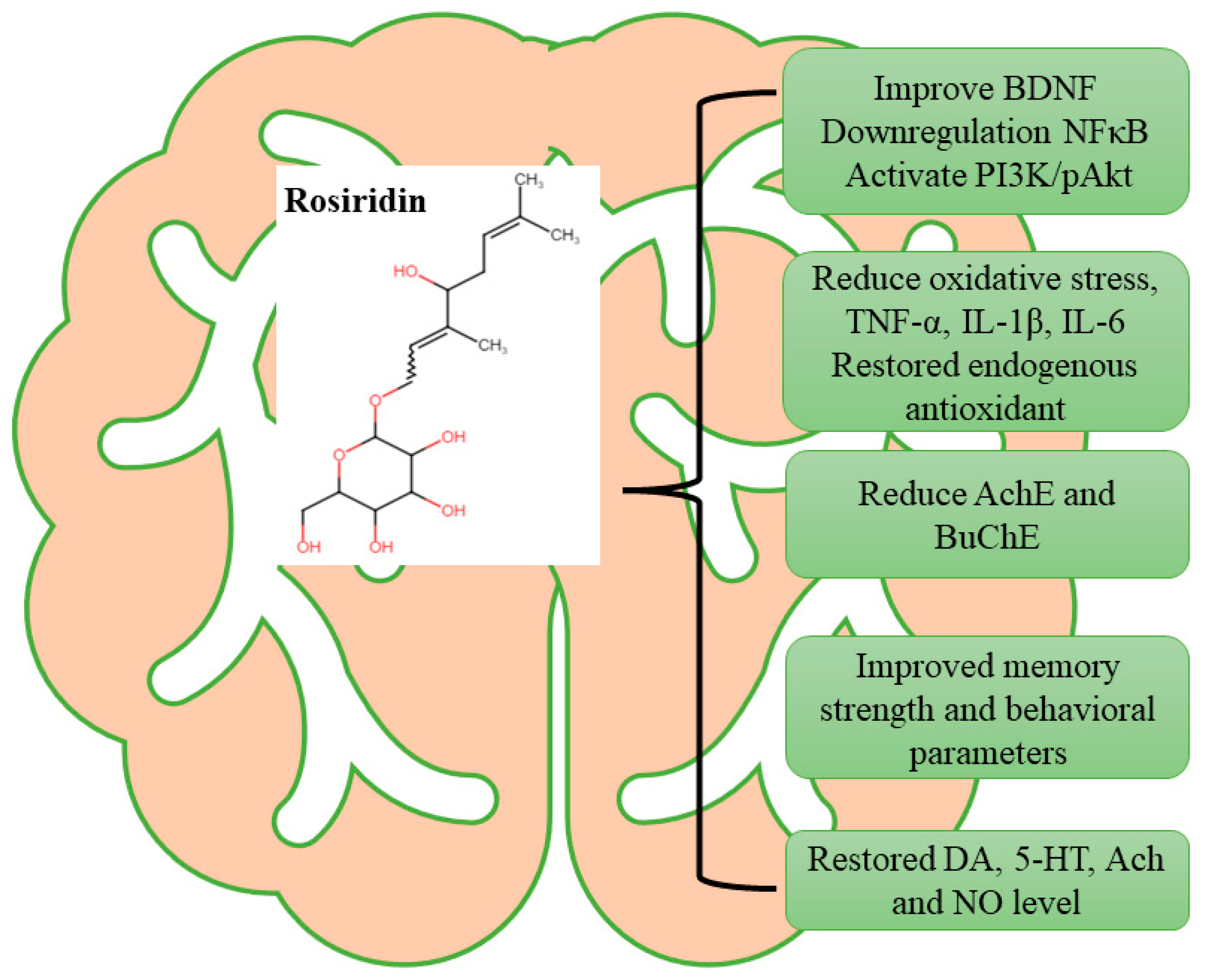
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, W.B.; Choi, H.J.; Kim, J.E.; Park, J.W.; Kang, M.J.; Bae, S.J.; Lee, Y.J.; Choi, Y.S.; Kim, K.S.; Jung, Y.-S.; et al. Comparison of scopolamine-induced cognitive impairment responses in three different ICR stocks. Lab. Anim. Res. 2018, 34, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; Kim, T.W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nature reviews. Neuroscience 2011, 12, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, L.H.; Langa, K.M.; Bynum, J.P.W.; Hsu, J.W. Financial Presentation of Alzheimer Disease and Related Dementias. JAMA Intern. Med. 2021, 181, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Shen, X.; Yu, H.; Sun, L.; Lin, W.; Zhang, C. Water-soluble ginseng oligosaccharides protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent. J. Ginseng Res. 2016, 40, 211–219. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Aykac, A.; Ozbeyli, D.; Uncu, M.; Ertaş, B.; Kılınc, O.; Şen, A.; Orun, O.; Sener, G. Evaluation of the protective effect of Myrtus communis in scopolamine-induced Alzheimer model through cholinergic receptors. Gene 2019, 689, 194–201. [Google Scholar] [CrossRef]
- Jafarian, S.; Ling, K.H.; Hassan, Z.; Perimal-Lewis, L.; Sulaiman, M.R.; Perimal, E.K. Effect of zerumbone on scopolamine-induced memory impairment and anxiety-like behaviours in rats. Alzheimer’s Dement. 2019, 5, 637–643. [Google Scholar] [CrossRef]
- Miu, A.C.; Benga, O. Aluminum and Alzheimer’s disease: A new look. J. Alzheimer’s Dis. 2006, 10, 179–201. [Google Scholar] [CrossRef]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav. Brain Res. 2009, 205, 384–390. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, L.; Sadir, S.; Batool, Z.; Tabassum, S.; Shahzad, S.; Afzal, A.; Haider, S. Acute aluminum chloride toxicity revisited: Study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci. 2019, 217, 202–211. [Google Scholar] [CrossRef] [PubMed]
- ELBini-Dhouib, I.; Doghri, R.; Ellefi, A.; Degrach, I.; Srairi-Abid, N.; Gati, A. Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease. Molecules 2021, 26, 3011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bal, A.; Gill, K.D. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Res. 2008, 1232, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Shur, B.; Kumar, A. Naringin protects memory impairment and mitochondrial oxidative damage against aluminum-induced neurotoxicity in rats. Int. J. Neurosci. 2013, 123, 636–645. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein Ameliorates Scopolamine-Induced Amnesia in Mice Through the Regulation of the Cholinergic Neurotransmission, Antioxidant System and the ERK/CREB/BDNF Signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef]
- Afzal, M.; Alzarea, S.; Qua, A.M.; Kazmi, I.; Zafar, A.; Imam, F.; Al-Harb, N.O.; Alhar, K.S.; Alruwaili, N.K. Boswellic Acid Attenuates Scopolamine-Induced Neurotoxicity and Dementia in Rats: Possible Mechanism of Action. Int. J. Pharmacol. 2021, 17, 499–505. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Mostafa, A.M.; Ashour, M.L.; Elhady, S.S. Neuroprotective Effects of Black Pepper Cold-Pressed Oil on Scopolamine-Induced Oxidative Stress and Memory Impairment in Rats. Antioxidants 2021, 10, 1993. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Muruganathan, U.; Srinivasan, S. Beneficial effect of carvone, a dietary monoterpene ameliorates hyperglycemia by regulating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2016, 84, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Arasteh, E.; Imenshahidi, M.; Iranshahi, M. Antihypertensive effect of auraptene, a monoterpene coumarin from the genus Citrus, upon chronic administration. Iran. J. Basic Med. Sci. 2015, 18, 153–158. [Google Scholar] [PubMed]
- Gonzalez Arbelaez, L.F.; Ciocci Pardo, A.; Fantinelli, J.C.; Rojano, B.; Schinella, G.R.; Mosca, S.M. Isoespintanol, a monoterpene isolated from oxandra cf xylopioides, ameliorates the myocardial ischemia-reperfusion injury by AKT/PKCε/eNOS-dependent pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Kaviyani, N.; Tavakol, S. Monoterpenes modulating autophagy: A review study. Basic Clin. Pharmacol. Toxicol. 2020, 126, 9–20. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chandrasekaran, B.; Namasivayam, N. Effect of geraniol, a plant derived monoterpene on lipids and lipid metabolizing enzymes in experimental hyperlipidemic hamsters. Mol. Cell Biochem. 2015, 398, 39–53. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.M.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Ganjewala, D.; Gupta, A.K.; Muhury, R. An Update on Bioactive Potential of a Monoterpene Aldehyde Citral. J. Biol. Act. Prod. Nat. 2012, 2, 186–199. [Google Scholar] [CrossRef]
- Saleem, S.; Naqvi, F.; Batool, A.; Naqvi, S.H.; Naqvi, F.; Batool, Z.; Tabassum, S.; Haider, S. Neuroprotective role of a monoterpene (thymol) on diazepam induced withdrawal symptoms in rats. Pak. J. Pharm. Sci. 2021, 34, 1615–1621. [Google Scholar]
- Javed, H.; Azimullah, S.; Meeran, M.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Kasprzak-Drozd, K.; Oniszczuk, A.; Borowicz-Reutt, K. Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies—A Non-Systematic Review. Int. J. Mol. Sci. 2021, 22, 7366. [Google Scholar] [CrossRef] [PubMed]
- Van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.-A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress-Protective Activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Yue, L.; Dang, X.; Chen, F.; Gong, Y.; Lin, X.; Luo, Y. Rosenroot (Rhodiola): Potential Applications in Aging-related Diseases. Aging Dis. 2019, 10, 134–146. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, Y.A.; Park, H.M.; Toh, S.H.; Lee, E.J.; Jang, H.D.; Kim, Y.H. Antioxidative phenolic compounds from the roots of Rhodiola sachalinensis A. Bor. Arch. Pharmacal Res. 2000, 23, 455–458. [Google Scholar] [CrossRef]
- Linh, P.T.; Kim, Y.H.; Hong, S.P.; Jian, J.J.; Kang, J.S. Quantitative determination of salidroside and tyrosol from the underground part of Rhodiola rosea by high performance liquid chromatography. Arch. Pharmacal Res. 2000, 23, 349–352. [Google Scholar] [CrossRef]
- Bol’shakova, I.V.; Lozovskaia, E.L.; Sapezhinskiĭ, I.I. Antioxidant properties of a series of extracts from medicinal plants. Biofizika 1997, 42, 480–483. [Google Scholar]
- Afzal, M.; Alzarea, S.I.; Alharbi, K.S.; Alzarea, A.I.; Alenezi, S.K.; Alshammari, M.S.; Alquraini, A.H.; Kazmi, I. Rosiridin Attenuates Scopolamine-Induced Cognitive Impairments in Rats via Inhibition of Oxidative and Nitrative Stress Leaded Caspase-3/9 and TNF-α Signaling Pathways. Molecules 2022, 27, 5888. [Google Scholar] [CrossRef]
- Afzal, M.; Sayyed, N.; Alharbi, K.S.; Alzarea, S.I.; Alshammari, M.S.; Alomar, F.A.; Alenezi, S.K.; Quazi, A.M.; Alzarea, A.I.; Kazmi, I. Anti-Huntington Effect of Rosiridin via Oxidative Stress/AchE Inhibition and Modulation of Succinate Dehydrogenase, Nitrite, and BDNF Levels against 3-Nitropropionic Acid in Rodents. Biomolecules 2022, 12, 1023. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; AlGhamdi, S.A.; Alghamdi, A.M.; Zeyadi, M.; Sheikh, R.A.; Gupta, G.; Sayyed, N. Influence of rosiridin on streptozotocin-induced diabetes in rodents through endogenous antioxidants-inflammatory cytokines pathway and molecular docking study. J. Biomol. Struct. Dyn. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abdelazem, H. Effect of Moringa oleifera on antioxidant enzymes and oxidative stress induced by aluminium exposure in male albino rat testes. Int. J. Cancer Biomed. Res. 2019, 3, 34–41. [Google Scholar] [CrossRef]
- Chacko, A.; Ittiyavirah, S.P. Neuroprotective effect of against aluminium-induced Gracilaria corticata neurotoxicity in the hippocampus and cerebral cortex of rat brain: Biochemical and histological approach. Asian J. Pharm. Pharmacol. 2019, 5, 604–613. [Google Scholar] [CrossRef]
- Galeano, P.; Martino Adami, P.V.; Do Carmo, S.; Blanco, E.; Rotondaro, C.; Capani, F.; Castaño, E.M.; Cuello, A.C.; Morelli, L. Longitudinal analysis of the behavioral phenotype in a novel transgenic rat model of early stages of Alzheimer’s disease. Front. Behav. Neurosci. 2014, 8, 321. [Google Scholar] [CrossRef]
- Morris, R.G.M.; Garrud, P.; Rawlins, J.N.; O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 1982, 297, 681–683. [Google Scholar] [CrossRef]
- Tiwari, V.; Kuhad, A.; Bishnoi, M.; Chopra, K. Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative–nitrosative stress in rats. Pharmacol. Biochem. Behav. 2009, 93, 183–189. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Tawari, S.; Patil, S.; Dixit, P.; Umathe, S.; Mundhada, D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011, 220, 30–41. [Google Scholar] [CrossRef]
- McNamara, R.K.; Skelton, R.W. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res. Rev. 1993, 18, 33–49. [Google Scholar] [CrossRef]
- Rafeeq, M.; Al-Abbasi, F.A.; Afzal, M.; Moglad, E.; Al-Qahtani, S.D.; Alzrea, S.I.; Almalki, N.A.; Imam, F.; Sayyed, N.; Kazmi, I. 6-Shogaol Abrogates Parkinson’s Disease in Rotenone-Induced Rodents: Based on In Silico Study and Inhibiting TNF-α/NF-κB/IL-1β/MAO-B. Pharmaceuticals 2024, 17, 1348. [Google Scholar] [CrossRef]
- Li, H.-Q.; Ip, S.-P.; Zheng, G.-Q.; Xian, Y.-F.; Lin, Z.-X. Isorhynchophylline alleviates learning and memory impairments induced by aluminum chloride in mice. Chin. Med. 2018, 13, 29. [Google Scholar] [CrossRef]
- Janeczek, M.; Gefen, T.; Samimi, M.; Kim, G.; Weintraub, S.; Bigio, E.; Rogalski, E.; Mesulam, M.-M.; Geula, C. Variations in acetylcholinesterase activity within human cortical pyramidal neurons across age and cognitive trajectories. Cereb. Cortex 2018, 28, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Nagakannan, P.; Shivasharan, B.; Thippeswamy, B.; Veerapur, V. Restoration of brain antioxidant status by hydroalcoholic extract of Mimusops elengi flowers in rats treated with monosodium glutamate. J. Environ. Pathol. Toxicol. Oncol. 2012, 31, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, R.A.; Afzal, M.; Al-Abbasi, F.A.; Bukhari, H.A.; Almalki, N.A.; Alqurashi, M.M.; Imam, F.; Sayyed, N.; Kazmi, I. Europinidin Attenuates Methamphetamine-induced Learning and Memory Impairments and Hippocampal Alterations in Rodents: Based on Molecular Docking through a Mechanism of Neuromodulatory Cytokines/Caspases-3/CREB/BDNF Pathway. Curr. Med. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Moshage, H.; Kok, B.; Huizenga, J.R.; Jansen, P.L. Nitrite and nitrate determinations in plasma: A critical evaluation. Clin. Chem. 1995, 41, 892–896. [Google Scholar] [CrossRef]
- Al-Amin, M.M.; Reza, H.M.; Saadi, H.M.; Mahmud, W.; Ibrahim, A.A.; Alam, M.M.; Kabir, N.; Saifullah, A.R.M.; Tropa, S.T.; Ruhul Quddus, A.H.M. Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Eur. J. Pharmacol. 2016, 777, 60–69. [Google Scholar] [CrossRef]
- Bawadood, A.S.; Afzal, M.; Tanko, A.I.; Al-Abbasi, F.A.; Zeyadi, M.; Alqurashi, M.M.; Sheikh, R.A.; Alzarea, S.I.; Sayyed, N.; Kazmi, I. Malvidin Improves Ethanol-Induced Memory Impairment by Targeting Cholinesterase, FRAP, and GABA Signaling: Based on Molecular Docking Study. J. Biol. Regul. Homeost. Agents 2024, 38, 2165–2179. [Google Scholar]
- Awad, H.H.; Desouky, M.A.; Zidan, A.; Bassem, M.; Qasem, A.; Farouk, M.; AlDeab, H.; Fouad, M.; Hany, C.; Basem, N.; et al. Neuromodulatory effect of vardenafil on aluminium chloride/D-galactose induced Alzheimer’s disease in rats: Emphasis on amyloid-beta, p-tau, PI3K/Akt/p53 pathway, endoplasmic reticulum stress, and cellular senescence. Inflammopharmacology 2023, 31, 2653–2673. [Google Scholar] [CrossRef]
- Kumar, V.; Gill, K.D. Aluminium neurotoxicity: Neurobehavioural and oxidative aspects. Arch. Toxicol. 2009, 83, 965–978. [Google Scholar] [CrossRef]
- Klotz, K.; Weistenhöfer, W.; Neff, F.; Hartwig, A.; van Thriel, C.; Drexler, H. The health effects of aluminum exposure. Dtsch. Ärzteblatt Int. 2017, 114, 653. [Google Scholar] [CrossRef]
- Aly, H.; Elrigal, N.; Ali, S.; Rizk, M.Z.; Ebrahim, N.A. Modulatory effects of Casimiroa edulis on aluminium nanoparticles-associated neurotoxicity in a rat model of induced Alzheimer’s disease. J. Mater. Environ. Sci. 2018, 9, 1931–1941. [Google Scholar]
- Auti, S.T.; Kulkarni, Y.A. Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front. Neurol. 2019, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Azib, L.; Debbache-Benaida, N.; Da Costa, G.; Atmani-Kilani, D.; Saidene, N.; Ayouni, K.; Richard, T.; Atmani, D. Pistacia lentiscus L. leaves extract and its major phenolic compounds reverse aluminium-induced neurotoxicity in mice. Ind. Crops Prod. 2019, 137, 576–584. [Google Scholar]
- Sun, B.; Spranger, I.; Yang, J.; Leandro, C.; Guo, L.; Canário, S.; Zhao, Y.; Wu, C. Red wine phenolic complexes and their in vitro antioxidant activity. J. Agric. Food Chem. 2009, 57, 8623–8627. [Google Scholar] [CrossRef]
- Speer, H.; D’cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health—A focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Luo, H.; Ding, L.; Jiang, X.; Li, X.; Jiao, R.; Bai, W. Comparative study on the stability and antioxidant activity of six pyranoanthocyanins based on malvidin-3-glucoside. J. Agric. Food Chem. 2020, 68, 2783–2794. [Google Scholar] [CrossRef]
- Zhao, Y.; Dang, M.; Zhang, W.; Lei, Y.; Ramesh, T.; Veeraraghavan, V.P.; Hou, X. Neuroprotective effects of Syringic acid against aluminium chloride induced oxidative stress mediated neuroinflammation in rat model of Alzheimer’s disease. J. Funct. Foods 2020, 71, 104009. [Google Scholar] [CrossRef]
- Kazmi, I.; Afzal, M.; Imam, F.; Alzarea, S.I.; Patil, S.; Mhaiskar, A.; Shah, U.; Almalki, W.H. Barbaloin’s Chemical Intervention in Aluminum Chloride Induced Cognitive Deficits and Changes in Rats through Modulation of Oxidative Stress, Cytokines, and BDNF Expression. ACS Omega 2024, 9, 6976–6985. [Google Scholar] [CrossRef]
- Firdaus, Z.; Kumar, D.; Singh, S.K.; Singh, T.D. Centella asiatica Alleviates AlCl3-induced Cognitive Impairment, Oxidative Stress, and Neurodegeneration by Modulating Cholinergic Activity and Oxidative Burden in Rat Brain. Biol. Trace Elem. Res. 2022, 200, 5115–5126. [Google Scholar] [CrossRef]
- Thenmozhi, A.J.; Raja, T.R.W.; Janakiraman, U.; Manivasagam, T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res. 2015, 40, 767–776. [Google Scholar] [CrossRef]
- Al-Otaibi, S.S.; Arafah, M.M.; Sharma, B.; Alhomida, A.S.; Siddiqi, N.J. Synergistic effect of quercetin and α-lipoic acid on aluminium chloride induced neurotoxicity in rats. J. Toxicol. 2018, 2018, 2817036. [Google Scholar] [CrossRef] [PubMed]
- Kasbe, P.; Jangra, A.; Lahkar, M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J. Trace Elem. Med. Biol. 2015, 31, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Aouey, B.; Kebieche, M.; Fetoui, H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chem. Biol. Interact. 2015, 239, 76–86. [Google Scholar] [CrossRef]
- Bais, S.; Kumari, R.; Prashar, Y. Ameliorative effect of trans-sinapic acid and its protective role in cerebral hypoxia in aluminium chloride induced dementia of alzheimer’s type. CNS Neurol. Disord.-Drug Targets 2018, 17, 144–154. [Google Scholar] [CrossRef]
- Alghamdi, B.S.A. Possible prophylactic anti-excitotoxic and anti-oxidant effects of virgin coconut oil on aluminium chloride-induced Alzheimer’s in rat models. J. Integr. Neurosci. 2018, 17, 593–607. [Google Scholar] [CrossRef]
- Zou, J.; Cai, P.-s.; Xiong, C.-m.; Ruan, J.-L. Neuroprotective effect of peptides extracted from walnut (Juglans Sigilata Dode) proteins on Aβ25-35-induced memory impairment in mice. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2016, 36, 21–30. [Google Scholar] [CrossRef]
- Jangra, A.; Kasbe, P.; Pandey, S.N.; Dwivedi, S.; Gurjar, S.S.; Kwatra, M.; Mishra, M.; Venu, A.K.; Sulakhiya, K.; Gogoi, R.; et al. Hesperidin and silibinin ameliorate aluminum-induced neurotoxicity: Modulation of antioxidants and inflammatory cytokines level in mice hippocampus. Biol. Trace Elem. Res. 2015, 168, 462–471. [Google Scholar] [CrossRef]
- Yu, L.; Zhai, Q.; Tian, F.; Liu, X.; Wang, G.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Lactobacillus plantarum CCFM639 can prevent aluminium-induced neural injuries and abnormal behaviour in mice. J. Funct. Foods 2017, 30, 142–150. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Chenodeoxycholic Acid Ameliorates AlCl3-Induced Alzheimer’s Disease Neurotoxicity and Cognitive Deterioration via Enhanced Insulin Signaling in Rats. Molecules 2019, 24, 1992. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, S.S.; Afzal, M.; Alharbi, K.S.; Alenezi, S.K.; Alsahli, T.G.; Zaidi, S.; Altyar, A.E.; Ghaboura, N.; Kazmi, I.; Mantargi, M.J.S.; et al. Rosiridin Protects Against Aluminum Chloride-Induced Memory Impairment via Modulation of BDNF/NFκB/PI3K/Akt Pathway in Rats. Medicina 2024, 60, 1812. https://doi.org/10.3390/medicina60111812
Alqarni SS, Afzal M, Alharbi KS, Alenezi SK, Alsahli TG, Zaidi S, Altyar AE, Ghaboura N, Kazmi I, Mantargi MJS, et al. Rosiridin Protects Against Aluminum Chloride-Induced Memory Impairment via Modulation of BDNF/NFκB/PI3K/Akt Pathway in Rats. Medicina. 2024; 60(11):1812. https://doi.org/10.3390/medicina60111812
Chicago/Turabian StyleAlqarni, Sana Saeed, Muhammad Afzal, Khalid Saad Alharbi, Sattam Khulaif Alenezi, Tariq G. Alsahli, Shafqat Zaidi, Ahmed Essam Altyar, Nehmat Ghaboura, Imran Kazmi, Mohammad Jaffar Sadiq Mantargi, and et al. 2024. "Rosiridin Protects Against Aluminum Chloride-Induced Memory Impairment via Modulation of BDNF/NFκB/PI3K/Akt Pathway in Rats" Medicina 60, no. 11: 1812. https://doi.org/10.3390/medicina60111812
APA StyleAlqarni, S. S., Afzal, M., Alharbi, K. S., Alenezi, S. K., Alsahli, T. G., Zaidi, S., Altyar, A. E., Ghaboura, N., Kazmi, I., Mantargi, M. J. S., & Imam, F. (2024). Rosiridin Protects Against Aluminum Chloride-Induced Memory Impairment via Modulation of BDNF/NFκB/PI3K/Akt Pathway in Rats. Medicina, 60(11), 1812. https://doi.org/10.3390/medicina60111812






