Predictive Value of NT-proBNP, FGF21, Galectin-3 and Copeptin in Advanced Heart Failure in Patients with Preserved and Mildly Reduced Ejection Fraction and Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Characteristics of the Groups
2.2. Biomarker Analysis
2.3. Echocardiographic Assessment
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Studied Sample of T2DM Patients
3.2. Correlations Between FGF21, NT-proBNP, Galectin-3 and Copeptin with Clinical and Echocardiographic Features
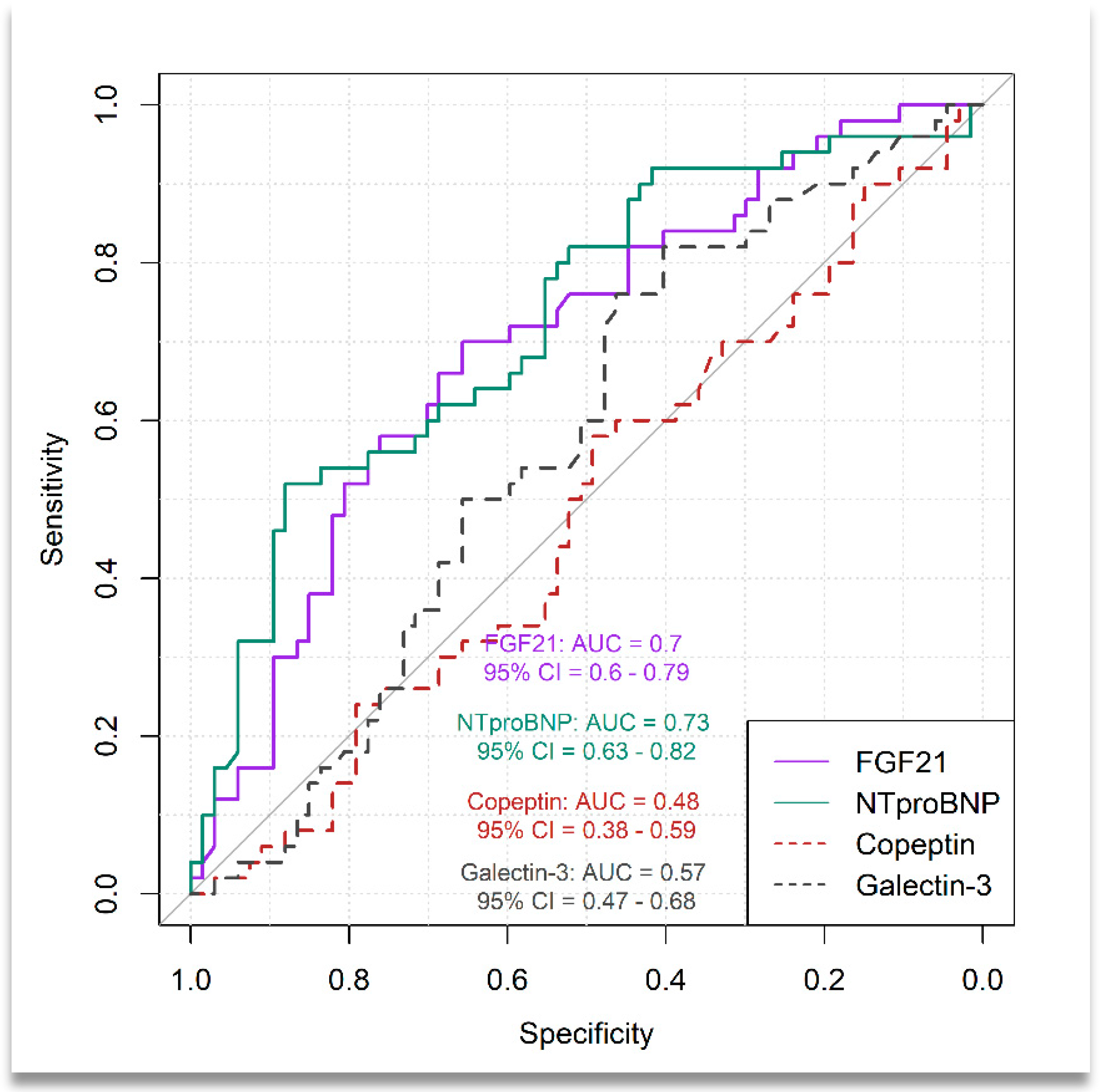
3.3. Distinguishing Between Patients with Moderate-to-Severe HF and Those with Mild HF
3.4. FGF21 and NT-proBNP as Independent Predictors for Advanced HF in T2DM Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Claude Mbanya, J.; et al. Erratum to “IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045” [Diabetes Res. Clin. Pract. 183 (2022) 109119]. Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to Diagnose Heart Failure with Preserved Ejection Fraction: The HFA-PEFF Diagnostic Algorithm: A Consensus Recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Ghilencea, L.-N.; Bejan, G.-C.; Zamfirescu, M.-B.; Stănescu, A.M.A.; Matei, L.-L.; Manea, L.-M.; Kilic, I.D.; Bălănescu, S.-M.; Popescu, A.-C.; Myerson, S.G. B-Type Natriuretic Peptide at Admission Is a Predictor of All-Cause Mortality at One Year after the First Acute Episode of New-Onset Heart Failure with Preserved Ejection Fraction. J. Pers. Med. 2022, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the Management of Cardiovascular Disease in Patients with Diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Hoek, A.G.; Dal Canto, E.; Wenker, E.; Bindraban, N.; Handoko, M.L.; Elders, P.J.M.; Beulens, J.W.J. Epidemiology of Heart Failure in Diabetes: A Disease in Disguise. Diabetologia 2024, 67, 574–601. [Google Scholar] [CrossRef]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; Ruijter, H.M.D.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The Prevalence of Left Ventricular Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction in Men and Women with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef]
- Bouthoorn, S.; Gohar, A.; Valstar, G.; den Ruijter, H.M.; Reitsma, J.B.; Hoes, A.W.; Rutten, F.H.; den Ruijter, H.M.; Rutten, F.H.; Hoes, A.W.; et al. Prevalence of Left Ventricular Systolic Dysfunction and Heart Failure with Reduced Ejection Fraction in Men and Women with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2018, 17, 58. [Google Scholar] [CrossRef]
- Lindman, B.R.; Dávila-Román, V.G.; Mann, D.L.; McNulty, S.; Semigran, M.J.; Lewis, G.D.; de las Fuentes, L.; Joseph, S.M.; Vader, J.; Hernandez, A.F.; et al. Cardiovascular Phenotype in HFpEF Patients with or without Diabetes: A RELAX Trial Ancillary Study. J. Am. Coll. Cardiol. 2014, 64, 541–549. [Google Scholar] [CrossRef]
- Lejeune, S.; Roy, C.; Slimani, A.; Pasquet, A.; Vancraeynest, D.; Vanoverschelde, J.-L.; Gerber, B.L.; Beauloye, C.; Pouleur, A.-C. Diabetic Phenotype and Prognosis of Patients with Heart Failure and Preserved Ejection Fraction in a Real Life Cohort. Cardiovasc. Diabetol. 2021, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Nikorowitsch, J.; Bei der Kellen, R.; Kirchhof, P.; Magnussen, C.; Jagodzinski, A.; Schnabel, R.B.; Blankenberg, S.; Wenzel, J.-P. Applying the ESC 2016, H2 FPEF, and HFA-PEFF Diagnostic Algorithms for Heart Failure with Preserved Ejection Fraction to the General Population. ESC Heart Fail. 2021, 8, 3603–3612. [Google Scholar] [CrossRef]
- Selvaraj, S.; Myhre, P.L.; Vaduganathan, M.; Claggett, B.L.; Matsushita, K.; Kitzman, D.W.; Borlaug, B.A.; Shah, A.M.; Solomon, S.D. Application of Diagnostic Algorithms for Heart Failure with Preserved Ejection Fraction to the Community. JACC Heart Fail. 2020, 8, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Sanders-van Wijk, S.; Barandiarán Aizpurua, A.; Brunner-La Rocca, H.-P.; Henkens, M.T.H.M.; Weerts, J.; Knackstedt, C.; Uszko-Lencer, N.; Heymans, S.; van Empel, V. The HFA-PEFF and H2 FPEF Scores Largely Disagree in Classifying Patients with Suspected Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2021, 23, 838–840. [Google Scholar] [CrossRef]
- Huis In ’t Veld, A.E.; de Man, F.S.; van Rossum, A.C.; Handoko, M.L. How to Diagnose Heart Failure with Preserved Ejection Fraction: The Value of Invasive Stress Testing. Neth. Heart J. 2016, 24, 244–251. [Google Scholar] [CrossRef]
- van de Bovenkamp, A.A.; Wijkstra, N.; Oosterveer, F.P.T.; Vonk Noordegraaf, A.; Bogaard, H.J.; van Rossum, A.C.; de Man, F.S.; Borlaug, B.A.; Handoko, M.L. The Value of Passive Leg Raise During Right Heart Catheterization in Diagnosing Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, e008935. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.P.; Redfield, M.M. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2010, 3, 588–595. [Google Scholar] [CrossRef]
- Tsutsui, H.; Albert, N.M.; Coats, A.J.S.; Anker, S.D.; Bayes-Genis, A.; Butler, J.; Chioncel, O.; Defilippi, C.R.; Drazner, M.H.; Felker, G.M.; et al. Natriuretic Peptides: Role in the Diagnosis and Management of Heart Failure: A Scientific Statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur. J. Heart Fail. 2023, 25, 616–631. [Google Scholar] [CrossRef]
- Remmelzwaal, S.; van Ballegooijen, A.J.; Schoonmade, L.J.; Dal Canto, E.; Handoko, M.L.; Henkens, M.T.H.M.; van Empel, V.; Heymans, S.R.B.; Beulens, J.W.J. Natriuretic Peptides for the Detection of Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction—A Systematic Review and Meta-Analysis. BMC Med. 2020, 18, 290. [Google Scholar] [CrossRef]
- Meijers, W.C.; Hoekstra, T.; Jaarsma, T.; van Veldhuisen, D.J.; de Boer, R.A. Patients with Heart Failure with Preserved Ejection Fraction and Low Levels of Natriuretic Peptides. Neth. Heart J. 2016, 24, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.V.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure with Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Vázquez-Carrera, M. Emerging Actors in Diabetic Cardiomyopathy: Heartbreaker Biomarkers or Therapeutic Targets? Trends Pharmacol. Sci. 2018, 39, 452–467. [Google Scholar] [CrossRef]
- Deng, J.; Yan, F.; Tian, J.; Qiao, A.; Yan, D. Potential Clinical Biomarkers and Perspectives in Diabetic Cardiomyopathy. Diabetol. Metab. Syndr. 2023, 15, 35. [Google Scholar] [CrossRef]
- Kirk, J.A.; Frangogiannis, N.G. Galectin-3 in the Pathogenesis of Heart Failure: A Causative Mediator or Simply a Biomarker? Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1256–H1258. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.-T.; de Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Chou, R.-H.; Huang, P.-H.; Hsu, C.-Y.; Chang, C.-C.; Leu, H.-B.; Huang, C.-C.; Chen, J.-W.; Lin, S.-J. Circulating Fibroblast Growth Factor 21 Is Associated with Diastolic Dysfunction in Heart Failure Patients with Preserved Ejection Fraction. Sci. Rep. 2016, 6, 33953. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Xu, X.; Tang, F.; Yi, P.; Qiu, B.; Hao, Y. A Review of Fibroblast Growth Factor 21 in Diabetic Cardiomyopathy. Heart Fail. Rev. 2019, 24, 1005–1017. [Google Scholar] [CrossRef]
- Mäkelä, J.; Tselykh, T.V.; Maiorana, F.; Eriksson, O.; Do, H.T.; Mudò, G.; Korhonen, L.T.; Belluardo, N.; Lindholm, D. Fibroblast Growth Factor-21 Enhances Mitochondrial Functions and Increases the Activity of PGC-1α in Human Dopaminergic Neurons via Sirtuin-1. Springerplus 2014, 3, 2. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, R.; Yan, L.; Lin, M.; Liu, X.; You, T. Copeptin in Heart Failure: Review and Meta-Analysis. Clin. Chim. Acta 2017, 475, 36–43. [Google Scholar] [CrossRef]
- Zimodro, J.M.; Gasecka, A.; Jaguszewski, M.; Amanowicz, S.; Szkiela, M.; Denegri, A.; Pruc, M.; Duchnowski, P.; Peacock, F.W.; Rafique, Z.; et al. Role of Copeptin in Diagnosis and Outcome Prediction in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Biomarkers 2022, 27, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Zile, M.; Pieske, B.; Voors, A.; Shah, A.; Kraigher-Krainer, E.; Shi, V.; Bransford, T.; Takeuchi, M.; Gong, J.; et al. The Angiotensin Receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction: A Phase 2 Double-Blind Randomised Controlled Trial. Lancet 2012, 380, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Ward, J.H.; Hernandez, A.F.; Lepage, S.; Morrow, D.A.; Sarwat, S.; Sharma, K.; Solomon, S.D.; Starling, R.C.; Velazquez, E.J.; et al. Rationale, Design and Baseline Characteristics of the PARAGLIDE-HF Trial: Sacubitril/Valsartan vs Valsartan in HFmrEF and HFpEF with a Worsening Heart Failure Event. J. Card. Fail. 2023, 29, 922–930. [Google Scholar] [CrossRef]
- Zamora, E.; Lupón, J.; Enjuanes, C.; Pascual-Figal, D.; de Antonio, M.; Domingo, M.; Comín-Colet, J.; Vila, J.; Peñafiel, J.; Farré, N.; et al. No Benefit from the Obesity Paradox for Diabetic Patients with Heart Failure. Eur. J. Heart Fail. 2016, 18, 851–858. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction in Patients with Normal Natriuretic Peptide Levels Is Associated with Increased Morbidity and Mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef]
- Madamanchi, C.; Alhosaini, H.; Sumida, A.; Runge, M.S. Obesity and Natriuretic Peptides, BNP and NT-proBNP: Mechanisms and Diagnostic Implications for Heart Failure. Int. J. Cardiol. 2014, 176, 611–617. [Google Scholar] [CrossRef]
- Löfman, I.; Szummer, K.; Evans, M.; Carrero, J.J.; Lund, L.H.; Jernberg, T. Incidence of, Associations With and Prognostic Impact of Worsening Renal Function in Heart Failure With Different Ejection Fraction Categories. Am. J. Cardiol. 2019, 124, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Palazzuoli, A.; Landolfo, M.; Cosentino, E. Hyperuricemia: A novel old disorder-relationship and potential mechanisms in heart failure. Heart Fail. Rev. 2020, 25, 43–51. [Google Scholar] [CrossRef]
- Zoungas, S.; Arima, H.; Gerstein, H.C.; Holman, R.R.; Woodward, M.; Reaven, P.; Hayward, R.A.; Craven, T.; Coleman, R.L.; Chalmers, J.; et al. Effects of Intensive Glucose Control on Microvascular Outcomes in Patients with Type 2 Diabetes: A Meta-Analysis of Individual Participant Data from Randomised Controlled Trials. Lancet Diabetes Endocrinol. 2017, 5, 431–437. [Google Scholar] [CrossRef]
- Control Group; Turnbull, F.M.; Abraira, C.; Anderson, R.J.; Byington, R.P.; Chalmers, J.P.; Duckworth, W.C.; Evans, G.W.; Gerstein, H.C.; Holman, R.R.; et al. Intensive Glucose Control and Macrovascular Outcomes in Type 2 Diabetes. Diabetologia 2009, 52, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Claggett, B.L.; de Denus, S.; Zile, M.R.; Huynh, T.; Desai, A.S.; Sirois, M.G.; Solomon, S.D.; Pitt, B.; Rouleau, J.L.; et al. Impact of Diabetes on Serum Biomarkers in Heart Failure with Preserved Ejection Fraction: Insights from the TOPCAT Trial. ESC Heart Fail. 2021, 8, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.-L.; Januszewski, A.S.; O’Connell, R.; Jenkins, A.J.; Xu, A.; Sullivan, D.R.; Barter, P.J.; Hung, W.-T.; Scott, R.S.; Taskinen, M.-R.; et al. The Relationship of Fibroblast Growth Factor 21 with Cardiovascular Outcome Events in the Fenofibrate Intervention and Event Lowering in Diabetes Study. Diabetologia 2015, 58, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, L.; Liu, Y.; Huang, P.; Song, H.; Zheng, P. The Potential Function and Clinical Application of FGF21 in Metabolic Diseases. Front. Pharmacol. 2022, 13, 1089214. [Google Scholar] [CrossRef]
- Ianoș, R.D.; Pop, C.; Iancu, M.; Rahaian, R.; Cozma, A.; Procopciuc, L.M. Diagnostic Performance of Serum Biomarkers Fibroblast Growth Factor 21, Galectin-3 and Copeptin for Heart Failure with Preserved Ejection Fraction in a Sample of Patients with Type 2 Diabetes Mellitus. Diagnostics 2021, 11, 1577. [Google Scholar] [CrossRef]
- Lenart-Lipińska, M.; Matyjaszek-Matuszek, B.; Gernand, W.; Nowakowski, A.; Solski, J. Serum Fibroblast Growth Factor 21 Is Predictive of Combined Cardiovascular Morbidity and Mortality in Patients with Type 2 Diabetes at a Relatively Short-Term Follow-Up. Diabetes Res. Clin. Pract. 2013, 101, 194–200. [Google Scholar] [CrossRef]
- de Boer, R.A.; Edelmann, F.; Cohen-Solal, A.; Mamas, M.A.; Maisel, A.; Pieske, B. Galectin-3 in Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2013, 15, 1095–1101. [Google Scholar] [CrossRef]
- Role of Galectin-3 in Obesity and Impaired Glucose Homeostasis—Menini—2016—Oxidative Medicine and Cellular Longevity—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1155/2016/9618092 (accessed on 4 September 2024).
- de Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; van der Harst, P. The Fibrosis Marker Galectin-3 and Outcome in the General Population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Vora, A.; de Lemos, J.A.; Ayers, C.; Grodin, J.L.; Lingvay, I. Association of Galectin-3 with Diabetes Mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019, 104, 4449–4458. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Bauer, S.; Farkas, S.; Scherer, M.N.; Schnitzbauer, A.; Schäffler, A.; Aslanidis, C.; Schölmerich, J.; et al. Serum Galectin-3 Is Elevated in Obesity and Negatively Correlates with Glycosylated Hemoglobin in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 1404–1411. [Google Scholar] [CrossRef]
- de Boer, R.A.; Nayor, M.; deFilippi, C.R.; Enserro, D.; Bhambhani, V.; Kizer, J.R.; Blaha, M.J.; Brouwers, F.P.; Cushman, M.; Lima, J.A.C.; et al. Association of Cardiovascular Biomarkers with Incident Heart Failure with Preserved and Reduced Ejection Fraction. JAMA Cardiol. 2018, 3, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Lou, Y.; Li, T.; Chen, H.; Liu, Q.; He, X. Serum Galectin-3: A Risk Factor for Vascular Complications in Type 2 Diabetes Mellitus. Chin. Med. J. 2013, 126, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.B.; Cheung, C.-L.; Lee, A.C.H.; Lam, J.K.Y.; Wong, Y.; Shiu, S.W.M. Galectin-3 and Risk of Cardiovascular Events and All-Cause Mortality in Type 2 Diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3093. [Google Scholar] [CrossRef]
- Lebedev, D.A.; Lyasnikova, E.A.; Vasilyeva, E.Y.; Babenko, A.Y.; Shlyakhto, E.V. Type 2 Diabetes Mellitus and Chronic Heart Failure with Midrange and Preserved Ejection Fraction: A Focus on Serum Biomarkers of Fibrosis. J. Diabetes Res. 2020, 2020, 6976153. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the Diagnosis and Management of Heart Failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Stoiser, B.; Mörtl, D.; Hülsmann, M.; Berger, R.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Pacher, R. Copeptin, a Fragment of the Vasopressin Precursor, as a Novel Predictor of Outcome in Heart Failure. Eur. J. Clin. Investig. 2006, 36, 771–778. [Google Scholar] [CrossRef]
| Mild HF (n = 67) | Advanced HF (n = 50) | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) a | 66.61 (9.89) | 67.56 (11.02) | 0.6261 |
| Male b | 34 (50.7) | 23 (46.0) | 0.6114 |
| Antropometrics | |||
| Body mass index (kg/m2) a | 30.90 (5.60) | 34.73 (6.62) | 0.00095 * |
| Body surface area (m2) a | 1.96 (0.21) | 2.06 (0.20) | 0.01251 * |
| Clinical characteristics | |||
| SBP (mmHg) c | 135 (125 to 140) | 130 (126 to 150) | 0.5383 |
| DBP(mmHg) c | 80 (70 to 80) | 80 (71 to 80) | 0.2940 |
| Smoking b | 15 (22.4) | 6 (12.0) | 0.1475 |
| PAD b | 6 (9.0) | 1 (2.0) | 0.2361 |
| Previous MI b | 38 (56.7) | 24 (48.0) | 0.4541 |
| Hypertension b | 53 (79.1) | 45 (90.0) | 0.1139 |
| Atrial fibrillation b | 4 (6.0) | 16 (32.0) | 0.00021 * |
| Paroxysmal atrial fibrillation b | 7 (10.4) | 4 (8.0) | 0.7563 |
| CCS b | 24 (35.8) | 14 (28.0) | 0.3715 |
| COPD b | 0 (0.0) | 10 (20.0) | 0.0001 * |
| Previous stroke b | 6 (9.0) | 4 (8.0) | 1.000 |
| Obesity (BMI ≥ 30 kg/m2) b | 35 (52.2) | 37 (74.0) | 0.0167 * |
| Coronary angiography b | 0.350 | ||
| Without lesions | 4 (6.0) | 1 (2.0) | |
| 1-vessel CAD | 17 (25.4) | 14 (28.0) | |
| 2-vessel CAD | 21 (31.3) | 22 (44.0) | |
| 3-vessel CAD | 25 (37.3) | 13 (26.0) | |
| T2DM severity b | 0.1149 | ||
| Controlled (HbA1c < 7%) | 19 (28.4) | 8 (16.0) | |
| Moderately controlled (7 ≤ HbA1c < 8%) | 14 (20.9) | 18 (36.0) | |
| Uncontrolled (HbA1c ≥ 8%) | 34 (50.7) | 24 (48.0) | |
| Mild HF (n = 67) | Advanced HF (n = 50) | p-Value | |
|---|---|---|---|
| Medication a | |||
| Metformin | 56 (83.6) | 34 (68.0) | 0.04780 * |
| GLP-1 receptor agonist | 2 (3.0) | 6 (12.0) | 0.07169 |
| SGLT2 inhibitor | 12 (17.9) | 11 (22.0) | 0.58190 |
| Insulin | 32 (47.8) | 33 (66.0) | 0.04952 * |
| Laboratory parameters | |||
| Hemoglobin b, g/dL | 13.65 (1.62) | 12.87 (1.94) | 0.01934 * |
| Creatinine c, mg/dL | 0.87 [0.72, 1.12] | 1.09 [0.84, 1.46] | 0.00381 * |
| eGFR c, mL/min/1.73 m2 by CKD-EPI | 83.0 [59.0, 101.0] | 69.5 [46.25, 86.75] | 0.01226 * |
| Urea c, mg/dL | 38.95 [30.32, 54.50] | 53.59 [38.49, 73.60] | 0.00389 * |
| Uric acid b, mg/dL | 6.29 (1.58) | 7.25 (2.12) | 0.00882 * |
| HDL cholesterol b, mg/dL | 8.47 (2.03) | 8.30 (1.59) | 0.29790 |
| Total cholesterol c, mg/dL | 164.0 [136.0, 204.0] | 153.5 [122.0, 194.3] | 0.17010 |
| LDL cholesterol c, mg/dL | 96.0 [69.10, 123.30] | 84.5 [61.85, 113.45] | 0.44050 |
| HbA1c b, % | 8.47 (2.03) | 8.30 (1.59) | 0.62960 |
| Glycemia b, mg/dL | 178.90 (72.26) | 191.24 (80.82) | 0.42620 |
| Variables | Mild HF (n = 67) | Advanced HF (n = 50) | p-Value |
|---|---|---|---|
| Copeptin a, pg/mL | 215.11 [155.83, 528.82] | 223.30 [153.85, 524.63] | 0.7745 |
| FGF21 a, ng/mL | 322.90 [178.70, 458.60] | 580.30 [329.90, 865.03] | 0.00027 * |
| Gal- 3 b, ng/mL | 12.06 (5.49) | 12.92 (4.16) | 0.3327 |
| NT-proBNP a, pg/mL | 1012.0 [327.0, 1704.5] | 2515.5 [1178.5, 3843.75] | 0.00003 * |
| LVEF b, % | 54.29 (6.08) | 52.58 (6.84) | 0.1576 |
| LV EDV b, mL | 113.83 (33.03) | 124.81 (39.51) | 0.1047 |
| LV ESV b, mL | 52.87 (20.03) | 60.36 (23.88) | 0.0680 |
| LVDd b, mm | 50.67 (4.39) | 50.42 (5.84) | 0.7989 |
| LVSd b, mm | 29.13 (5.46) | 30.46 (7.32) | 0.2846 |
| E/e ‘ mean ratio b | 11.06 (2.77) | 12.16 (4.01) | 0.0997 |
| Lad b, mm | 41.53 (5.67) | 44.62 (5.52) | 0.0039 * |
| LAV b, mL | 63.41 (17.81) | 79.44 (26.10) | 0.0003 * |
| LAVi a, mL/m2 | 32.06 [26.92, 37.89] | 35.31 [30.74, 43.21] | 0.0126 * |
| LAS b, cm2 | 22.95 (5.85) | 26.18 (5.62) | 0.0033 * |
| PWT b, mm | 11.97 (1.17) | 12.14 (1.31) | 0.4722 |
| IVST b, mm | 12.36 (1.46) | 12.26 (1.37) | 0.7127 |
| RWT b | 0.47 (0.05) | 0.48 (0.08) | 0.2992 |
| EDT b, msec | 221.39 (42.76) | 211.68 (38.36) | 0.2071 |
| TR velocity b | 2.45 (0.46) | 2.76 (0.57) | 0.0012 * |
| RV-RA gradient b,mmHg | 26.57 (8.41) | 33.19 (11.79) | 0.0011 * |
| PASP b, mm HG | 33.14 (9.10) | 42.28 (14.77) | 0.00023 * |
| LV GLS b, % | 13.69 (2.46) | 13.08 (2.64) | 0.2028 |
| Groups /Variables | FGF21 | NT-proBNP | Galectin-3 | Copeptin |
|---|---|---|---|---|
| Advanced HF Group | ||||
| FGF21, ng/mL | ||||
| NTproBNP, pg/mL | −0.05 (0.7208) | |||
| Gal-3, ng/mL | 0.05 (0.7268) | −0.14 (0.3339) | ||
| Copeptin, pg/mL | 0.01 (0.9588) | −0.16 (0.9588) | −0.14 (0.3247) | |
| Age, years | −0.27 (0.0628) | 0.31 (0.0273 *) | 0.002 (0.9860) | 0.004 (0.9781) |
| BMI, kg/m2 | 0.01 (0.9369) | −0.32 (0.0232 *) | 0.18 (0.2096) | 0.001 (0.9947) |
| SBP, mmHg | −0.10 (0.4840) | 0.10 (0.5099) | 0.20 (0.1603) | 0.12 (0.3888) |
| DBP, mmHg | −0.06 (0.6971) | 0.04 (0.7824) | −0.02 (0.9159) | 0.10 (0.4800) |
| HbA1C, % | 0.06 (0.7027) | −0.11 (0.4283) | 0.07 (0.6054) | −0.13 (0.3588) |
| Hemoglobin, g/dL | −0.07 (0.6402) | −0.28 (0.0514) | 0.002 (0.9884) | −0.19 (0.1928) |
| eGFR, mL/min/1.73 m2 by CKD-EPI | 0.35 (0.0125 *) | −0.26 (0.0701) | −0.04 (0.760) | −0.007 (0.9592) |
| Urea, mg/dL | −0.16 (0.2581) | 0.29 (0.0401 *) | 0.18 (0.2194) | −0.14 (0.3446) |
| Uric acid, mg/dL | −0.19 (0.1779) | 0.30 (0.0338 *) | 0.20 (0.1674) | −0.17 (0.2371) |
| HDL cholesterol, mg/dL | −0.29 (0.0386 *) | 0.08 (0.5583) | 0.12 (0.3941) | −0.20 (0.1643) |
| Total cholesterol, mg/dL | 0.22 (0.1196) | −0.32 (0.0247 *) | 0.03 (0.8206) | 0.04 (0.7751) |
| LDL cholesterol, mg/dL | 0.21 (0.1413) | −0.29 (0.0379 *) | 0.04 (0.7980) | 0.09 (0.5170) |
| Triglycerides/dL | 0.28 (0.0465 *) | −0.07 (0.6271) | −0.13 (0.3553) | 0.02 (0.8660) |
| E/e’ ratio | −0.21 (0.1522) | −0.01 (0.9406) | 0.09 (0.5542) | 0.19 (0.1892) |
| LAVi, mL/m2 | −0.15 (0.2891) | 0.10 (0.4762) | 0.08 (0.5780) | −0.03 (0.8415) |
| LAS, cm2 | −0.23 (0.1141) | 0.17 (0.2403) | 0.02 (0.9144) | −0.08 (0.5954) |
| TR velocity, mmHg | −0.27 (0.0604) | 0.38 (0.0068 *) | −0.18 (0.2231) | −0.10 (0.4696) |
| RV-RA gradient, mm Hg | −0.30 (0.0358 *) | 0.31 (0.0301 *) | −0.17 (0.2329) | −0.09 (0.5015) |
| PASP, mmHg | −0.21 (0.1356) | 0.39 (0.0053 *) | −0.12 (0.4058) | −0.14 (0.3399) |
| Mild HF Group | ||||
| FGF-21, ng/mL | ||||
| NTproBNP, pg/mL | −0.37 (0.0022 *) | |||
| Gal-3, ng/mL | 0.05 (0.6703) | −0.17 (0.1752) | ||
| Copeptin, pg/mL | −0.02 (0.8829) | 0.03 (0.7949) | −0.32 (0.0078 *) | |
| Age, years | −0.15 (0.2351) | 0.11 (0.3965) | 0.02 (0.8498) | 0.22 (0.06725) |
| BMI, kg/m2 | −0.04 (0.770) | −0.05 (0.6994) | 0.46 (0.0001 *) | −0.25 (0.0417 *) |
| SBP, mmHg | −0.03 (0.7810) | 0.14 (0.2508) | 0.04 (0.7233) | −0.10 (0.4035) |
| DBP, mmHg | −0.06 (0.6085) | 0.21 (0.0910) | −0.005 (0.9668) | 0.008 (0.9477) |
| HbA1C, % | −0.09 (0.4530) | 0.07 (0.5839) | −0.18 (0.1357) | −0.08 (0.4977) |
| Hemoglobin | 0.06 (0.5888) | −0.08 (0.5294) | −0.29 (0.0154 *) | −0.15 (0.2304) |
| eGFR, mL/min/1.73 m2 by CKD-EPI | 0.07 (0.5615) | −0.14 (0.2522) | −0.18 (0.1495) | −0.001 (0.9918) |
| Urea, mg/dL | 0.17 (0.1569) | 0.007 (0.9522) | 0.06 (0.6266) | 0.07 (0.5560) |
| Uric acid, mg/dL | −0.01 (0.9672) | 0.15 (0.2388) | −0.04 (0.7275) | 0.13 (0.3061) |
| HDL cholesterol/dL | 0.01 (0.9321) | −0.21 (0.0936) | −0.19 (0.1299) | 0.27 (0.0244 *) |
| Total cholesterol/dL | 0.12 (0.3397) | −0.10 (0.4402) | −0.07 (0.5515) | 0.12 (0.3459) |
| LDL cholesterol, mg/dL | 0.05 (0.6688) | −0.03 (0.8322) | −0.06 (0.6107) | 0.05 (0.6975) |
| Triglycerides, mg/dL | 0.15 (0.2229) | −0.11 (0.3785) | 0.06 (0.6341) | 0.06 (0.6166) |
| E/e’ ratio | −0.29 (0.0182 *) | 0.09 (0.4618) | 0.10 (0.4289) | −0.08 (0.4973) |
| LAVi, mL/m2 | −0.13 (0.3071) | 0.05 (0.7004) | −0.05 (0.7148) | −0.11 (0.3625) |
| LAS, cm2 | −0.15 (0.2358) | 0.23 (0.0573) | 0.13 (0.3045) | −0.24 (0.0523) |
| TR velocity, m/s | −0.24 (0.0470 *) | 0.15 (0.2153) | 0.08 (0.5289) | 0.09 (0.4826) |
| RV-RA gradient, mmHg | −0.24 (0.0472 *) | 0.12 (0.3256) | 0.14 (0.2762) | 0.03 (0.8407) |
| PAPS, mm HG | −0.23 (0.06014) | 0.16 (0.2009) | 0.12 (0.3217) | 0.01 (0.9214) |
| FGF21 (ng/mL) | OR [95% CI] | p-Value | NT-proBNP (pg/mL) | OR [95% CI] | p-Value |
|---|---|---|---|---|---|
| Model 1 | Model 1 | ||||
| <377.50 | Reference | <2379 | Reference | ||
| ≥377.50 | 4.46 [2.07, 10.94] | 0.00019 * | ≥2379 | 7.99 [3.29, 21.24] | 0.00001 * |
| Model 2 | Model 2 | ||||
| <377.50 | Reference | <2379 | Reference | ||
| ≥377.50 | 5.06 [2.27, 12.03] | 0.00012 * | ≥2379 | 10.35 [3.92, 30.89] | 0.000008 * |
| Model 3 | Model 3 | ||||
| <377.50 | Reference | <2379 | Reference | ||
| ≥377.50 | 8.19 [3.14, 24.03] | 0.00004 * | ≥2379 | 17.01 [5.11, 70.27] | 0.00002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianos, R.D.; Iancu, M.; Pop, C.; Lucaciu, R.L.; Hangan, A.C.; Rahaian, R.; Cozma, A.; Negrean, V.; Mercea, D.; Procopciuc, L.M. Predictive Value of NT-proBNP, FGF21, Galectin-3 and Copeptin in Advanced Heart Failure in Patients with Preserved and Mildly Reduced Ejection Fraction and Type 2 Diabetes Mellitus. Medicina 2024, 60, 1841. https://doi.org/10.3390/medicina60111841
Ianos RD, Iancu M, Pop C, Lucaciu RL, Hangan AC, Rahaian R, Cozma A, Negrean V, Mercea D, Procopciuc LM. Predictive Value of NT-proBNP, FGF21, Galectin-3 and Copeptin in Advanced Heart Failure in Patients with Preserved and Mildly Reduced Ejection Fraction and Type 2 Diabetes Mellitus. Medicina. 2024; 60(11):1841. https://doi.org/10.3390/medicina60111841
Chicago/Turabian StyleIanos, Raluca Diana, Mihaela Iancu, Calin Pop, Roxana Liana Lucaciu, Adriana Corina Hangan, Rodica Rahaian, Angela Cozma, Vasile Negrean, Delia Mercea, and Lucia Maria Procopciuc. 2024. "Predictive Value of NT-proBNP, FGF21, Galectin-3 and Copeptin in Advanced Heart Failure in Patients with Preserved and Mildly Reduced Ejection Fraction and Type 2 Diabetes Mellitus" Medicina 60, no. 11: 1841. https://doi.org/10.3390/medicina60111841
APA StyleIanos, R. D., Iancu, M., Pop, C., Lucaciu, R. L., Hangan, A. C., Rahaian, R., Cozma, A., Negrean, V., Mercea, D., & Procopciuc, L. M. (2024). Predictive Value of NT-proBNP, FGF21, Galectin-3 and Copeptin in Advanced Heart Failure in Patients with Preserved and Mildly Reduced Ejection Fraction and Type 2 Diabetes Mellitus. Medicina, 60(11), 1841. https://doi.org/10.3390/medicina60111841






.jpg)



