The Significance of Plant Nutrition in the Creation of the Intestinal Microbiota—Prevention of Chronic Diseases: A Narrative Review
Abstract
:1. Introduction
The Microbiome of a Newborn
2. Nutrition and the Microbiome
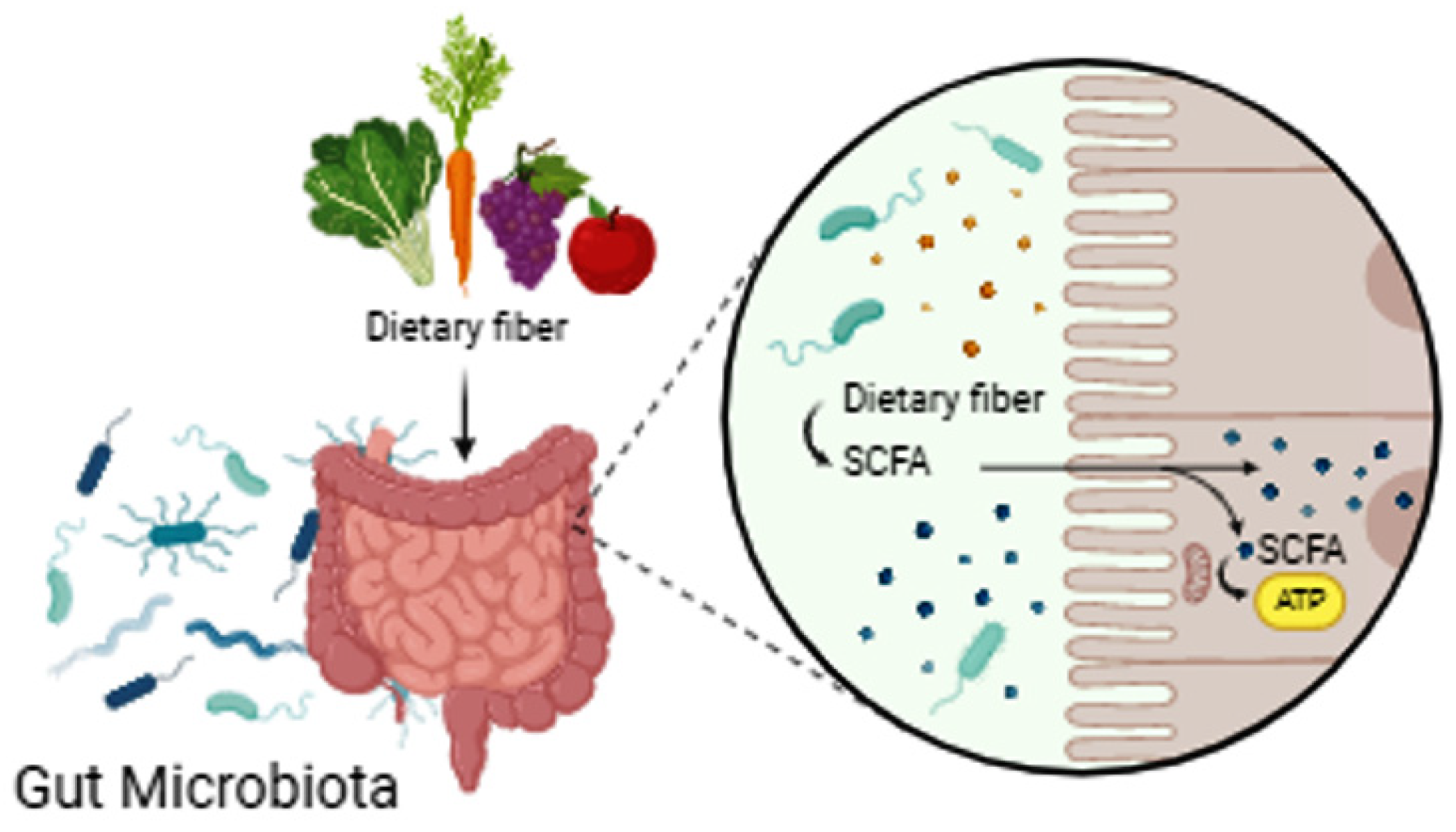
3. Plant-Based Nutrition
Disadvantages of Plant-Based Nutrition
4. Mediterranean Diet
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.W.; Arrieta, M.C. The intestinal mycobiome as a determinant of host immune and metabolic health. Curr. Opin. Microbiol. 2021, 62, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rintarhat, P.; Cho, Y.J.; Koh, H.; Park, S.; Lee, E.J.; Lim, H.; Noh, J.; Lee, D.W.; Jung, W.H. Assessment of DNA extraction methods for human gut mycobiome analysis. R. Soc. Open Sci. 2024, 11, 231129. [Google Scholar] [CrossRef]
- Lai, T.T.; Liou, C.W.; Tsai, Y.H.; Lin, Y.Y.; Wu, W.L. Butterflies in the gut: The interplay between intestinal microbiota and stress. J. Biomed. Sci. 2023, 30, 92. [Google Scholar] [CrossRef]
- Konturek, P.; Konturek, K.; Zopf, Y.; Harsch, I.A. Intestinale Mikrobiota—Ein lebenswichtiges “Organ” mit vielfältigen Funktionen [Intestinal microbiota—A vital “organ” with manifold functions]. MMW Fortschr Med. 2020, 162 (Suppl. S4), 9–14. [Google Scholar] [CrossRef]
- Reynolds, H.M.; Bettini, M.L. Early-life microbiota-immune homeostasis. Front. Immunol. 2023, 14, 1266876. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Maslin, K. Commentary on a Cochrane Review of Early Additional Food and Fluids for Healthy Breastfed Full-Term Infants. Nurs. Womens Health 2016, 20, 347–349. [Google Scholar] [CrossRef]
- Łuszczki, E.; Boakye, F.; Zielińska, M.; Dereń, K.; Bartosiewicz, A.; Oleksy, Ł.; Stolarczyk, A. Vegan diet: Nutritional components, implementation, and effects on adults’ health. Front. Nutr. 2023, 10, 1294497. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Liu, Y.; Wang, S.; Ma, J.; Yang, H. Distribution characteristics of intestinal microbiota during pregnancy and postpartum in healthy women. J. Matern. Fetal Neonatal Med. 2022, 35, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef]
- Bittinger, K.; Zhao, C.; Li, Y.; Ford, E.; Friedman, E.S.; Ni, J.; Kulkarni, C.V.; Cai, J.; Tian, Y.; Liu, Q.; et al. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol. 2020, 5, 838–847. [Google Scholar] [CrossRef]
- Lin, C.; Lin, Y.; Zhang, H.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Intestinal ‘Infant-Type’ Bifidobacteria Mediate Immune System Development in the First 1000 Days of Life. Nutrients 2022, 14, 1498. [Google Scholar] [CrossRef]
- Seki, D.; Mayer, M.; Hausmann, B.; Pjevac, P.; Giordano, V.; Goeral, K.; Unterasinger, L.; Klebermaß-Schrehof, K.; De Paepe, K.; Van de Wiele, T.; et al. Aberrant gut-microbiota-immune-brain axis development in premature neonates with brain damage. Cell Host Microbe 2021, 29, 1558–1572. [Google Scholar] [CrossRef]
- Lynch, C.M.K.; Cowan, C.S.M.; Bastiaanssen, T.F.S.; Moloney, G.M.; Theune, N.; van de Wouw, M.; Florensa Zanuy, E.; Ventura-Silva, A.P.; Codagnone, M.G.; Villalobos-Manríquez, F.; et al. Critical windows of early-life microbiota disruption on behaviour, neuroimmune function, and neurodevelopment. Brain Behav. Immun. 2023, 108, 309–327. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Zhang, T.; Sidorchuk, A.; Sevilla-Cermeño, L.; Vilaplana-Pérez, A.; Chang, Z.; Larsson, H.; Mataix-Cols, D.; Fernández de la Cruz, L. Association of Cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: A systematic review and meta-analysis. JAMA Netw. Open 2019, 2, e1910236. [Google Scholar] [CrossRef]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef] [PubMed]
- Jovandaric, M.Z.; Dugalic, S.; Babic, S.; Babovic, I.R.; Milicevic, S.; Mihajlovic, D.; Culjic, M.; Zivanovic, T.; Trklja, A.; Markovic, B.; et al. Programming Factors of Neonatal Intestinal Dysbiosis as a Cause of Disease. Int. J. Mol. Sci. 2023, 24, 5723. [Google Scholar] [CrossRef] [PubMed]
- Nyangahu, D.D.; Jaspan, H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019, 198, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Shalon, D.; Culver, R.N.; Grembi, J.A.; Folz, J.; Treit, P.V.; Shi, H.; Rosenberger, F.A.; Dethlefsen, L.; Meng, X.; Yaffe, E.; et al. Profiling the human intestinal environment under physiological conditions. Nature 2023, 617, 581–591. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Walsh, L.; Hill, C.; Ross, R.P. Impact of glyphosate (RoundupTM) on the composition and functionality of the gut microbiome. Gut Microbes 2023, 15, 2263935. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Cao, G.; Chen, D.Q.; Vaziri, N.D.; Chen, L.; Zhang, J.; Wang, M.; Guo, Y.; Zhao, Y.Y. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol. Life Sci. 2019, 76, 4961–4978. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Dilmore, A.H.; Burcham, Z.M.; Metcalf, J.L.; Jeste, D.; Knight, R. Microbiota succession throughout life from the cradle to the grave. Nat. Rev. Microbiol. 2022, 20, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Conz, A.; Salmona, M.; Diomede, L. Effect of Non-Nutritive Sweeteners on the Gut Microbiota. Nutrients 2023, 15, 1869. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Hullar, M.A.J.; Maskarinec, G.; Monroe, K.R.; Shepherd, J.A.; Franke, A.A.; Randolph, T.W.; Wilkens, L.R.; Boushey, C.J.; Le Marchand, L.; et al. The Gut Microbiome Is Associated with Circulating Dietary Biomarkers of Fruit and Vegetable Intake in a Multiethnic Cohort. J. Acad. Nutr. Diet. 2022, 122, 78–98. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Khalid, W.; Arshad, M.S.; Jabeen, A.; Muhammad Anjum, F.; Qaisrani, T.B.; Suleria, H.A.R. Fiber-enriched botanicals: A therapeutic tool against certain metabolic ailments. Food Sci. Nutr. 2022, 10, 3203–3218. [Google Scholar] [CrossRef]
- Precup, G.; Pocol, C.B.; Teleky, B.E.; Vodnar, D.C. Awareness, Knowledge, and Interest about Prebiotics-A Study among Romanian Consumers. Int. J. Environ. Res. Public. Health 2022, 19, 1208. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. Technol. 2021, 117, 1. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Bhardwaj, A. β-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Gasparre, N.; Mefleh, M.; Boukid, F. Nutritional Facts and Health/Nutrition Claims of Commercial Plant-Based Infant Foods: Where Do We Stand? Plants 2022, 11, 2531. [Google Scholar] [CrossRef]
- Haider, S.; Sima, A.; Kühn, T.; Wakolbinger, M. The Association between Vegan Dietary Patterns and Physical Activity-A Cross-Sectional Online Survey. Nutrients 2023, 15, 1847. [Google Scholar] [CrossRef]
- Puhlmann, M.L.; de Vos, W.M. Intrinsic dietary fibers and the gut microbiome: Rediscovering the benefits of the plant cell matrix for human health. Front. Immunol. 2022, 13, 954845. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Xie, Z.; He, W.; Gobbi, A.; Bertram, H.C.; Nielsen, D.S. The effect of in vitro simulated colonic pH gradients on microbial activity and metabolite production using common prebiotics as substrates. BMC Microbiol. 2024, 24, 83. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Shalunkhe, A.; Duhduh, A.; Walton, G.E.; Gibson, G.R.; Pereira, D.I.; Wijeyesekera, A.; Andrews, S.C. Impact of inorganic iron and haem on the human gut microbiota; An in vitro batch-culture approach. Front. Microbiol. 2023, 14, 1074637. [Google Scholar] [CrossRef] [PubMed]
- Cuisiniere, T.; Calvé, A.; Fragoso, G.; Oliero, M.; Hajjar, R.; Gonzalez, E.; Santos, M.M. Oral iron supplementation after antibiotic exposure induces a deleterious recovery of the gut microbiota. BMC Microbiol. 2021, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Kai, Y. Intestinal villus structure contributes to even shedding of epithelial cells. Biophys. J. 2021, 120, 699–710. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Lachmansingh, D.A.; Valderrama, B.; Bastiaanssen, T.; Cryan, J.; Clarke, G.; Lavelle, A. Impact of dietary fiber on gut microbiota composition, function and gut-brain-modules in healthy adults—A systematic review protocol. HRB Open Res. 2024, 6, 62. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The microbiota-gut-brain axis and neurodevelopmental disorders. Protein Cell. 2023, 14, 762–775. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Wiebe, N.; Stenvinkel, P.; Tonelli, M. Associations of Chronic Inflammation, Insulin Resistance, and Severe Obesity with Mortality, Myocardial Infarction, Cancer, and Chronic Pulmonary Disease. JAMA Netw. Open 2019, 2, e10456. [Google Scholar]
- Shivakoti, R.; Biggs, M.L.; Djoussé, L.; Durda, P.J.; Kizer, J.R.; Psaty, B.; Reiner, A.P.; Tracy, R.P.; Siscovick, D.; Mukamal, K.J. Intake and Sources of Dietary Fiber, Inflammation, and Cardiovascular Disease in Older US Adults. JAMA Netw. Open 2022, 5, e225012. [Google Scholar] [CrossRef]
- Insuela, D.B.R.; Carvalho, V.F. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur. J. Pharmacol. 2017, 812, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; Alva, V. PII-like signaling proteins: A new paradigm in orchestrating cellular homeostasis. Curr. Opin. Microbiol. 2024, 79, 102453. [Google Scholar] [CrossRef] [PubMed]
- Niero, M.; Bartoli, G.; De Colle, P.; Scarcella, M.; Zanetti, M. Impact of Dietary Fiber on Inflammation and Insulin Resistance in Older Patients: A Narrative Review. Nutrients 2023, 15, 2365. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Wen, J.; Yammine, L.; Culver, J.; Parida, I.S.; Garren, J.; Xue, L.; Hales, K.; Xiang, Q.; Birnbaum, M.J.; et al. Regulated dynamic subcellular GLUT4 localization revealed by proximal proteome mapping in human muscle cells. J. Cell Sci. 2023, 136, jcs261454. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- Zhelev, Z.; Georgieva, E.; Lazarova, D.; Semkova, S.; Aoki, I.; Gulubova, M.; Higashi, T.; Bakalova, R. “Redox Imaging to Distinguish Cells with Different Proliferative Indexes: Superoxide, Hydroperoxides, and Their Ratio as Potential Biomarkers. Oxid. Med. Cell Longev. 2019, 2019, 6373685. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, L.; Song, B.; Cui, Z.; Chen, G.; Yu, Z.; Song, B. Insulin-like Growth Factor-2 (IGF-2) in Fibrosis. Biomolecules 2022, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, G.; Sela, Y. The Ideal Diet for Humans to Sustainably Feed the Growing Population—Review, Meta-Analyses, and Policies for Change. F1000Research 2023, 10, 1135. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, V. Plant carotenoids evolution during cultivation, postharvest storage, and food processing: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1561–1604. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika, S.A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent. Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, H. Anthocyanins in Plant Food: Current Status, Genetic Modification, and Future Perspectives. Molecules 2023, 28, 866. [Google Scholar] [CrossRef]
- Sami, A.; Han, S.; Haider, M.Z.; Khizar, R.; Ali, Q.; Shafiq, M.; Tabassum, J.; Khalid, M.N.; Javed, M.A.; Sajid, M.; et al. Genetics aspect of vitamin C (Ascorbic Acid) biosynthesis and signaling pathways in fruits and vegetables crops. Funct. Integr. Genom. 2024, 24, 73. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Noguchi, N. Antioxidant action of vitamin E in vivo as assessed from its reaction products with multiple biological oxidants. Free Radic. Res. 2021, 55, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Han, S.N. Vegetarian Diet for Cardiovascular Disease Risk Reduction: Cons. J. Lipid Atheroscler. 2023, 12, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.D. Nutritional Considerations for the Vegetarian and Vegan Dancer. J. Danc. Med. Sci. 2018, 22, 44–53. [Google Scholar] [CrossRef]
- Sutter, D.O.; Bender, N. Nutrient status and growth in vegan children. Nutr. Res. 2021, 91, 13–25. [Google Scholar] [CrossRef]
- Müller, P. Vegan Diet in Young Children. Nestle Nutr. Inst. Workshop Ser. 2020, 93, 103–110. [Google Scholar]
- Chouraqui, J.P. Vegetarian diets and diets which restrict animal-source foods during childhood in high-income countries. Paediatr. Int. Child. Health 2023, 43, 57–82. [Google Scholar] [CrossRef]
- Fernandes, S.; Oliveira, L.; Pereira, A.; Costa, M.D.C.; Raposo, A.; Saraiva, A.; Magalhães, B. Exploring Vitamin B12 Supplementation in the Vegan Population: A Scoping Review of the Evidence. Nutrients 2024, 16, 1442. [Google Scholar] [CrossRef]
- Chouraqui, J.P. Risk Assessment of Micronutrients Deficiency in Vegetarian or Vegan Children: Not So Obvious. Nutrients 2023, 15, 2129. [Google Scholar] [CrossRef]
- Meulenbroeks, D.; Otten, E.; Smeets, S.; Groeneveld, L.; Jonkers, D.; Eussen, S.; Scheepers, H.; Gubbels, J. The Association of a Vegan Diet during Pregnancy with Maternal and Child Outcomes: A Systematic Review. Nutrients 2024, 16, 3329. [Google Scholar] [CrossRef]
- Avnon, T.; Paz Dubinsky, E.; Lavie, I.; Ben-Mayor Bashi, T.; Anbar, R.; Yogev, Y. The impact of a vegan diet on pregnancy outcomes. J. Perinatol. 2021, 41, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Jebasingh, F.; Thomas, N. Barker Hypothesis and Hypertension. Front. Public. Health 2022, 9, 767545. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.; De Rosso, S.; Mueller, C.; Philippe, K.; Pickard, A.; Nicklaus, S.; van Kleef, E.; Varela, P. Development of food literacy in children and adolescents: Implications for the design of strategies to promote healthier and more sustainable diets. Nutr. Rev. 2024, 82, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.E. Risks and benefits of vegan and vegetarian diets in children. Proc. Nutr. Soc. 2021, 80, 159–164. [Google Scholar] [CrossRef]
- Corkins, M.R.; Daniels, S.R.; de Ferranti, S.D.; Golden, N.H.; Kim, J.H.; Magge, S.N.; Schwarzenberg, S.J. Nutrition in Children and Adolescents. Med. Clin. N. Am. 2016, 100, 1217–1235. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A.; Santolaria-Piedrafita, S.; Cañamares-Orbis, P.; García-Erce, J.A. Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients 2021, 13, 3437. [Google Scholar] [CrossRef]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Stuppia, L.; Connelly, S.T.; Herbst, K.L.; et al. Modern vision of the Mediterranean diet. J. Prev. Med. Hyg. 2022, 63, E36–E43. [Google Scholar]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits-A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Klement, R.J.; Pazienza, V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina 2019, 55, 84. [Google Scholar] [CrossRef]
- Soldán, M.; Argalášová, Ľ.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Brichacek, A.L.; Florkowski, M.; Abiona, E.; Frank, K.M. Ultra-Processed Foods: A Narrative Review of the Impact on the Human Gut Microbiome and Variations in Classification Methods. Nutrients 2024, 16, 1738. [Google Scholar] [CrossRef]
- Longo, V.D.; Anderson, R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell 2022, 185, 1455–1470. [Google Scholar] [CrossRef]


| Children Up to 12 Months of Life | Breastfeeding + Varied Diet + Supplements (Doctor’s Control) |
|---|---|
| Children up to 3 years of age | Varied diet + supplements (doctor’s control) |
| Children older than 3 years and adolescents | Varied diet (vegetable diet + supplements only under the supervision of a doctor) |
| 20–30 years | Plant-based diet + supplements (nutritionist control) or varied diet |
| 30–40 years | Plant-based diet + supplements (nutritionist control) or varied diet |
| 40–60 years | Plant-based diet + supplements (nutritionist control) or varied diet |
| Over 60 years | Plant-based diet + supplements (nutritionist control) or varied diet |
| Pregnant women | Varied diet + supplements (under the control of a doctor) |
| Women who are breastfeeding | Varied diet + supplements (under the control of a doctor) |
| Special pathological conditions (diseases of the gastrointestinal tract, carcinoma, allergy to certain types of food) | Varied diet + supplements (under the control of a doctor) |
| Athletes | Plant-based diet + supplements (supervised by a nutritionist) or varied diet + supplements (under the supervision of a doctor) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovandaric, M.Z.; Jovanović, K.; Raus, M.; Babic, S.; Igic, T.; Kotlica, B.; Milicevic, S. The Significance of Plant Nutrition in the Creation of the Intestinal Microbiota—Prevention of Chronic Diseases: A Narrative Review. Medicina 2024, 60, 1969. https://doi.org/10.3390/medicina60121969
Jovandaric MZ, Jovanović K, Raus M, Babic S, Igic T, Kotlica B, Milicevic S. The Significance of Plant Nutrition in the Creation of the Intestinal Microbiota—Prevention of Chronic Diseases: A Narrative Review. Medicina. 2024; 60(12):1969. https://doi.org/10.3390/medicina60121969
Chicago/Turabian StyleJovandaric, Miljana Z., Kristina Jovanović, Misela Raus, Sandra Babic, Tamara Igic, Boba Kotlica, and Srboljub Milicevic. 2024. "The Significance of Plant Nutrition in the Creation of the Intestinal Microbiota—Prevention of Chronic Diseases: A Narrative Review" Medicina 60, no. 12: 1969. https://doi.org/10.3390/medicina60121969
APA StyleJovandaric, M. Z., Jovanović, K., Raus, M., Babic, S., Igic, T., Kotlica, B., & Milicevic, S. (2024). The Significance of Plant Nutrition in the Creation of the Intestinal Microbiota—Prevention of Chronic Diseases: A Narrative Review. Medicina, 60(12), 1969. https://doi.org/10.3390/medicina60121969







