Interventions Targeting Insulin Resistance in Patients with Type 1 Diabetes: A Narrative Review
Abstract
1. Background and Objectives
1.1. Diabetes—A Global Public Health Issue
1.2. Type 1 Diabetes vs. Type 2 Diabetes
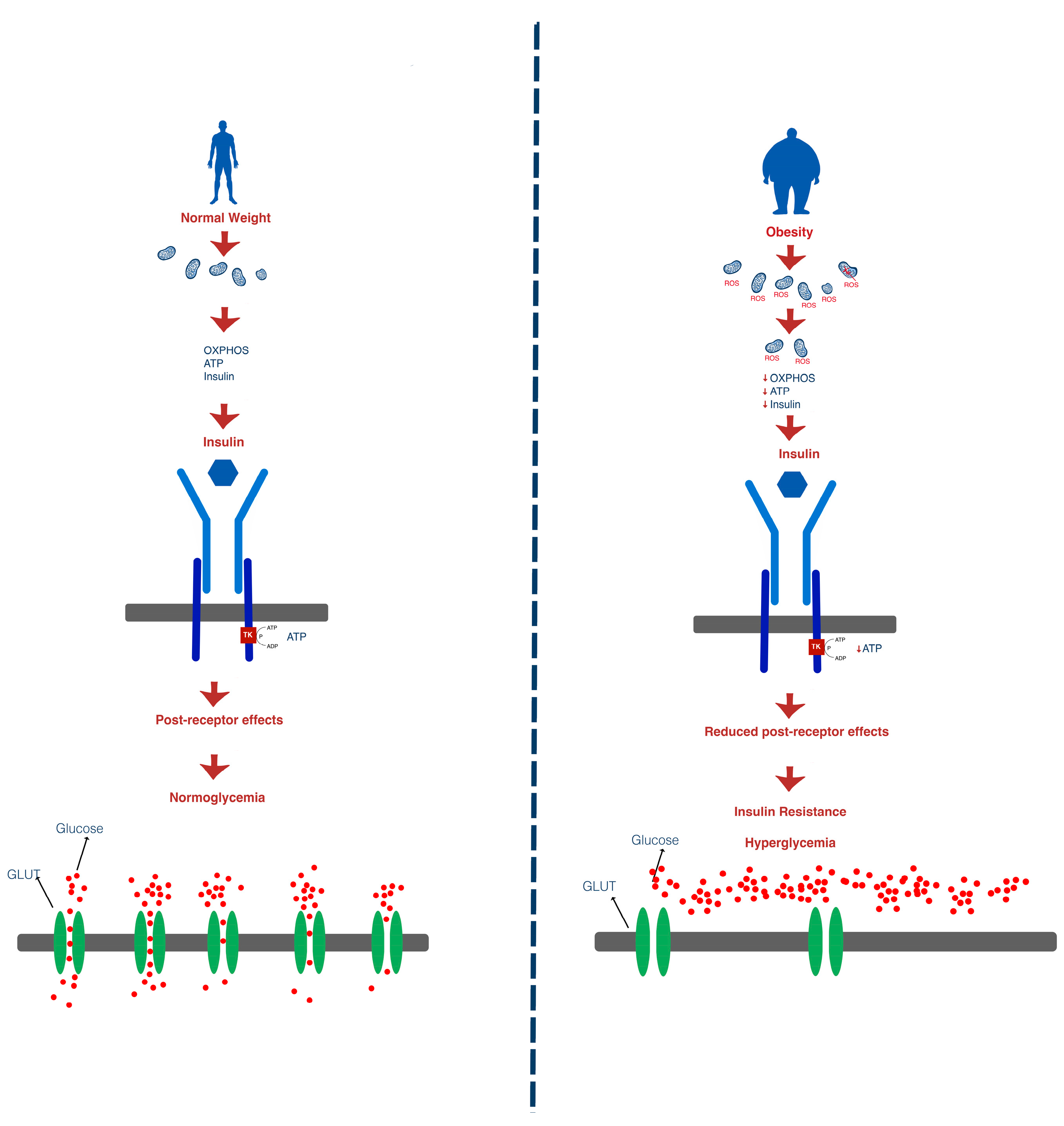
1.3. Insulin Resistance
1.4. Consequences of Insulin Resistance
1.4.1. Obesity
1.4.2. Diabetes Mellitus
1.4.3. Non-Alcoholic Fatty Liver Disease
1.4.4. Polycystic Ovary Syndrome
1.4.5. Cardiovascular Disease
1.4.6. Hypertension
2. Insulin Resistance in T1DM
3. Results: Interventions Targeting Insulin Resistance
3.1. Dietary Interventions
3.2. Physical Activity
3.3. Pharmacological Therapies Targeting Insulin Resistance in T1DM
3.3.1. Metformin
3.3.2. Sodium–Glucose Cotransporter 2 Inhibitors (SGLT2is)
3.3.3. Glucagon-like Peptide-1 Receptor Agonists (GLP1-RAs)
3.3.4. Thiazolidinediones
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes Mellitus, the Fastest Growing Global Public Health Concern: Early Detection Should Be Focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Husein, M.J.; Depkes, R.I.; Soelistijo, S.A.; Novida, H.; Rudijanto, A.; BPJS; Of, S.; Carediabetes, M.; Ceriello, A.; Gavin, J.R.; et al. Classification of Diabetes Mellitus. Dep. Manag. NCD Disabil. Violence Inj. Prev. 2018, 138, 271–281. [Google Scholar]
- Matthew Giwa, A.; Ahmed, R.; Omidian, Z.; Majety, N.; Ege Karakus, K.; Omer, S.M.; Donner, T.; Rahim Hamad, A.A.; Professor, A. Current Understandings of the Pathogenesis of Type 1 Diabetes: Genetics to Environment. World J. Diabetes 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2021, 46, 15. [Google Scholar] [CrossRef]
- Priya, G.; Kalra, S. A Review of Insulin Resistance in Type 1 Diabetes: Is There a Place for Adjunctive Metformin? Diabetes Ther. 2018, 9, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Jose, P.S.; Rubio, M.Á.; Lecube, A. Obesity in Patients with Type 1 Diabetes: Links, Risks and Management Challenges. Diabetes Metab. Syndr. Obes. 2021, 14, 2807–2827. [Google Scholar] [CrossRef]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Courtney, C.H.; Olefsky, J.M. Insulin Resistance. In Mechanisms of Insulin Action: Medical Intelligence Unit; Landes Bioscience: Austin, TX, USA, 2024; pp. 185–209. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.A.; Gavin, J.R.; Aguilar, R.B. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care 2016, 39, 179–186. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Wagg, C.S.; Altamimi, T.R.; Uddin, G.M.; Ho, K.L.; Darwesh, A.M.; Seubert, J.M.; Lopaschuk, G.D. Insulin Directly Stimulates Mitochondrial Glucose Oxidation in the Heart. Cardiovasc. Diabetol. 2020, 19, 207. [Google Scholar] [CrossRef]
- Bouzakri, K.; Koistinen, H.; Zierath, J. Molecular Mechanisms of Skeletal Muscle Insulin Resistance in Type 2 Diabetes. Curr. Diabetes Rev. 2005, 1, 167–174. [Google Scholar] [CrossRef]
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance; StatPearls: Tampa, FL, USA, 2024. [Google Scholar]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The Pathogenesis of Insulin Resistance: Integrating Signaling Pathways and Substrate Flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte Lipolysis and Insulin Resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 1. [Google Scholar] [CrossRef]
- Avram, V.F.; Merce, A.P.; Hâncu, I.M.; Bătrân, A.D.; Kennedy, G.; Rosca, M.G.; Muntean, D.M. Impairment of Mitochondrial Respiration in Metabolic Diseases: An Overview. Int. J. Mol. Sci. 2022, 23, 8852. [Google Scholar] [CrossRef] [PubMed]
- Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Available online: https://www.spandidos-publications.com/10.3892/ijmm.2019.4188 (accessed on 10 November 2024).
- Boushel, R.; Gnaiger, E.; Schjerling, P.; Skovbro, M.; Kraunsøe, R.; Dela, F. Patients with Type 2 Diabetes Have Normal Mitochondrial Function in Skeletal Muscle. Diabetologia 2007, 50, 790–796. [Google Scholar] [CrossRef]
- Mangmool, S.; Denkaew, T.; Parichatikanond, W.; Kurose, H. β-Adrenergic Receptor and Insulin Resistance in the Heart. Biomol. Ther. 2017, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zorzano, A. Intracellular Signaling Mechanisms Involved in Insulin Action. In The Metabolic Syndrome at the Beginning of the XXI Century: A Genetic and Molecular Approach; Elsevier: Barcelona, Spain, 2005; pp. 15–42. [Google Scholar] [CrossRef]
- Navale, A.M.; Paranjape, A.N. Glucose Transporters: Physiological and Pathological Roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef]
- Vargas, E.; Podder, V.; Sepulveda, M.A.C. Physiology, Glucose Transporter Type 4. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Mechanisms of Insulin Resistance in Humans and Possible Links with Inflammation. Hypertension 2005, 45, 828–833. [Google Scholar] [CrossRef]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin Resistance (HOMA-IR) Cut-off Values and the Metabolic Syndrome in a General Adult Population: Effect of Gender and Age: EPIRCE Cross-Sectional Study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Baneu, P.; Văcărescu, C.; Drăgan, S.R.; Cirin, L.; Lazăr-Höcher, A.I.; Cozgarea, A.; Faur-Grigori, A.A.; Crișan, S.; Gaiță, D.; Luca, C.T.; et al. The Triglyceride/HDL Ratio as a Surrogate Biomarker for Insulin Resistance. Biomedicines 2024, 12, 1493. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K. Hyperinsulinemic-Euglycemic Clamp to Assess Insulin Sensitivity in Vivo. Methods Mol. Biol. 2009, 560, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Mcauley, K.A.; Williams, S.M.; Mann, J.I.; Walker, R.J.; Lewis-Barned, N.J.; Temple, L.A.; Duncan, A.W. Diagnosing Insulin Resistance in the General Population. Diabetes Care 2001, 24, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Insulin Resistance and Diabetes | ADA. Available online: https://diabetes.org/health-wellness/insulin-resistance (accessed on 28 October 2024).
- Gerich, J.E. Is Reduced First-Phase Insulin Release the Earliest Detectable Abnormality in Individuals Destined to Develop Type 2 Diabetes? Diabetes 2002, 51 (Suppl. S1), S117–S121. [Google Scholar] [CrossRef] [PubMed]
- Al-Badrani, S.M.; Al-Sowayan, N.S.; Al-Badrani, S.M.; Al-Sowayan, N.S. Consequences of Insulin Resistance Long Term in the Body and Its Association with the Development of Chronic Diseases. J. Biosci. Med. 2022, 10, 96–109. [Google Scholar] [CrossRef]
- Klobučar, S.; Detel, D.; Igrec, M.; Bergoč, A.; Rahelić, V.; Rahelić, D. Overweight and Obesity in Adults with Type 1 Diabetes: A Growing Challenge. Diabetology 2024, 5, 234–245. [Google Scholar] [CrossRef]
- Van der Schueren, B.; Ellis, D.; Faradji, R.N.; Al-Ozairi, E.; Rosen, J.; Mathieu, C. Obesity in People Living with Type 1 Diabetes. Lancet Diabetes Endocrinol. 2021, 9, 776–785. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin Resistance in Obesity: An Overview of Fundamental Alterations. Eat. Weight Disord. 2018, 23, 149–157. [Google Scholar] [CrossRef]
- Al-Sowayan, N.S.; Al-Sowayan, N.S. Exploration of the Relationship between Adipocytokines, Tradition Risk Markers, Nontraditional Risk Markers and Anthropometric Measurements in T2DM Patients. J. Biomed. Sci. Eng. 2015, 8, 184–200. [Google Scholar] [CrossRef][Green Version]
- Guo, S. Insulin Signaling, Resistance, and the Metabolic Syndrome: Insights from Mouse Models into Disease Mechanisms. J. Endocrinol. 2014, 220, T1. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.P.; Cusi, K. Role of Insulin Resistance in the Development of Nonalcoholic Fatty Liver Disease in People with Type 2 Diabetes: From Bench to Patient Care. Diabetes Spectr. 2024, 37, 20–28. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to Diabetes and from Diabetes to NASH: Mechanisms and Treatment Options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of Fatty Acids Stored in Liver and Secreted via Lipoproteins in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E.; et al. Polycystic Ovary Syndrome in Insulin-Resistant Adolescents with Obesity: The Role of Nutrition Therapy and Food Supplements as a Strategy to Protect Fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]
- Unluhizarci, K.; Karaca, Z.; Kelestimur, F. Role of Insulin and Insulin Resistance in Androgen Excess Disorders. World J. Diabetes 2021, 12, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Foley, J.E. Rationale and Application of Fatty Acid Oxidation Inhibitors in Treatment of Diabetes Mellitus. Diabetes Care 1992, 15, 773–784. [Google Scholar] [CrossRef]
- Ginsberg, H.N. REVIEW: Efficacy and Mechanisms of Action of Statins in the Treatment of Diabetic Dyslipidemia. J. Clin. Endocrinol. Metab. 2006, 91, 383–392. [Google Scholar] [CrossRef]
- Semenkovich, C.F. Insulin Resistance and Atherosclerosis. J. Clin. Investig. 2006, 116, 1813. [Google Scholar] [CrossRef]
- Mancusi, C.; Izzo, R.; di Gioia, G.; Losi, M.A.; Barbato, E.; Morisco, C. Insulin Resistance the Hinge Between Hypertension and Type 2 Diabetes. High Blood Press. Cardiovasc. Prev. 2020, 27, 515–526. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.; do Carmo, J.M.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can. J. Cardiol. 2020, 36, 671–682. [Google Scholar] [CrossRef]
- Sasaki, N.; Ozono, R.; Higashi, Y.; Maeda, R.; Kihara, Y. Association of Insulin Resistance, Plasma Glucose Level, and Serum Insulin Level with Hypertension in a Population with Different Stages of Impaired Glucose Metabolism. J. Am. Heart Assoc. 2020, 9, e015546. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Molęda, P.; Pius-Sadowska, E.; Machaliński, B. Double Diabetes-When Type 1 Diabetes Meets Type 2 Diabetes: Definition, Pathogenesis and Recognition. Cardiovasc. Diabetol. 2024, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Sammut, M.J.; Dotzert, M.S.; Melling, C.W.J. Mechanisms of Insulin Resistance in Type 1 Diabetes Mellitus: A Case of Glucolipotoxicity in Skeletal Muscle. J. Cell. Physiol. 2024, e31419. [Google Scholar] [CrossRef] [PubMed]
- Donga, E.; van Dijk, M.; Hoogma, R.P.L.M.; Corssmit, E.P.M.; Romijn, J.A. Insulin Resistance in Multiple Tissues in Patients with Type 1 Diabetes Mellitus on Long-Term Continuous Subcutaneous Insulin Infusion Therapy. Diabetes Metab. Res. Rev. 2013, 29, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Howard, D.; Schauer, I.E.; Maahs, D.M.; Snell-Bergeon, J.K.; Eckel, R.H.; Perreault, L.; Rewers, M. Features of Hepatic and Skeletal Muscle Insulin Resistance Unique to Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Klupa, T. Metformin in Type 1 Diabetes Mellitus? Revisiting Treatment Dogmas in Diabetes. Pol. Arch. Med. Wewn. 2016, 126, 461–462. [Google Scholar] [CrossRef][Green Version]
- Wolosowicz, M.; Lukaszuk, B.; Chabowski, A. The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention? Int. J. Environ. Res. Public Health 2020, 17, 8651. [Google Scholar] [CrossRef]
- Todd, J.A.; Bell, J.I.; McDevitt, H.O. HLA-DQ Beta Gene Contributes to Susceptibility and Resistance to Insulin-Dependent Diabetes Mellitus. Nature 1987, 329, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Edge, J.A.; Dunger, D.B.; Matthews, D.R.; Gilbert, J.P.; Smith, C.P. Increased Overnight Growth Hormone Concentrations in Diabetic Compared with Normal Adolescents. J. Clin. Endocrinol. Metab. 1990, 71, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Somatomedin-C/IGF-I Measured by Radioimmunoassay and Somatomedin Bioactivity in Adolescents with Insulin Dependent Diabetes Compared with Puberty Matched Controls. Diabetes Res. 1988, 9, 177–181. Available online: https://pubmed.ncbi.nlm.nih.gov/3248365/ (accessed on 3 November 2024).
- Jesus, N.; Tavares, P.; Alexandrino, H.; Silva, J.; Goncalves, M.; Sousa, A.; Rocha, G.; Ferreira, M.; Correia, S.; Monteiro, S.; et al. Estimated Glucose Disposal Rate as a Predictor of Chronic Complications in Type 1 Diabetes—A Cross-Sectional Study. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2024; Volume 99. [Google Scholar] [CrossRef]
- Epstein, E.J.; Osman, J.L.; Cohen, H.W.; Rajpathak, S.N.; Lewis, O.; Crandall, J.P. Use of the Estimated Glucose Disposal Rate as a Measure of Insulin Resistance in an Urban Multiethnic Population with Type 1 Diabetes. Diabetes Care 2013, 36, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Cutruzzolà, A.; Parise, M.; Scavelli, F.B.; Fiorentino, R.; Lucà, S.; Di Molfetta, S.; Gnasso, A.; Irace, C. The Potential of Glucose Management Indicator for the Estimation of Glucose Disposal Rate in People with Type 1 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Karamanakos, G.; Barmpagianni, A.; Kapelios, C.J.; Kountouri, A.; Bonou, M.; Makrilakis, K.; Lambadiari, V.; Barbetseas, J.; Liatis, S. The Association of Insulin Resistance Measured through the Estimated Glucose Disposal Rate with Predictors of Micro-and Macrovascular Complications in Patients with Type 1 Diabetes. Prim. Care Diabetes 2022, 16, 837–843. [Google Scholar] [CrossRef]
- Palomo Atance, E.; Ballester Herrera, M.J.; Giralt Muiña, P.; Ruiz Cano, R.; León Martín, A.; Giralt Muiña, J. Estimated Glucose Disposal Rate in Patients under 18 Years of Age with Type 1 Diabetes Mellitus and Overweight or Obesity. Endocrinol. Nutr. (Engl. Ed.) 2013, 60, 379–385. [Google Scholar] [CrossRef]
- Chillarón, J.J.; Goday, A.; Flores-Le-Roux, J.A.; Benaiges, D.; Carrera, M.J.; Puig, J.; Cano-Pérez, J.F.; Pedro-Botet, J. Estimated Glucose Disposal Rate in Assessment of the Metabolic Syndrome and Microvascular Complications in Patients with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 3530–3534. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q.; Hokanson, J.E.; Marcovina, S.M.; Steffes, M.W.; Cleary, P.A.; Brunzell, J.D. Effect of Excessive Weight Gain with Intensive Therapy of Type 1 Diabetes on Lipid Levels and Blood Pressure: Results from the DCCT. Diabetes Control and Complications Trial. JAMA 1998, 280, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Arafat, A.M.; Weickert, M.O.; Adamidou, A.; Otto, B.; Perschel, F.H.; Spranger, J.; Möhlig, M.; Pfeiffer, A.F.H. The Impact of Insulin-Independent, Glucagon-Induced Suppression of Total Ghrelin on Satiety in Obesity and Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2013, 98, 4133–4142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Depoortere, I. Targeting the Ghrelin Receptor to Regulate Food Intake. Regul. Pept. 2009, 156, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Harpsøe, M.C.; Basit, S.; Andersson, M.; Nielsen, N.M.; Frisch, M.; Wohlfahrt, J.; Nohr, E.A.; Linneberg, A.; Jess, T. Body Mass Index and Risk of Autoimmune Diseases: A Study within the Danish National Birth Cohort. Int. J. Epidemiol. 2014, 43, 843–855. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in Autoimmune Diseases: Not a Passive Bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Cedillo, M.; Libman, I.M.; Arena, V.C.; Zhou, L.; Trucco, M.; Ize-Ludlow, D.; Pietropaolo, M.; Becker, D.J. Obesity, Islet Cell Autoimmunity, and Cardiovascular Risk Factors in Youth at Onset of Type 1 Autoimmune Diabetes. J. Clin. Endocrinol. Metab. 2014, 100, E82. [Google Scholar] [CrossRef] [PubMed]
- Grabia, M.; Markiewicz-żukowska, R.; Socha, K.; Polkowska, A.; Zasim, A.; Boruch, K.; Bossowski, A. Prevalence of Metabolic Syndrome in Relation to Cardiovascular Biomarkers and Dietary Factors among Adolescents with Type 1 Diabetes Mellitus. Nutrients 2022, 14, 2435. [Google Scholar] [CrossRef]
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional Management in Children and Adolescents with Diabetes. Pediatr. Diabetes 2018, 19 (Suppl. S27), 136–154. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.; Oza, C.; Mondkar, S.A. Insulin Resistance in Adolescents and Youth with Type 1 Diabetes: A Review of Problems and Solutions. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231206730. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S.; Brehm, B.J.; Bucher, H.C. Effects of Low-Carbohydrate vs Low-Fat Diets on Weight Loss and Cardiovascular Risk Factors: A Meta-Analysis of Randomized Controlled Trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef]
- Natto, Z.S.; Yaghmoor, W.; Alshaeri, H.K.; Van Dyke, T.E. Omega-3 Fatty Acids Effects on Inflammatory Biomarkers and Lipid Profiles among Diabetic and Cardiovascular Disease Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 18867. [Google Scholar] [CrossRef]
- Rosenfalck, A.M.; Almdal, T.; Viggers, L.; Madsbad, S.; Hilsted, J. A Low-Fat Diet Improves Peripheral Insulin Sensitivity in Patients with Type 1 Diabetes. Diabet. Med. 2006, 23, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Annan, S.F.; Higgins, L.A.; Jelleryd, E.; Hannon, T.; Rose, S.; Salis, S.; Baptista, J.; Chinchilla, P.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional Management in Children and Adolescents with Diabetes. Pediatr. Diabetes 2022, 23, 1297–1321. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, A.S.; Leroux, C.; Mircescu, H.; Rabasa-Lhoret, R. Physical Activity Level and Body Composition among Adults with Type 1 Diabetes. Diabet. Med. 2012, 29, e402–e408. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Laan, R.; Dassau, E.; Kerr, D. Physical Activity and Type 1 Diabetes: Time for a Rewire? J. Diabetes Sci. Technol. 2015, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, R.; Hellinckx, T.; Van Laethem, C.; Stegen, S.; De Schepper, J.; Van Aken, S.; Dewolf, D.; Calders, P. Influence of Combined Aerobic and Resistance Training on Metabolic Control, Cardiovascular Fitness and Quality of Life in Adolescents with Type 1 Diabetes: A Randomized Controlled Trial. Clin. Rehabil. 2011, 25, 349–359. [Google Scholar] [CrossRef]
- Yki-Jarvinen, H.; DeFronzo, R.A.; Koivisto, V.A. Normalization of Insulin Sensitivity in Type I Diabetic Subjects by Physical Training during Insulin Pump Therapy. Diabetes Care 1984, 7, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Landt, K.W.; Campaigne, B.N.; James, F.W.; Sperling, M.A. Effects of Exercise Training on Insulin Sensitivity in Adolescents with Type I Diabetes. Diabetes Care 1985, 8, 461–465. [Google Scholar] [CrossRef]
- Wallberg-Henriksson, H.; Gunnarsson, R.; Henriksson, J.; DeFronzo, R.; Felig, P.; Ostman, J.; Wahren, J. Increased Peripheral Insulin Sensitivity and Muscle Mitochondrial Enzymes but Unchanged Blood Glucose Control in Type I Diabetics after Physical Training. Diabetes 1982, 31, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Gregg, B. Metformin; a Review of Its History and Future: From Lilac to Longevity. Pediatr. Diabetes 2017, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, S.J.; Levin, D.; Looker, H.C.; Lindsay, R.S.; Wild, S.H.; Joss, N.; Leese, G.; Leslie, P.; McCrimmon, R.J.; Metcalfe, W.; et al. Estimated Life Expectancy in a Scottish Cohort with Type 1 Diabetes, 2008–2010. JAMA 2015, 313, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gin, H.; Messerchmitt, C.; Brottier, E.; Aubertin, J. Metformin Improved Insulin Resistance in Type I, Insulin-Dependent, Diabetic Patients. Metabolism 1985, 34, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Schäfer, M.; Truong, U.; Cree-Green, M.; Pyle, L.; Baumgartner, A.; Reyes, Y.G.; Maniatis, A.; Nayak, S.; Wadwa, R.P.; et al. Metformin Improves Insulin Sensitivity and Vascular Health in Youth with Type 1 Diabetes Mellitus. Circulation 2018, 138, 2895–2907. [Google Scholar] [CrossRef] [PubMed]
- Maffei, P.; Bettini, S.; Busetto, L.; Dassie, F. SGLT2 Inhibitors in the Management of Type 1 Diabetes (T1D): An Update on Current Evidence and Recommendations. Diabetes, Metab. Syndr. Obes. 2023, 16, 3579. [Google Scholar] [CrossRef]
- Urakami, T.; Yoshida, K.; Suzuki, J. Efficacy of Low-Dose Dapagliflozin in Young People with Type 1 Diabetes. Intern. Med. 2023, 62, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Zinman, B.; Hemmingsson, J.U.; Woo, V.; Colman, P.; Christiansen, E.; Linder, M.; Bode, B. Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care 2016, 39, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B.; Hirsch, I.B.; Pieber, T.R.; Mathieu, C.; Gomez-Peralta, F.; Hansen, T.K.; Philotheou, A.; Birch, S.; Christiansen, E.; Jensen, T.J.; et al. Efficacy and Safety of Liraglutide Added to Capped Insulin Treatment in Subjects with Type 1 Diabetes: The ADJUNCT TWO Randomized Trial. Diabetes Care 2016, 39, 1693–1701. [Google Scholar] [CrossRef]
- Nesto, R.W.; Bell, D.; Bonow, R.O.; Fonseca, V.; Grundy, S.M.; Horton, E.S.; Le Winter, M.; Porte, D.; Semenkovich, C.F.; Smith, S.; et al. Thiazolidinedione Use, Fluid Retention, and Congestive Heart Failure: A Consensus Statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004, 27, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [PubMed]

| Authors | Study | Main Ideas |
|---|---|---|
| A M Arafat et al. [55] | The impact of insulin-independent, glucagon-induced suppression of total ghrelin on satiety in obesity in type 1 diabetes mellitus |
|
| Maria C Harpsøe et al. [57] | Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort |
|
| Mathilde Versini et al. [58] | Obesity in autoimmune disease: not a passive bystander |
|
| Daniel Vestberg et al. [60] | Relationship between overweight and obesity with hospitalization for heart failure in 20,985 patients with type 1 diabetes: a population-based study from the Swedish National Diabetes Registry |
|
| Sarah A Price [61] | Obesity is associated with retinopathy and macrovascular disease in type 1 diabetes |
|
| Orit Pinhas-Hamiel et al. [62] | Prevalence of overweight, obesity and metabolic syndrome components in children, adolescents and young adults with type 1 diabetes mellitus |
|
| Sarah K Holt et al. [63] | Prevalence of low testosterone and predisposing risk factors in men with type 1 diabetes mellitus: findings from the DCCT/EDIC |
|
| Pawel Burchardt et al. [64] | Metformin added to intensive insulin therapy reduces plasma levels of glycated but not oxidized low-density lipoprotein in young patients with type 1 diabetes and obesity in comparison with insulin alone: a pilot study |
|
| S Vella et al. [65] | The use of metformin in type 1 diabetes: a systematic review of efficacy |
|
| Bo Ahrén et al. [66] | Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial |
|
| Juan J Chaillarón et al. [51] | Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herascu, A.; Avram, V.-F.; Gaita, L.; Alexandra, S.; Reurean-Pintilei, D.-V.; Timar, B. Interventions Targeting Insulin Resistance in Patients with Type 1 Diabetes: A Narrative Review. Medicina 2024, 60, 2067. https://doi.org/10.3390/medicina60122067
Herascu A, Avram V-F, Gaita L, Alexandra S, Reurean-Pintilei D-V, Timar B. Interventions Targeting Insulin Resistance in Patients with Type 1 Diabetes: A Narrative Review. Medicina. 2024; 60(12):2067. https://doi.org/10.3390/medicina60122067
Chicago/Turabian StyleHerascu, Andreea, Vlad-Florian Avram, Laura Gaita, Sima Alexandra, Delia-Viola Reurean-Pintilei, and Bogdan Timar. 2024. "Interventions Targeting Insulin Resistance in Patients with Type 1 Diabetes: A Narrative Review" Medicina 60, no. 12: 2067. https://doi.org/10.3390/medicina60122067
APA StyleHerascu, A., Avram, V.-F., Gaita, L., Alexandra, S., Reurean-Pintilei, D.-V., & Timar, B. (2024). Interventions Targeting Insulin Resistance in Patients with Type 1 Diabetes: A Narrative Review. Medicina, 60(12), 2067. https://doi.org/10.3390/medicina60122067






