The Effect of Training Experience on Cardiac Morphology in Resistance Exercise Practitioners: A Study on Left Ventricular Systolic and Diastolic Parameters and Left Atrium Mechanical Functions
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection Tools
2.2.1. Body Surface Area
2.2.2. Systolic and Diastolic Blood Pressure and Heart Rate
2.2.3. ECHO Measurements
2.3. Statistical Analysis
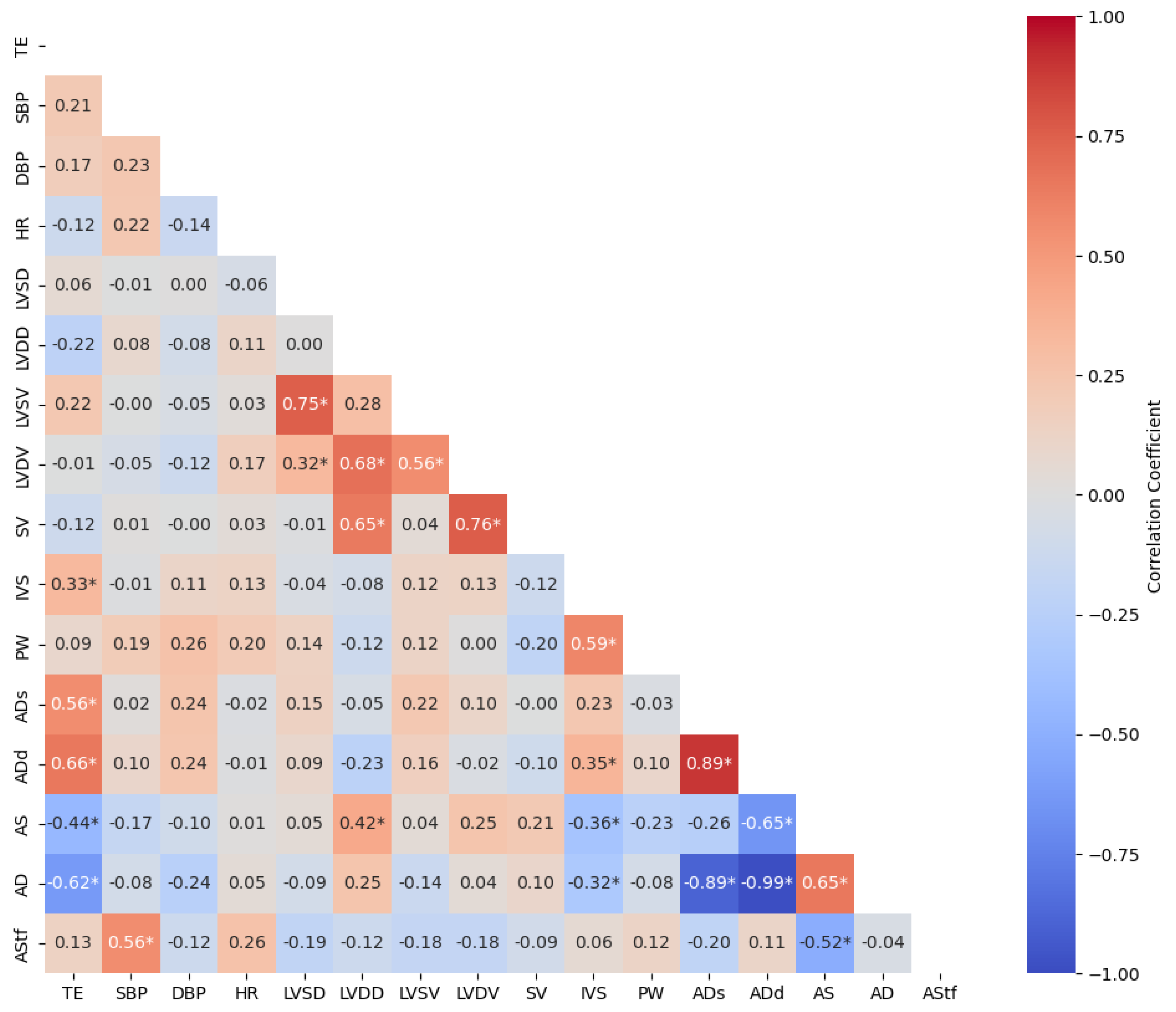
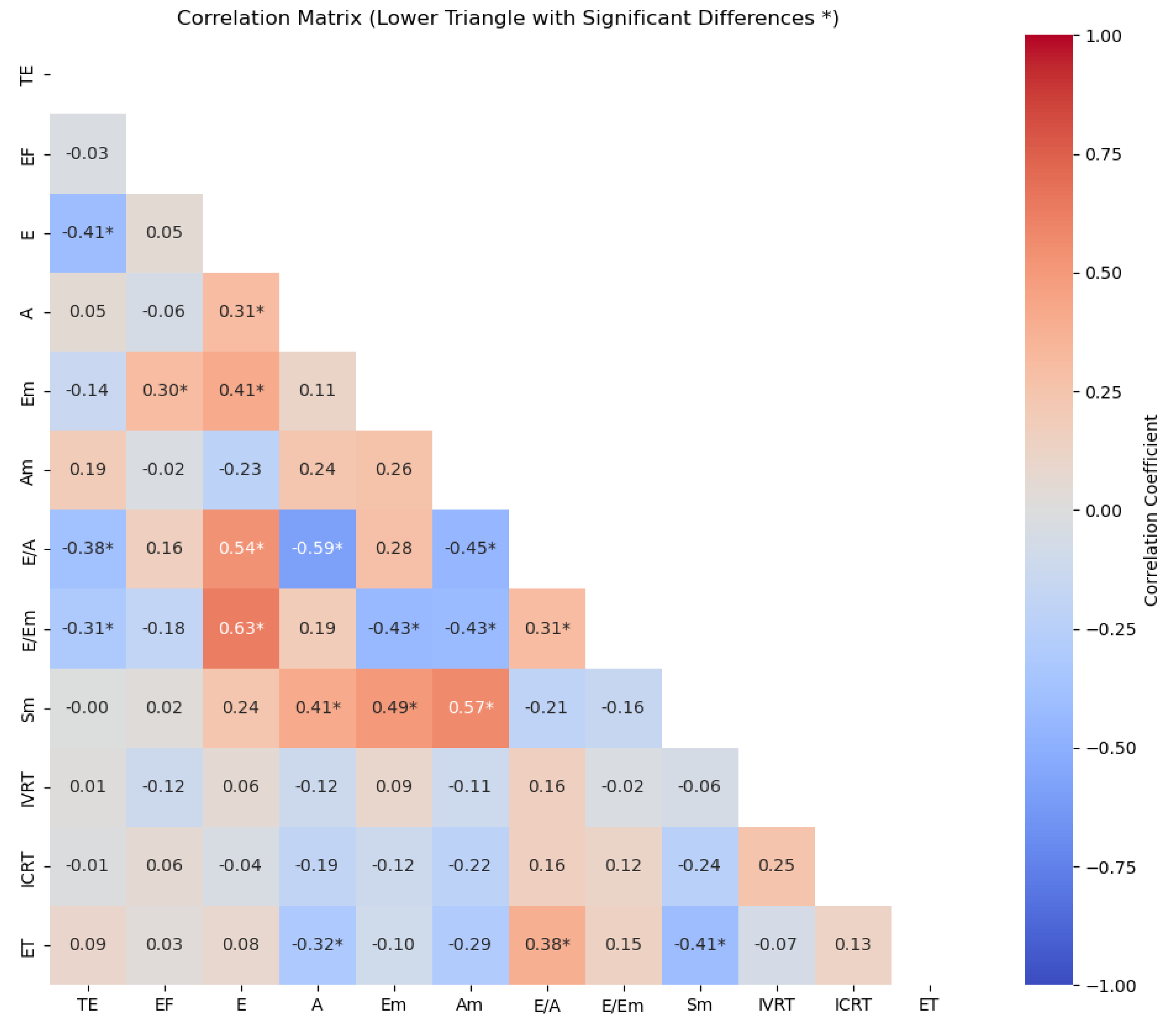
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, M.W.; Kim, J.H.; Shah, A.B.; Phelan, D.; Emery, M.S.; Wasfy, M.M.; Fernandez, A.B.; Bunch, T.J.; Dean, P.; Danielian, A.; et al. Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 78, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Dockerill, C.; Lapidaire, W.; Lewandowski, A.J.; Leeson, P. Cardiac Remodelling and Exercise: What Happens with Ultra-Endurance Exercise? Eur. J. Prev. Cardiol. 2020, 27, 1464–1466. [Google Scholar] [CrossRef] [PubMed]
- Kleinnibbelink, G.; Panhuyzen-Goedkoop, N.; Hulshof, H.; Van Dijk, A.; George, K.; Somauroo, J.; Oxborough, D.; Thijssen, D.H.J. Exercise Training Induces Left- but Not Right-Sided Cardiac Remodelling in Olympic Rowers. Int. J. Sports Med. 2022, 43, 151–160. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Focardi, M.; Mondillo, S. Atrial Enlargement in the Athlete’s Heart: Assessment of Atrial Function May Help Distinguish Adaptive from Pathologic Remodeling. J. Am. Soc. Echocardiogr. 2018, 31, 148–157. [Google Scholar] [CrossRef]
- Slankamenac, J.; Milovancev, A.; Klasnja, A.; Gavrilovic, T.; Sekulic, D.; Kesic, M.G.; Trivic, T.; Kolarov, V.; Drid, P. Echocardiographic Characterization of Left Heart Morphology and Function in Highly Trained Male Judo Athletes. Int. J. Environ. Res. Public Health 2022, 19, 8842. [Google Scholar] [CrossRef]
- Cooke, S.; Samuel, T.J.; Cooper, S.; Stöhr, E.J. Adaptation of Myocardial Twist in the Remodelled Athlete’s Heart Is Not Related to Cardiac Output. Exp. Physiol. 2018, 103, 1456–1468. [Google Scholar] [CrossRef]
- Kurtoğlu, A.; Akgümüş, A.; Balun, A.; Aydın, E.; Kurtoğlu, E.; Çar, B.; Konar, N.; Eken, Ö.; Nobari, H. Investigation of Left Atrial Mechanical Function and Left Ventricular Systolic and Diastolic Parameters in Athletes Performing Resistance Exercise and Combined Exercise. BMC Cardiovasc. Disord. 2024, 24, 237. [Google Scholar] [CrossRef]
- Stöhr, E.J.; Stembridge, M.; Shave, R.; Samuel, T.J.; Stone, K.; Esformes, J.I. Systolic and Diastolic Left Ventricular Mechanics during and after Resistance Exercise. Med. Sci. Sports Exerc. 2017, 49, 2025–2031. [Google Scholar] [CrossRef]
- Petek, B.J.; Groezinger, E.Y.; Pedlar, C.R.; Baggish, A.L. Cardiac Effects of Detraining in Athletes: A Narrative Review. Ann. Phys. Rehabil. Med. 2022, 65, 101581. [Google Scholar] [CrossRef]
- Levy, W.C.; Cerqueira, M.D.; Abrass, I.B.; Schwartz, R.S.; Stratton, J.R. Endurance Exercise Training Augments Diastolic Filling at Rest and during Exercise in Healthy Young and Older Men. Circulation 1993, 88, 116–126. [Google Scholar] [CrossRef]
- Brown, B.; Millar, L.; Somauroo, J.; George, K.; Sharma, S.; La Gerche, A.; Forsythe, L.; Oxborough, D. Left Ventricular Remodeling in Elite and Sub-elite Road Cyclists. Scand. J. Med. Sci. Sports 2020, 30, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Soci, U.P.R.; Oliveira, E.M. Eccentric and Concentric Cardiac Hypertrophy Induced by Exercise Training: microRNAs and Molecular Determinants. Braz. J. Med. Biol. Res. 2011, 44, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.X.; Howard, L. Left Ventricular Hypertrophy in Athletes: Differentiating Physiology From Pathology. Curr. Treat Options Cardio. Med. 2018, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Deng, W.; Liu, T.; Huang, L.; Zhang, R.; Zhang, Y.; Fu, Y.; Fang, L.; Li, Y.; Zhang, L.; et al. Left Atrial Strain Brings New Insights for Evaluating Early Diastolic Dysfunction in Patients with Well-functioning Bicuspid Aortic Valve. Echocardiography 2023, 40, 1243–1250. [Google Scholar] [CrossRef]
- Akgümüş, A.; Kurtoğlu, A.; Aydın, E.; Balun, A.; Çar, B.; Eken, Ö.; Aldhahi, M.I. The Insufficiency of Recreational Exercises in Improving Cardiovascular Fitness: An Investigation of Ventricular Systolic and Diastolic Parameters and Left Atrial Mechanical Functions. BMC Cardiovasc. Disord. 2023, 23, 486. [Google Scholar] [CrossRef]
- Kishi, T. Clinical Implication of Left Ventricular Preload and Afterload Reduction during Venoarterial Extracorporeal Membrane Oxygenation. Int. J. Cardiol. 2020, 320, 124–125. [Google Scholar] [CrossRef]
- Blazek, D.; Stastny, P.; Maszczyk, A.; Krawczyk, M.; Matykiewicz, P.; Petr, M. Systematic Review of Intra-Abdominal and Intrathoracic Pressures Initiated by the Valsalva Manoeuvre during High-Intensity Resistance Exercises. Biol. Sport. 2019, 36, 373–386. [Google Scholar] [CrossRef]
- Gong, F.F.; Coller, J.M.; McGrady, M.; Boffa, U.; Shiel, L.; Liew, D.; Stewart, S.; Owen, A.J.; Krum, H.; Reid, C.M.; et al. Age-related Longitudinal Change in Cardiac Structure and Function in Adults at Increased Cardiovascular Risk. ESC Heart Fail. 2020, 7, 1344–1361. [Google Scholar] [CrossRef]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef]
- Kowallick, J.T.; Silva Vieira, M.; Kutty, S.; Lotz, J.; Hasenfuß, G.; Chiribiri, A.; Schuster, A. Left Atrial Performance in the Course of Hypertrophic Cardiomyopathy: Relation to Left Ventricular Hypertrophy and Fibrosis. Investig. Radiol. 2017, 52, 177–185. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kitano, T.; Nabeshima, Y.; Otsuji, Y.; Otani, K. Left Ventricular and Left Atrial Volume Ratio Assessed by Three-dimensional Echocardiography: Novel Indices for Evaluating Age-related Change in Left Heart Chamber Size. Physiol. Rep. 2019, 7, e14300. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, G.; Cienszkowska, K.; Ludwiczak, M.; Szmigielski, C. Age-Related Values of Aortic Pulse Wave Velocity in Healthy Subjects Measured by Doppler Echocardiography. J. Hum. Hypertens. 2021, 35, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Grilo, G.A.; Shaver, P.R.; Stoffel, H.J.; Morrow, C.A.; Johnson, O.T.; Iyer, R.P.; De Castro Brás, L.E. Age- and Sex-Dependent Differences in Extracellular Matrix Metabolism Associate with Cardiac Functional and Structural Changes. J. Mol. Cell. Cardiol. 2020, 139, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Abdin, A.; Mahfoud, F.; Brueckmann, M.; Gollop, N.D.; Iwata, T.; et al. Empagliflozin Improves Outcomes in Patients With Heart Failure and Preserved Ejection Fraction Irrespective of Age. J. Am. Coll. Cardiol. 2022, 80, 1–18. [Google Scholar] [CrossRef]
- Santoro, A.; Alvino, F.; Antonelli, G.; Cassano, F.E.; De Vito, R.; Cameli, M.; Mondillo, S. Age Related Diastolic Function in Amateur Athletes. Int. J. Cardiovasc. Imaging 2015, 31, 567–573. [Google Scholar] [CrossRef]
- Saheera, S.; Krishnamurthy, P. Cardiovascular Changes Associated with Hypertensive Heart Disease and Aging. Cell Transpl. 2020, 29, 096368972092083. [Google Scholar] [CrossRef]
- Kesmodel, U.S. Cross-sectional Studies—What Are They Good For? Acta Obstet. Gynecol. Scand. 2018, 97, 388–393. [Google Scholar] [CrossRef]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer/Lippincott Williams Wilkins Heal: Philadelphia, PA, USA, 2018. [Google Scholar]
- Zafrir, B.; Salman, N.; Crespo-Leiro, M.G.; Anker, S.D.; Coats, A.J.; Ferrari, R.; Filippatos, G.; Maggioni, A.P.; Mebazaa, A.; Piepoli, M.F.; et al. Body Surface Area as a Prognostic Marker in Chronic Heart Failure Patients: Results from the Heart Failure Registry of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 859–868. [Google Scholar] [CrossRef]
- Johnson, K.A.; Partsch, D.J.; Gleason, P.; Makay, K. Comparison of Two Home Blood Pressure Monitors with a Mercury Sphygmomanometer in an Ambulatory Population. Pharmacotherapy 1999, 19, 333–339. [Google Scholar] [CrossRef]
- Lang, R.; Bierig, M.; Devereux, R.; Flachskampf, F.; Foster, E.; Pellikka, P.; Picard, M.; Roman, M.; Seward, J.; Shanewise, J. Recommendations for Chamber Quantification☆. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Ujino, K.; Barnes, M.E.; Cha, S.S.; Langins, A.P.; Bailey, K.R.; Seward, J.B.; Tsang, T.S.M. Two-Dimensional Echocardiographic Methods for Assessment of Left Atrial Volume. Am. J. Cardiol. 2006, 98, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, S.N. Valsalva Maneuver in Echocardiography. J. Echocardiogr. 2017, 15, 1–5. [Google Scholar] [CrossRef]
- Devereux, R.B.; Reichek, N. Echocardiographic Determination of Left Ventricular Mass in Man. Anatomic Validation of the Method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Stratos, C.; Boudoulas, H.; Kourouklis, C.; Toutouzas, P. Distensibility of the Ascending Aorta: Comparison of Invasive and Non-Invasive Techniques in Healthy Men and in Men with Coronary Artery Disease. Eur. Heart J. 1990, 11, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef]
- Yilmaz, M.; Arican Ozluk, F.O.; Akgumus, A.; Peker, T.; Karaagac, K.; Vatansever, F.; Bekler, A. Left Atrial Mechanical Functions in Patients with the Metabolic Syndrome. Acta Cardiol. 2013, 68, 133–137. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making Meaningful Inferences about Magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef]
- Seyedizadeh, S.H.; Cheragh-Birjandi, S.; Hamedi Nia, M.R. The Effects of Combined Exercise Training (Resistance-Aerobic) on Serum Kinesin and Physical Function in Type 2 Diabetes Patients with Diabetic Peripheral Neuropathy (Randomized Controlled Trials). J. Diabetes Res. 2020, 2020, 6978128. [Google Scholar] [CrossRef]
- Awick, E.A.; Ehlers, D.K.; Aguiñaga, S.; Daugherty, A.M.; Kramer, A.F.; McAuley, E. Effects of a Randomized Exercise Trial on Physical Activity, Psychological Distress and Quality of Life in Older Adults. Gen. Hosp. Psychiatry 2017, 49, 44–50. [Google Scholar] [CrossRef]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical Activity Programs for Balance and Fall Prevention in Elderly: A Systematic Review. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T. Echocardiography and Circulatory Response to Progressive Endurance Exercise. Sports Med. 2008, 38, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Morganroth, J. Comparative Left Ventricular Dimensions in Trained Athletes. Ann. Intern. Med. 1975, 82, 521. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Samuel, T.J.; Nelson, M.D.; La Gerche, A. Athlete’s Heart: Is the Morganroth Hypothesis Obsolete? Heart Lung Circ. 2018, 27, 1037–1041. [Google Scholar] [CrossRef]
- Stephenson, C.; McCarthy, J.; Vikelis, E.; Shave, R.; Whyte, G.; Gaze, D.; George, K. The Effect of Weightlifting upon Left Ventricular Function and Markers of Cardiomyocyte Damage. Ergonomics 2005, 48, 1585–1593. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Liang, Y.; Pechter, D.; Jones, L.W.; McAlister, F.A.; Clark, A.M. A Meta-Analysis of the Effect of Exercise Training on Left Ventricular Remodeling in Heart Failure Patients. J. Am. Coll. Cardiol. 2007, 49, 2329–2336. [Google Scholar] [CrossRef]
- Lentini, A.C.; McKelvie, R.S.; McCartney, N.; Tomlinson, C.W.; MacDougall, J.D. Left Ventricular Response in Healthy Young Men during Heavy-Intensity Weight-Lifting Exercise. J. Appl. Physiol. 1993, 75, 2703–2710. [Google Scholar] [CrossRef]
- Matthews, E.L.; Guers, J.J.; Ramick, M.G.; Hosick, P.A. Inverse Association between Exercising Blood Pressure Response and Left Ventricular Chamber Size and Mass in Women Who Habitually Resistance Train. Healthcare 2024, 12, 353. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Dressendorfer, R.; Taylor, D.; Mandic, S.; Humen, D. Resistance Training and Cardiac Hypertrophy: Unravelling the Training Effect. Sports Med. 2002, 32, 837–849. [Google Scholar] [CrossRef]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic Review and Meta-Analysis of Training Mode, Imaging Modality and Body Size Influences on the Morphology and Function of the Male Athlete’s Heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef]
- Rose, J.-L.; Lalande, A.; Bouchot, O.; Bourennane, E.-B.; Walker, P.M.; Ugolini, P.; Revol-Muller, C.; Cartier, R.; Brunotte, F. Influence of Age and Sex on Aortic Distensibility Assessed by MRI in Healthy Subjects. Magn. Reson. Imaging 2010, 28, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Clemons, J.M.; He, Q.; Liu, S. Effects of Resistance Exercise and Cycling on Recovery Blood Pressure. J. Sports Sci. 1994, 12, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Niederhoffer, N.; Kieffer, P.; Desplanches, D.; Lartaud-Idjouadiene, I.; Sornay, M.-H.; Atkinson, J. Physical Exercise, Aortic Blood Pressure, and Aortic Wall Elasticity and Composition in Rats. Hypertension 2000, 35, 919–924. [Google Scholar] [CrossRef] [PubMed]
- McCallinhart, P.E.; Lee, Y.U.; Lee, A.; Anghelescu, M.; Tonniges, J.R.; Calomeni, E.; Agarwal, G.; Lincoln, J.; Trask, A.J. Dissociation of Pulse Wave Velocity and Aortic Wall Stiffness in Diabetic Db/Db Mice: The Influence of Blood Pressure. Front. Physiol. 2023, 14, 1154454. [Google Scholar] [CrossRef]
- Nabati, M.; Namazi, S.S.; Yazdani, J.; Sharif Nia, H. Relation Between Aortic Stiffness Index and Distensibility with Age in Hypertensive Patients. Int. J. Gen. Med. 2020, 13, 297–303. [Google Scholar] [CrossRef]
- Ryffel, C.P.; Eser, P.; Marcin, T.; Herrsche, D.; Brugger, N.; Trachsel, L.D.; Wilhelm, M. Young Endurance Training Starting Age in Non-Elite Athletes Is Associated with Higher Proximal Aortic Distensibility. Open Heart 2022, 9, e001771. [Google Scholar] [CrossRef]
- Mohabeer, A.L.; Kroetsch, J.T.; McFadden, M.; Khosraviani, N.; Broekelmann, T.J.; Hou, G.; Zhang, H.; Zhou, Y.-Q.; Wang, M.; Gramolini, A.O.; et al. Deletion of Type VIII Collagen Reduces Blood Pressure, Increases Carotid Artery Functional Distensibility and Promotes Elastin Deposition. Matrix Biol. Plus 2021, 12, 100085. [Google Scholar] [CrossRef]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A Test in Context. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, W.; Pan, Y.; Li, T.; Cui, M.; Chen, B. The Correlation between Maternal Age, Parity, Cardiac Diastolic Function and Occurrence Rate of Pre-Eclampsia. Sci. Rep. 2021, 11, 8842. [Google Scholar] [CrossRef]
- Barretti, D.L.M.; Melo, S.F.S.; Oliveira, E.M.; Barauna, V.G. Resistance Training Attenuates Salt Overload-Induced Cardiac Remodeling and Diastolic Dysfunction in Normotensive Rats. Braz. J. Med. Biol. Res. 2017, 50, e6146. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kim, T.-H.; Shim, C.-Y.; Mun, H.-S.; Uhm, J.S.; Joung, B.; Hong, G.-R.; Lee, M.-H.; Pak, H.-N. The Ratio of Early Transmitral Flow Velocity (E) to Early Mitral Annular Velocity (Em) Predicts Improvement in Left Ventricular Systolic and Diastolic Function 1 Year after Catheter Ablation for Atrial Fibrillation. Europace 2015, 17, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, G.; Perrone, M.A.; Iellamo, F.; D’Antoni, V.; Catena, M.; Franchini, A.; Volterrani, M. Acute Left Atrial Response to Different Eccentric Resistance Exercise Loads in Patients with Heart Failure with Middle Range Ejection Fraction: A Pilot Study. J. Pers. Med. 2022, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Di Biase, L.; Leung, M.; Romero, J.; Tops, L.F.; Casadei, B.; Marrouche, N.; Bax, J.J. Structure and Function of the Left Atrium and Left Atrial Appendage. J. Am. Coll. Cardiol. 2017, 70, 3157–3172. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Dini, F.L.; Henein, M.; Mondillo, S. Left Atrial Strain: A New Parameter for Assessment of Left Ventricular Filling Pressure. Heart. Fail. Rev. 2016, 21, 65–76. [Google Scholar] [CrossRef]
- Gottlieb, L.A.; Vaillant, F.; Abell, E.; Belterman, C.; Loyer, V.; El Hamrani, D.; Naulin, J.; Constantin, M.; Quesson, B.; Boukens, B.J.; et al. Localized Pulmonary Vein Scar Promotes Atrial Fibrillation in High Left Atrial Pressure. Front. Physiol. 2021, 12, 709844. [Google Scholar] [CrossRef]
- Teo, S.G.; Yang, H.; Chai, P.; Yeo, T.C. Impact of Left Ventricular Diastolic Dysfunction on Left Atrial Volume and Function: A Volumetric Analysis. Eur. J. Echocardiogr. 2010, 11, 38–43. [Google Scholar] [CrossRef][Green Version]

| Parameters | Mean ± SD | %25 Quartile | %75 Quartile |
|---|---|---|---|
| Age (year) | 29.91 ± 10.30 | 21.0 | 36.50 |
| Height (cm) | 178.37 ± 5.49 | 175.0 | 181.50 |
| Weight (kg) | 83.15 ± 13.91 | 73.0 | 91.50 |
| BMI (kg/m2) | 26.03 ± 3.42 | 23.55 | 27.77 |
| TE (year) | 7.28 ± 6.49 | 1.25 | 12.50 |
| SBP (mmHg) | 124.17 ± 10.35 | 120.0 | 130.0 |
| DBP (mmHg) | 75.60 ± 9.48 | 70.0 | 81.0 |
| HR (beat) | 79.04 ± 9.34 | 72.0 | 85.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurtoğlu, A.; Kurtoğlu, E.; Çar, B.; Eken, Ö.; Muracki, J.; Setiawan, E.; Alotaibi, M.H.; Elkholi, S.M. The Effect of Training Experience on Cardiac Morphology in Resistance Exercise Practitioners: A Study on Left Ventricular Systolic and Diastolic Parameters and Left Atrium Mechanical Functions. Medicina 2024, 60, 2008. https://doi.org/10.3390/medicina60122008
Kurtoğlu A, Kurtoğlu E, Çar B, Eken Ö, Muracki J, Setiawan E, Alotaibi MH, Elkholi SM. The Effect of Training Experience on Cardiac Morphology in Resistance Exercise Practitioners: A Study on Left Ventricular Systolic and Diastolic Parameters and Left Atrium Mechanical Functions. Medicina. 2024; 60(12):2008. https://doi.org/10.3390/medicina60122008
Chicago/Turabian StyleKurtoğlu, Ahmet, Ertuğrul Kurtoğlu, Bekir Çar, Özgür Eken, Jarosław Muracki, Edi Setiawan, Madawi H. Alotaibi, and Safaa M. Elkholi. 2024. "The Effect of Training Experience on Cardiac Morphology in Resistance Exercise Practitioners: A Study on Left Ventricular Systolic and Diastolic Parameters and Left Atrium Mechanical Functions" Medicina 60, no. 12: 2008. https://doi.org/10.3390/medicina60122008
APA StyleKurtoğlu, A., Kurtoğlu, E., Çar, B., Eken, Ö., Muracki, J., Setiawan, E., Alotaibi, M. H., & Elkholi, S. M. (2024). The Effect of Training Experience on Cardiac Morphology in Resistance Exercise Practitioners: A Study on Left Ventricular Systolic and Diastolic Parameters and Left Atrium Mechanical Functions. Medicina, 60(12), 2008. https://doi.org/10.3390/medicina60122008









