Predictive and Prognostic Value of Inflammatory and Nutritional Indexes in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
- (a)
- Invasive breast cancer, confirmed using core needle biopsy prior to NAC.
- (b)
- Surgery received after NAC.
- (c)
- Complete clinical records and follow-up information.
- (d)
- Blood samples obtained prior to NAC.
- (a)
- Patients with an inflammatory or infectious disease.
- (b)
- Patients with missing follow-up information.
- (c)
- Patients with bilateral breast cancer or secondary malignancy.
- (d)
- The absence of laboratory results before NAC.
2.3. Data Collection, Blood Samples, and Indexes
- NLR: neutrophil count (103/mm3)/lymphocyte count (103/mm3).
- PIV: neutrophil count (103/mm3) × platelet count (103/mm3) × monocyte count (103/mm3)/lymphocyte count (103/mm3).
- PNI: serum albumin (g/L) + 5 × lymphocyte count (103/mm3).
- LAR: lactate dehydrogenase (U/L)/serum albumin (g/L).
2.4. Treatment and Follow-Up
2.5. Pathological Assessments
2.6. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. ROC Analysis
3.3. Predictive Factors for pCR
3.4. Univariate and Multivariate Analyses for DFS and OS
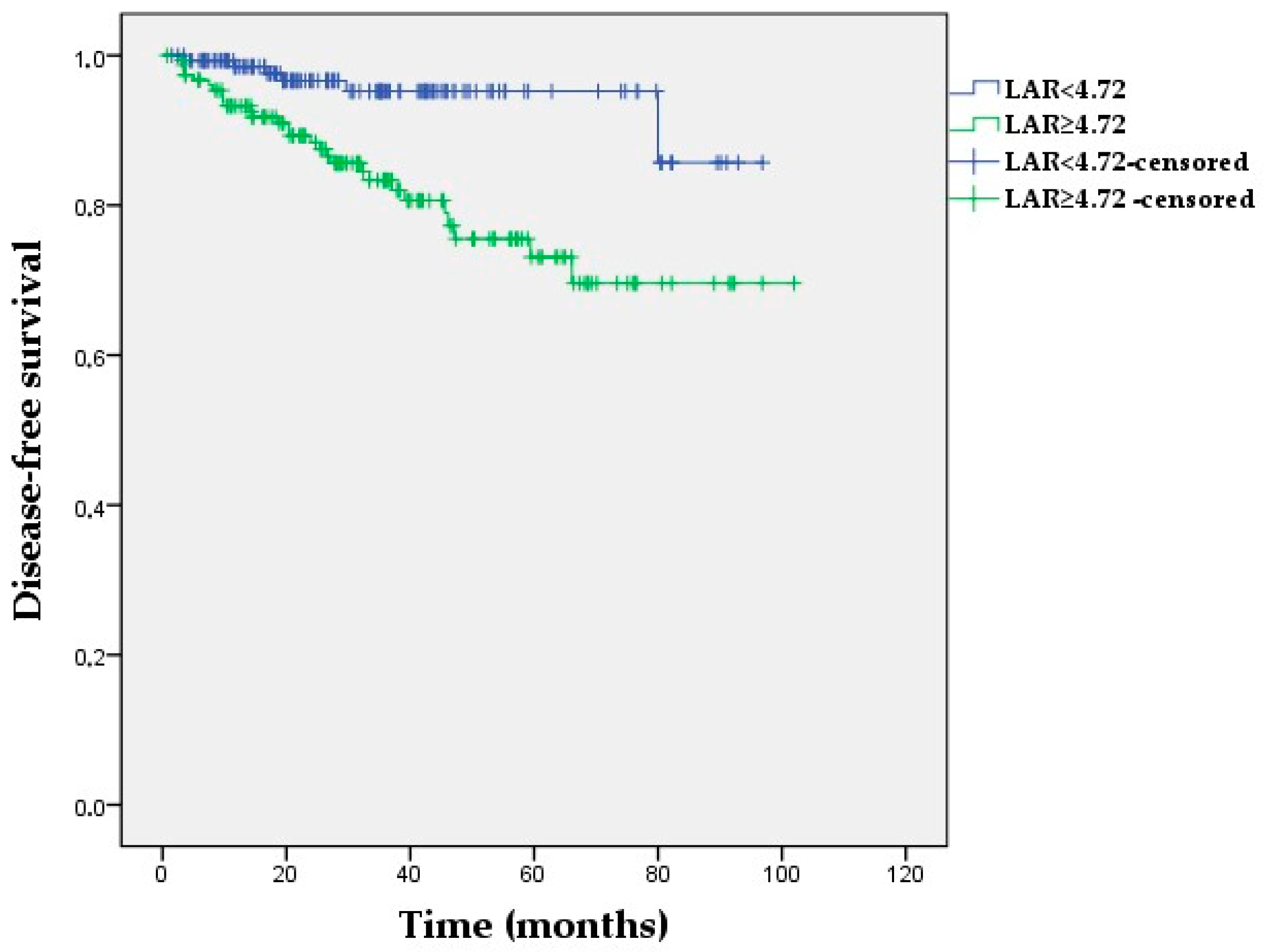
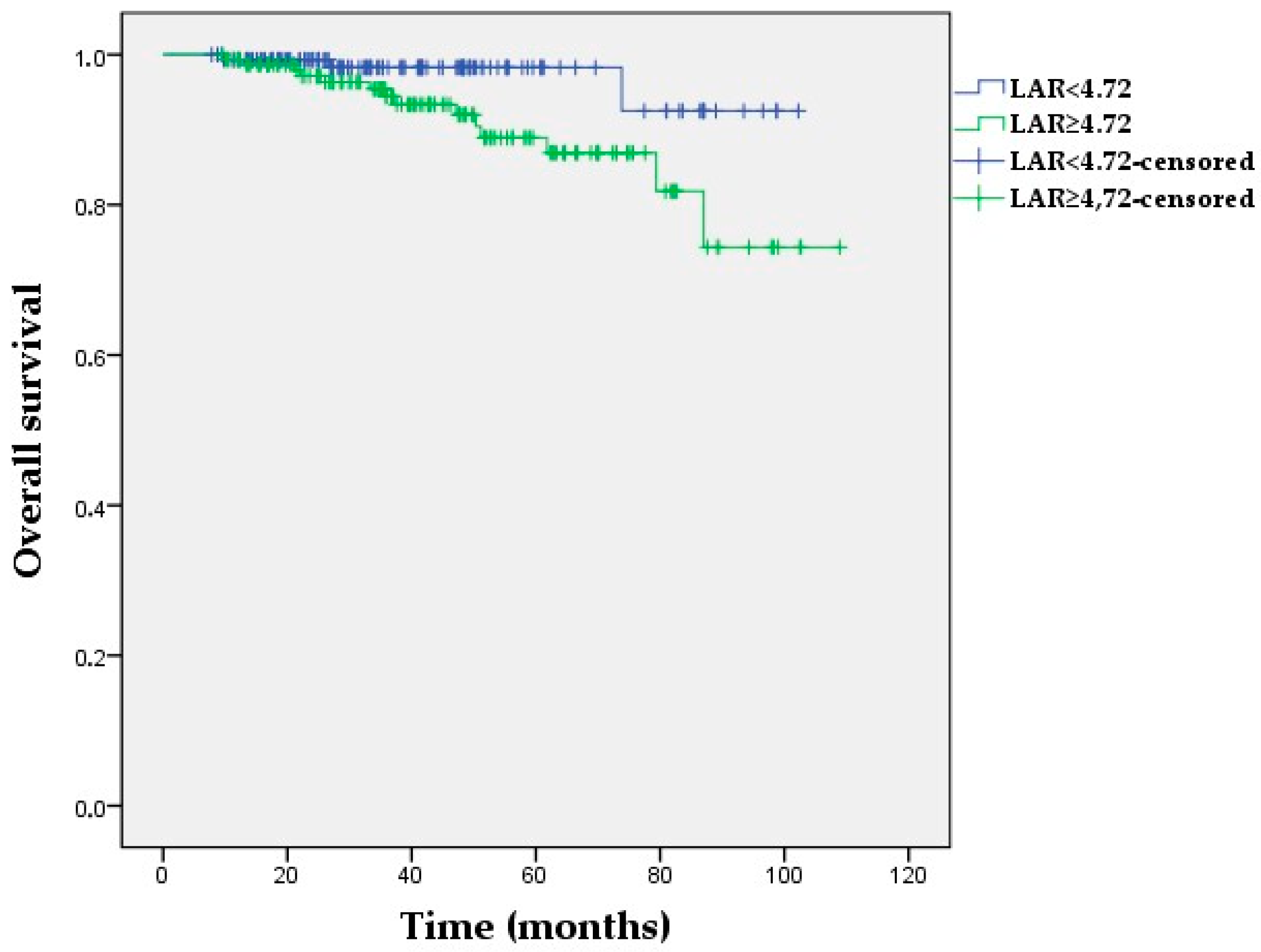
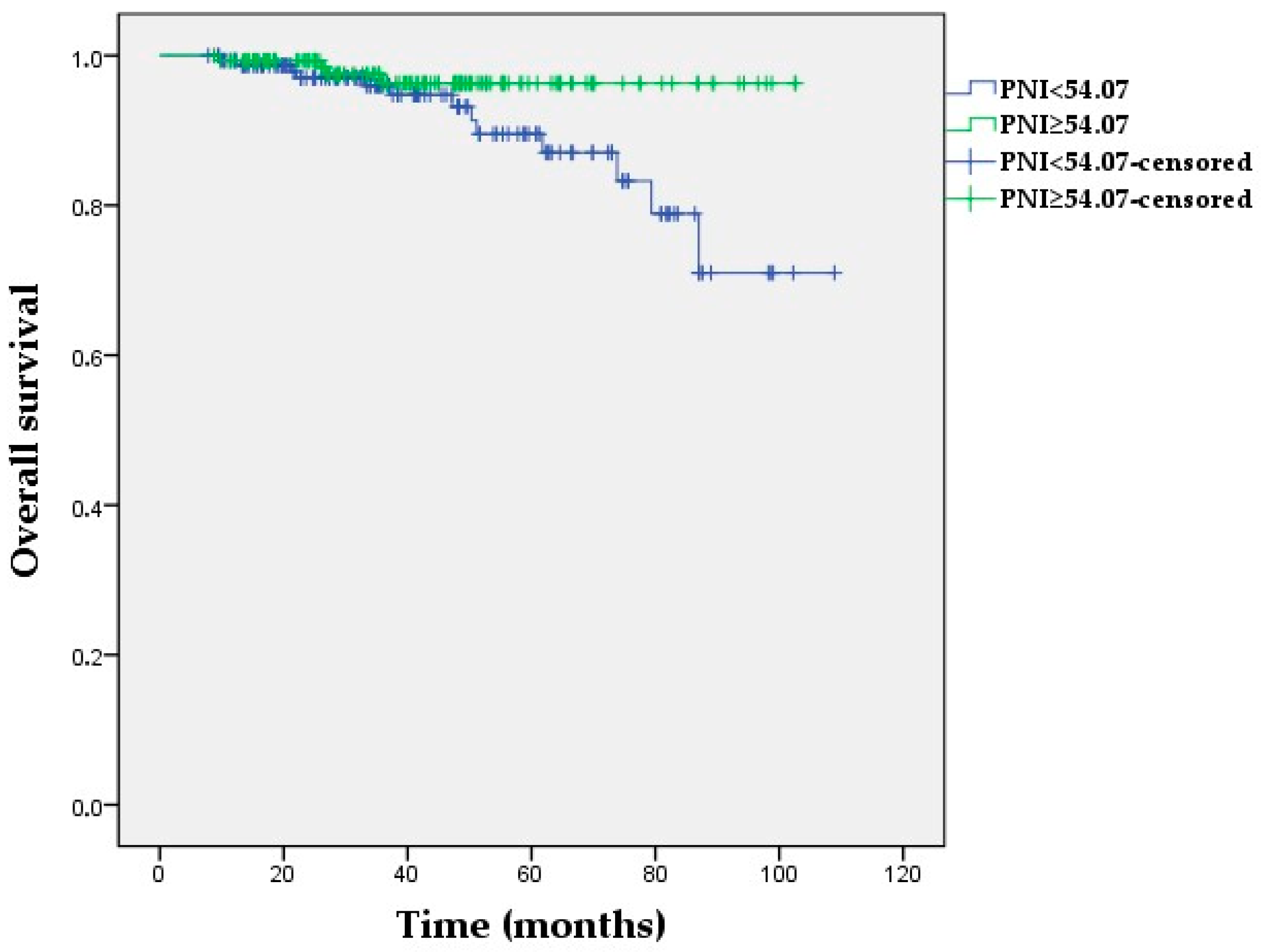
3.5. Kaplan–Meier Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tabor, S.; Szostakowska-Rodzos, M.; Fabisiewicz, A.; Grzybowska, E.A. How to Predict Metastasis in Luminal Breast Cancer? Current Solutions and Future Prospects. Int. J. Mol. Sci. 2020, 21, 8415. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.; DeMichele, A.M.; Yau, C.; Isaacs, C.; Symmans, W.F.; Albain, K.S.; Chen, Y.Y.; Krings, G.; Wei, S.; Harada, S.; et al. Association of Event-Free and Distant Recurrence-Free Survival With Individual-Level Pathologic Complete Response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: Three-Year Follow-up Analysis for the I-SPY2 Adaptively Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1355–1362. [Google Scholar] [CrossRef]
- Asaoka, M.; Gandhi, S.; Ishikawa, T.; Takabe, K. Neoadjuvant Chemotherapy for Breast Cancer: Past, Present, and Future. Breast Cancer 2020, 14, 1178223420980377. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Haque, W.; Verma, V.; Hatch, S.; Suzanne Klimberg, V.; Brian Butler, E.; Teh, B.S. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2018, 170, 559–567. [Google Scholar] [CrossRef]
- Derouane, F.; van Marcke, C.; Berlière, M.; Gerday, A.; Fellah, L.; Leconte, I.; Van Bockstal, M.R.; Galant, C.; Corbet, C.; Duhoux, F.P. Predictive Biomarkers of Response to Neoadjuvant Chemotherapy in Breast Cancer: Current and Future Perspectives for Precision Medicine. Cancers 2022, 14, 3876. [Google Scholar] [CrossRef]
- Xie, J.; Guo, Z.; Zhu, Y.; Ma, M.; Jia, G. Peripheral blood inflammatory indexes in breast cancer: A review. Medicine 2023, 102, e36315. [Google Scholar] [CrossRef]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qiao, B.; Song, T.; Huang, D.; Zhang, H.; Liu, Y.; Jin, Q.; Yang, M.; Liu, D. Clinical utility of the pan-immune-inflammation value in breast cancer patients. Front. Oncol. 2023, 13, 1223786. [Google Scholar] [CrossRef] [PubMed]
- Şahin, A.B.; Cubukcu, E.; Ocak, B.; Deligonul, A.; Oyucu Orhan, S.; Tolunay, S.; Gokgoz, M.S.; Cetintas, S.; Yarbas, G.; Senol, K.; et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 2021, 11, 14662. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Karp, J.E.; Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017, 19, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, Y.H.; Li, B.G.; Yang, Q.; Zhang, P.Y.; Wang, H.T. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 9800. [Google Scholar] [CrossRef]
- Gremese, E.; Bruno, D.; Varriano, V.; Perniola, S.; Petricca, L.; Ferraccioli, G. Serum Albumin Levels: A Biomarker to Be Repurposed in Different Disease Settings in Clinical Practice. J. Clin. Med. 2023, 12, 6017. [Google Scholar] [CrossRef]
- Sun, K.; Chen, S.; Xu, J.; Li, G.; He, Y. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1537–1549. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Cao, Y.; Wang, H.; Yang, Y.; Jiang, R.; Gong, Q.; Zhou, Q. Nomograms based on lactate dehydrogenase to albumin ratio for predicting survival in colorectal cancer. Int. J. Med. Sci. 2022, 19, 1003–1012. [Google Scholar] [CrossRef]
- He, J.; Tong, L.; Wu, P.; Wu, Y.; Shi, W.; Chen, L. Prognostic Significance of Preoperative Lactate Dehydrogenase to Albumin Ratio in Breast Cancer: A Retrospective Study. Int. J. Gen. Med. 2023, 16, 507–514. [Google Scholar] [CrossRef]
- Hua, X.; Long, Z.Q.; Huang, X.; Deng, J.P.; He, Z.Y.; Guo, L.; Zhang, W.W.; Lin, H.X. The Value of Prognostic Nutritional Index (PNI) in Predicting Survival and Guiding Radiotherapy of Patients With T1-2N1 Breast Cancer. Front. Oncol. 2019, 9, 1562. [Google Scholar] [CrossRef]
- Wang, Y.; Battseren, B.; Yin, W.; Lin, Y.; Zhou, L.; Yang, F.; Wang, Y.; Sun, L.; Lu, J. Predictive and prognostic value of prognostic nutritional index for locally advanced breast cancer. Gland. Surg. 2019, 8, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, B.; Hou, L.; Xie, Y.; Cao, X. Pre-operative prognostic nutritional index predicts the outcomes for triple-negative breast cancer. Tumour Biol. 2014, 35, 12165–12171. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Luo, Y.; Peng, Y.; Yu, H.; Sun, L.; Liu, S.; Zeng, X. Construction and validation of a prognostic nutritional index-based nomogram for predicting pathological complete response in breast cancer: A two-center study of 1,170 patients. Front. Immunol. 2023, 14, 1335546. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Dogan, A.; Akdag, G.; Yüksel Yasar, Z.; Bal, H.; Kinikoglu, O.; Oksuz, S.; Ozkerim, U.; Tunbekici, S.; Yildiz, H.S.; et al. The role of laboratory indices on treatment response and survival in breast cancer receiving neoadjuvant chemotherapy. Sci. Rep. 2024, 14, 12123. [Google Scholar] [CrossRef]
- Büyükşimşek, M.; Oğul, A.; Mirili, C.; Paydaş, S. Inflammatory Markers Predicting Pathological Complete Response in Cases with Breast Cancer Treated by Neoadjuvant Chemotherapy. Eur. J. Breast Health 2020, 16, 229–234. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.; Xiao, X.; Nie, Y.; Qu, S.; Gong, C.; Su, F.; Song, E. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: A retrospective study. BMC Cancer 2016, 16, 320. [Google Scholar] [CrossRef]
- Chen, L.; Bai, P.; Kong, X.; Huang, S.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Prognostic Nutritional Index (PNI) in Patients With Breast Cancer Treated With Neoadjuvant Chemotherapy as a Useful Prognostic Indicator. Front. Cell Dev. Biol. 2021, 9, 656741. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, C.; Pan, F.; Chen, Y.; Xiong, L.; Li, Y.; Chu, X.; Huang, G. Platelets in the tumor microenvironment and their biological effects on cancer hallmarks. Front. Oncol. 2023, 13, 1121401. [Google Scholar] [CrossRef]
- Mantzorou, M.; Koutelidakis, A.; Theocharis, S.; Giaginis, C. Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr. Cancer 2017, 69, 1151–1176. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Albasini, S.; Truffi, M.; Favilla, K.; Tagliaferri, B.; Piccotti, F.; Bossi, D.; Armatura, G.; Calcinotto, A.; Chiappa, C.; et al. Low neutrophil-to-lymphocyte ratio and pan-immune-inflammation-value predict nodal pathologic complete response in 1274 breast cancer patients treated with neoadjuvant chemotherapy: A multicenter analysis. Ther. Adv. Med. Oncol. 2023, 15, 17588359231193732. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Sottotetti, F.; Gafni, N.; Albasini, S.; Piccotti, F.; Morasso, C.; Tibollo, V.; Mocchi, M.; Zanella, V.; Corsi, F. Prognostic Potential of Immune Inflammatory Biomarkers in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Cancers 2022, 14, 5287. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Smith, H.; Board, M.; Pellagatti, A.; Turley, H.; Boultwood, J.; Callaghan, R. The Effects of Severe Hypoxia on Glycolytic Flux and Enzyme Activity in a Model of Solid Tumors. J. Cell Biochem. 2016, 117, 1890–1901. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Wu, C.; Zhang, L.; Mei, Q.; Hu, G.; Long, G.; Sun, W. Prognostic significance of serum lactate dehydrogenase in patients with breast cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 3611–3619. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Fujii, T.; Tokuda, S.; Nakazawa, Y.; Kurozumi, S.; Obayashi, S.; Yajima, R.; Shirabe, K. Implications of Low Serum Albumin as a Prognostic Factor of Long-term Outcomes in Patients With Breast Cancer. In Vivo 2020, 34, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Zhang, M.X.; Wang, J.X.; Fu, Y.P.; Huang, J.L.; Yi, Y.; Jing, C.Y.; Fan, J.; Zhou, J.; Qiu, S.J. Prognostic impact of lactic dehydrogenase to albumin ratio in hepatocellular carcinoma patients with Child-Pugh I who underwent curative resection: A prognostic nomogram study. Cancer Manag. Res. 2018, 10, 5383–5394. [Google Scholar] [CrossRef]
- Nakazawa, N.; Sohda, M.; Yamaguchi, A.; Watanabe, T.; Saito, H.; Ubukata, Y.; Kuriyama, K.; Sano, A.; Sakai, M.; Yokobori, T.; et al. An Elevated Serum Lactate Dehydrogenase-to-albumin Ratio Is a Useful Poor Prognostic Predictor of Nivolumab in Patients With Gastric Cancer. Anticancer Res. 2021, 41, 3925–3931. [Google Scholar] [CrossRef]
- Peng, R.R.; Liang, Z.G.; Chen, K.H.; Li, L.; Qu, S.; Zhu, X.D. Nomogram Based on Lactate Dehydrogenase-to-Albumin Ratio (LAR) and Platelet-to-Lymphocyte Ratio (PLR) for Predicting Survival in Nasopharyngeal Carcinoma. J. Inflamm. Res. 2021, 14, 4019–4033. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhou, H.; Wang, L.; Wu, Y. The Significance of the preoperative lactate dehydrogenase/albumin Ratio in the Prognosis of Colon Cancer: A retrospective study. PeerJ 2022, 10, e13091. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Ding, Q.; Zhong, K.; Wang, S.; Wang, S.; Huang, L. Low pretreatment prognostic nutritional index predicts poor survival in breast cancer patients: A meta-analysis. PLoS ONE 2023, 18, e0280669. [Google Scholar] [CrossRef]
- Prasetiyo, P.D.; Baskoro, B.A.; Hariyanto, T.I. The role of nutrition-based index in predicting survival of breast cancer patients: A systematic review and meta-analysis. Heliyon 2024, 10, e23541. [Google Scholar] [CrossRef] [PubMed]
| Variable | n (%) |
|---|---|
| Age | |
| Median (range), years | 50 (23–78) |
| Menopausal status | |
| Premenopausal | 136 (44.7) |
| Postmenopausal | 168 (55.3) |
| Clinical stage | |
| Stage II | 154 (50.7) |
| Stage III | 150 (49.3) |
| Pre-treatment histology | |
| Invasive carcinoma, NST | 274 (90.1) |
| Others * | 30 (9.9) |
| ER status | |
| Positive | 196 (64.5) |
| Negative | 108 (35.5) |
| HER2 status | |
| Positive | 92 (30.3) |
| Negative | 212 (69.7) |
| Biological subtype | |
| Lum A | 40 (13.2) |
| Lum B, HER2-negative | 106 (34.9) |
| Lum B, HER2-positive | 56 (18.4) |
| HR-negative, HER2-positive | 36 (11.8) |
| Triple-negative | 66 (21.7) |
| Ki-67 index (%) | |
| Median (range) | 30 (3–100) |
| NAC regimen | |
| Anthracycline + Taxane | 207 (68.1) |
| Anthracycline + Taxane + Anti HER2 | 75 (24.7) |
| Anthracycline-free | 22 (7.2) |
| pCR status | |
| pCR | 126 (41.4) |
| Non-pCR | 178 (58.6) |
| Optimal Cut-Off | AUC | 95% CI | Specificity (%) | Sensitivity (%) | p-Value | |
|---|---|---|---|---|---|---|
| NLR | 2.09 | 0.587 | 0.523–0.652 | 56.3 | 56.2 | 0.009 |
| PIV | 317.5 | 0.618 | 0.556–0.681 | 58.7 | 59.0 | <0.001 |
| PNI | 54.07 | 0.455 | 0.389–0.520 | 46.8 | 45.5 | 0.177 |
| LAR | 4.72 | 0.579 | 0.514–0.645 | 55.6 | 55.6 | 0.018 |
| pCR (n = 126) | Non-pCR (n = 178) | p-Value | |
|---|---|---|---|
| Age group | 0.343 | ||
| <50 | 65 (51.6) | 82 (46.1) | |
| ≥50 | 61 (48.4) | 96 (53.9) | |
| Menopausal status | 0.187 | ||
| Premenopausal | 62 (49.2) | 74 (41.6) | |
| Postmenopausal | 64 (50.8) | 104 (58.4) | |
| Clinical stage | 0.018 | ||
| Stage II | 74 (58.7) | 80 (44.9) | |
| Stage III | 52 (41.3) | 98 (55.1) | |
| Pre-treatment histology | 0.004 | ||
| Invasive carcinoma, NST | 121 (96.0) | 153 (86.0) | |
| Others | 5 (4.0) | 25 (14.0) | |
| ER status | <0.001 | ||
| Positive | 58 (46.0) | 138 (77.5) | |
| Negative | 68 (54.0) | 40 (22.5) | |
| HER2 status | <0.001 | ||
| Positive | 62 (49.2) | 30 (16.9) | |
| Negative | 64 (50.8) | 148 (83.1) | |
| Biological subtypes | <0.001 | ||
| Lum A | 4 (3.2) | 36 (20.2) | |
| Lum B, HER-2-negative | 25 (19.8) | 81 (45.5) | |
| Lum B, HER-2-positive | 34 (27.0) | 22 (12.4) | |
| HR-negative, HER2-positive | 28 (22.2) | 8 (4.5) | |
| Triple-negative | 35 (27.8) | 31 (17.4) | |
| Ki-67 group | 0.001 | ||
| <20% | 13 (10.3) | 45 (25.3) | |
| ≥20% | 113 (89.7) | 133 (74.7) | |
| NLR | 0.031 | ||
| <2.09 | 71 (56.3) | 78 (43.8) | |
| ≥2.09 | 55 (43.7) | 100 (56.2) | |
| PIV | 0.002 | ||
| <317.5 | 74 (58.7) | 73 (41.0) | |
| ≥317.5 | 52 (41.3) | 105 (59.0) | |
| PNI | 0.188 | ||
| <54.07 | 59 (46.8) | 97 (54.5) | |
| ≥54.07 | 67 (53.2) | 81 (45.5) | |
| LAR | 0.055 | ||
| <4.72 | 70 (55.6) | 79 (44.4) | |
| ≥4.72 | 56 (44.4) | 99 (55.6) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Risk Factor | OR (95%CI) | p-Value | OR (95%CI) | p-Value |
| Age group (>50 vs. ≤50) | 0.80 (0.5–1.26) | 0.343 | ||
| Clinical stage (Stage 3 vs. 2) | 0.57 (0.36–0.91) | 0.018 | 0.52 (0.31–0.90) | 0.020 |
| ER (negative vs. positive) | 4.05 (2.46–6.46) | 0.001 | 3.77 (2.17–6.54) | 0.001 |
| HER2 (negative vs. positive) | 4.78 (2.82–8.08) | 0.001 | 5.56 (3.10–9.96) | 0.001 |
| Ki-67 (≥20 vs. <20) | 2.95 (1.51–5.72) | 0.002 | 2.26 (1.05–4.85) | 0.036 |
| NLR (≥2.09 vs. <2.09) | 0.60 (0.38–0.95) | 0.032 | 0.89 (0.50–1.59) | 0.697 |
| PIV (<317.5 vs. ≥317.5) | 2.04 (1.28–3.25) | 0.002 | 2.06 (1.20–3.52) | 0.008 |
| PNI (≥54.07 vs. <54.07) | 0.19 (0.86–2.15) | 0.188 | ||
| LAR (≥4.72 vs. <4.72) | 0.64 (0.40–1.01) | 0.055 | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Risk Factor | OR (95%CI) | p-Value | OR (95%CI) | p-Value |
| Age group (>50 vs. ≤50) | 0.88 (0.44–1.72) | 0.713 | ||
| Clinical stage (Stage 3 vs. 2) | 2.18 (1.06–4.48) | 0.033 | 2.20 (1.07–4.54) | 0.032 |
| ER (negative vs. positive) | 1.55 (0.78–3.08) | 0.208 | ||
| HER2 (negative vs. positive) | 1.37 (0.67–2.78) | 0.378 | ||
| Ki-67 (≥20 vs. <20) | 2.18 (0.83–5.69) | 0.111 | ||
| Pathologic status (non-pCR vs. pCR) | 3.40 (1.40–8.22) | 0.006 | 2.84 (1.17–6.88) | 0.021 |
| NLR (≥2.09 vs. <2.09) | 2.20 (0.96–4.24) | 0.061 | ||
| PIV (≥317.5 vs. <317.5) | 1.61 (0.79–3.25) | 0.184 | ||
| PNI (≥54.07 vs. <54.07) | 0.55 (0.27–1.12) | 0.104 | ||
| LAR (≥4.72 vs. <4.72) | 4.08 (1.68–9.86) | 0.002 | 3.98 (1.64–9.65) | 0.002 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Risk Factor | OR (95%CI) | p-Value | OR (95%CI) | p-Value |
| Age group (>50 vs. ≤50) | 1.12 (0.43–2.91) | 0.811 | ||
| Clinical stage (Stage 3 vs. 2) | 1.70 (0.62–4.62) | 0.294 | ||
| ER (negative vs. positive) | 0.85 (0.31–2.31) | 0.755 | ||
| HER2 (negative vs. positive) | 0.74 (0.24–2.29) | 0.608 | ||
| Ki-67 (≥20 vs. <20) | 3.12 (0.70–13.79) | 0.134 | ||
| Pathologic status (non-pCR vs. pCR) | 4.83 (1.10–21.16) | 0.037 | 4.29 (0.98–18.86) | 0.054 |
| NLR (≥2.09 vs. <2.09) | 2.39 (0.77–7.38) | 0.104 | ||
| PIV (≥317.5 vs. <317.5) | 1.46 (0.54–3.97) | 0.453 | ||
| PNI (<54.07 vs. ≥54.07) | 2.99 (0.97–9.18) | 0.056 | ||
| LAR (≥4.72 vs. <4.72) | 3.61(1.03–12.61) | 0.044 | 3.17 (0.90–11.10) | 0.071 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arici, M.O.; Kivrak Salim, D.; Kocer, M.; Alparslan, A.S.; Karakas, B.R.; Ozturk, B. Predictive and Prognostic Value of Inflammatory and Nutritional Indexes in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. Medicina 2024, 60, 1849. https://doi.org/10.3390/medicina60111849
Arici MO, Kivrak Salim D, Kocer M, Alparslan AS, Karakas BR, Ozturk B. Predictive and Prognostic Value of Inflammatory and Nutritional Indexes in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. Medicina. 2024; 60(11):1849. https://doi.org/10.3390/medicina60111849
Chicago/Turabian StyleArici, Mustafa Ozgur, Derya Kivrak Salim, Murat Kocer, Ahmet Sukru Alparslan, Baris Rafet Karakas, and Banu Ozturk. 2024. "Predictive and Prognostic Value of Inflammatory and Nutritional Indexes in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy" Medicina 60, no. 11: 1849. https://doi.org/10.3390/medicina60111849
APA StyleArici, M. O., Kivrak Salim, D., Kocer, M., Alparslan, A. S., Karakas, B. R., & Ozturk, B. (2024). Predictive and Prognostic Value of Inflammatory and Nutritional Indexes in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. Medicina, 60(11), 1849. https://doi.org/10.3390/medicina60111849






