Mass Spectrometry Investigation of Some ATP-Binding Cassette (ABC) Proteins
Abstract
1. Introduction
2. Discussion
2.1. Developments Which Enhanced the Role of MS in Proteins Characterization
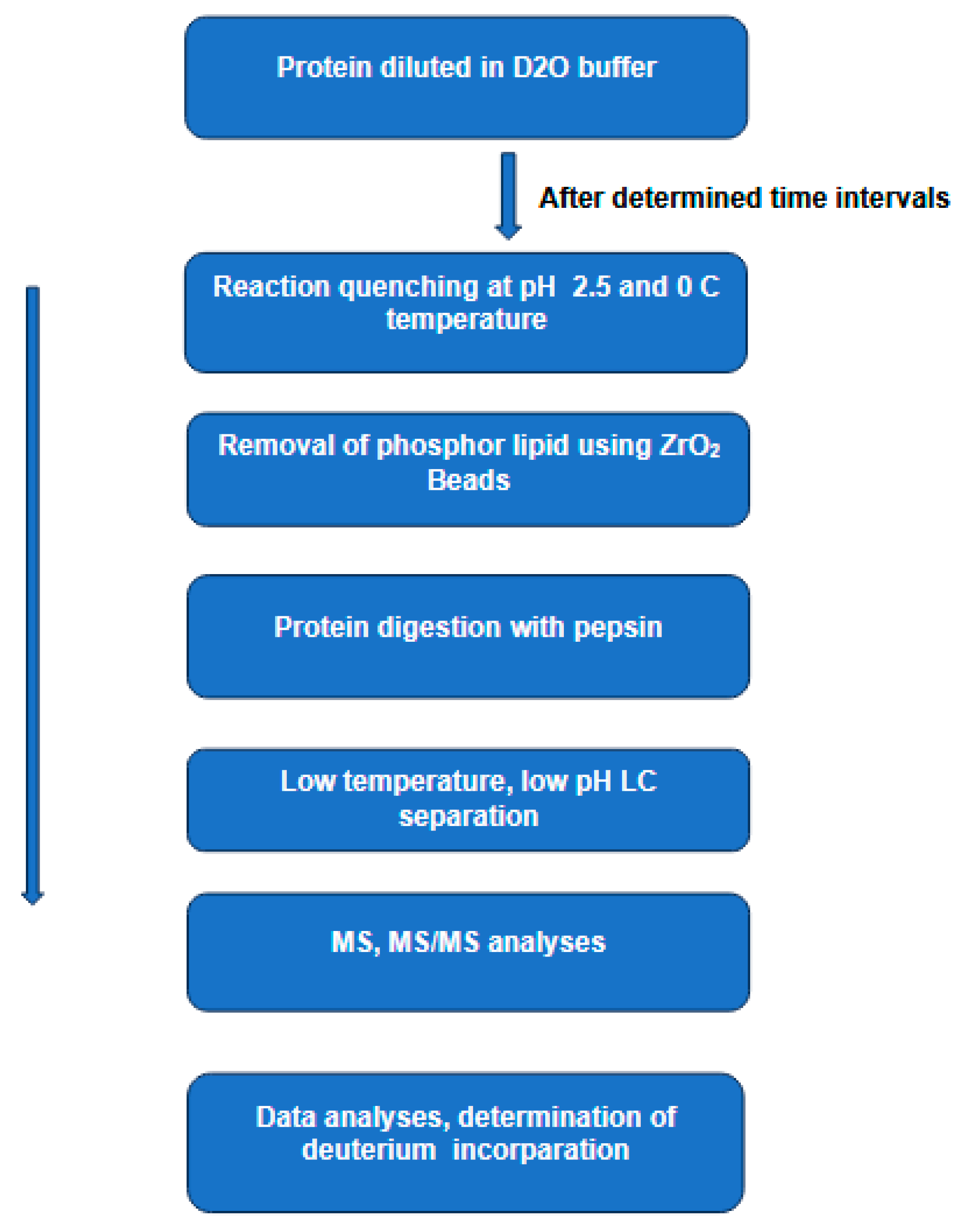
2.1.1. Hydrogen–Deuterium Exchange Mass Spectrometry(H/DX-MS)
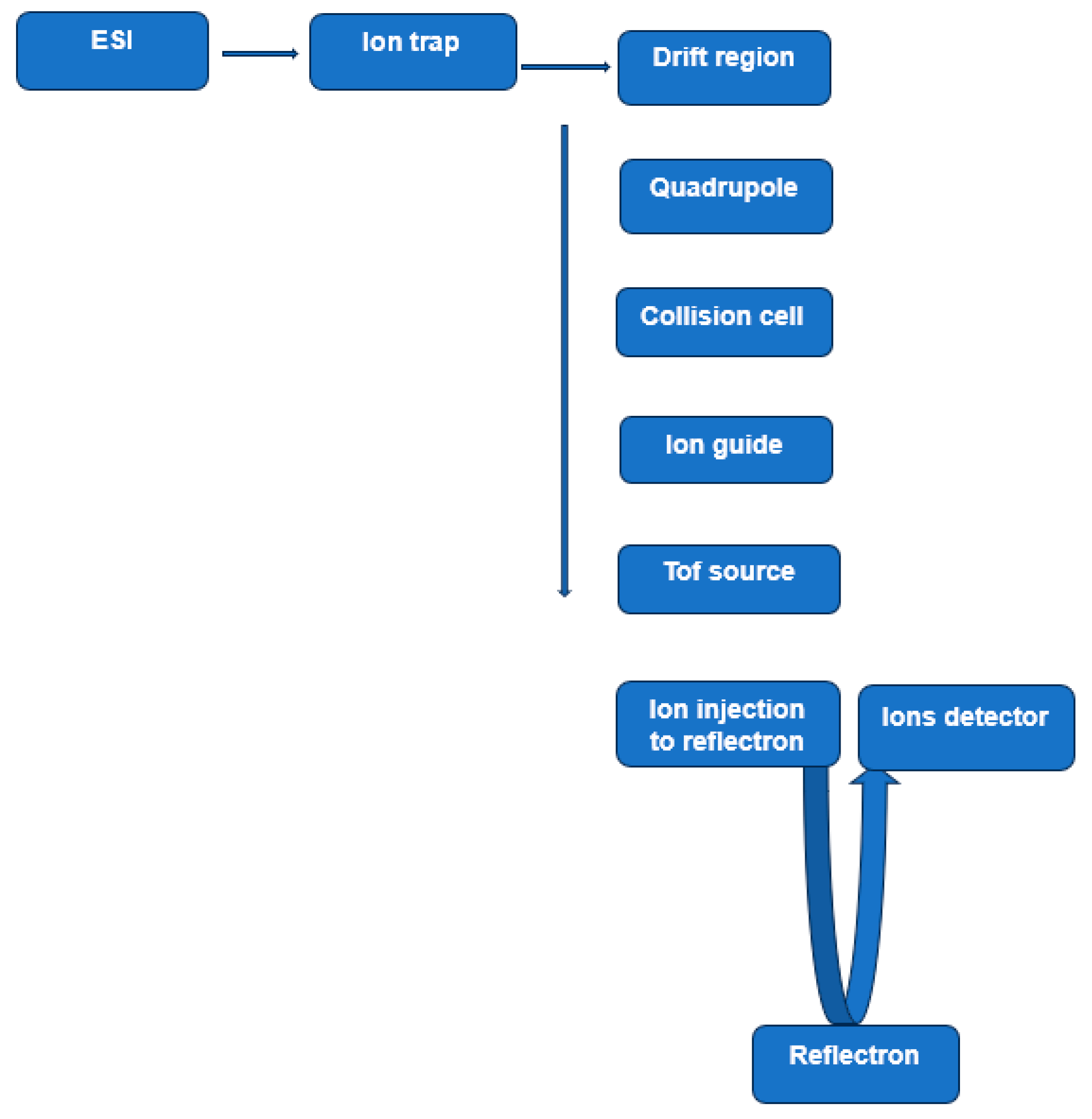
2.1.2. Ion Mobility–Mass Spectrometry
2.1.3. More Efficient Activation Methods of Macromolecules
2.1.4. Electron Capture Dissociation (ECD)
2.1.5. Electron Transfer Dissociation (ETD)
2.1.6. Photodissociation Methods
3. Analysis of Some ATP-Binding Cassette (ABC) Transporters
3.1. Monitoring the Conformation of P-glycoprotein
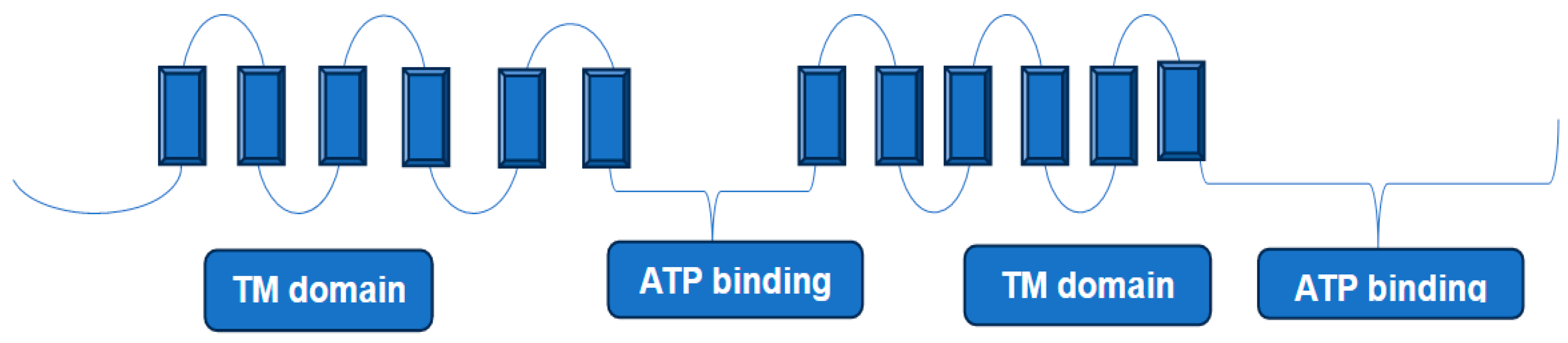
3.2. Breast Cancer Resistance Protein (ABCG2)
4. Commenting on Inhibitors of P-glycoprotein
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| MDR | Multidrug resistance |
| RND | Resistance-nodulation-division |
| SMR | Small multidrug resistance |
| MATE | Multi antimicrobial extrusion |
| MFS | Major facilitator superfamily |
| ATP | Adenosine triphosphate |
| MDR1. ABCB1. | Multidrug resistance protein1 (P-glycoprotein) |
| MDR | associated protein 1: MRP1; ABCC1 |
| BCRP, ABCG2 | Cancer resistance protein |
| MS | Mass spectrometry |
| ESI | Electrospray ionization |
| HDX-MS | Hydrogen/deuterium exchange mass spectrometry |
| TCA | Trichloroacetic acid |
| TCEP | Tris(2-carboxyethyl) phosphine |
| PTMs | Post-translational modifications |
| ECD | Electron capture dissociation |
| ETD | Electron transfer dissociation |
| IM-MS | Ion mobility–mass spectrometry |
| CCS | Collision cross-section |
| Q-TOF | Quadrupole/time-of-flight instrument |
| FT-ICR | Fourier transform ion cyclotron resonance |
| CID | Collision-induced dissociation |
| IRMPD | Infrared multiphoton dissociation |
| UVPD | Ultraviolet photodissociation |
| MALDI | Matrix-assisted laser desorption/ionization; |
| NBD | Nucleotide-binding domain; |
| TMD | Transmembrane domain; |
| FDA | Food and Drug Administration; |
| EMA | European Medicines Agency; |
| HCC | Hepatocellular carcinoma; |
| RT-PCR | Reverse transcription–polymerase chain reaction; |
| IHC | Immunohistochemistry; |
| NSCLC | Non-small cell lung cancer. |
References
- Almén, M.S.; Nordström, K.J.; Fredriksson, R.; Schiöth, H.B. Mapping the human membrane proteome: A majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Wallin, E.; von Heijne, G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998, 7, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Arinaminpathy, Y.; Khurana, E.; Engelman, D.M.; Gerstein, M.B. Computational analysis of membrane proteins: The largest class of drug targets. Drug Discov. Today 2009, 14, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. Opinion—How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Billings, E.; Lundquist, K.; Overly, C.; Srinivasan, K.; Noinaj, N. Structure Determination of Membrane Proteins Using X-ray Crystallography. Methods Mol. Biol. 2021, 2302, 101–136. [Google Scholar] [PubMed]
- Hernando-Amado, S.; Blanco, P.; Alcalde-Rico, M.; Corona, F.; Reales-Calderón, J.A.; Sánchez, M.B.; Martínez, J.L. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist. Update 2016, 28, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.R.; Rozen, D.E. Non-inherited antibiotic resistance. Nat. Rev. Microbiol. 2006, 4, 556–562. [Google Scholar] [CrossRef]
- Corona, F.; Martinez, J.L. Phenotypic Resistance to Antibiotics. Antibiotics 2013, 2, 237–255. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef]
- Choi, C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. The Resolution Revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef]
- Thompson, R.F.; Walker, M.; Siebert, C.A.; Muench, S.P.; Ranson, N.A. An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology. Methods 2016, 100, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Konermann, L.; Pan, J.; Liu, Y.H. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem. Soc. Rev. 2011, 40, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, A.N.; Radford, S.E. Mass spectrometry-enabled structural biology of membrane proteins. Methods 2018, 147, 187–205. [Google Scholar] [CrossRef]
- Laganowsky, A.; Reading, E.; Hopper, J.T.S.; Robinson, C.V. Mass spectrometry of intact membrane protein complexes. Nat. Protoc. 2013, 8, 639–651. [Google Scholar] [CrossRef]
- Lanucara, F.; Holman, S.W.; Gray, C.J.; Eyers, C.E. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem. 2014, 6, 281–294. [Google Scholar] [CrossRef]
- Konijnenberg, A.; Butterer, A.; Sobott, F. Native ion mobility-mass spectrometry and related methods in structural biology. BBA—Proteins Proteom. 2013, 1834, 1239–1256. [Google Scholar] [CrossRef]
- Zhong, Y.; Hyung, S.-J.; Ruotolo, B.T. Ion mobility–mass spectrometry for structural proteomics. Expert Rev. Proteom. 2012, 9, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Engen, J.R.; Wales, T.E. Analytical Aspects of Hydrogen Exchange Mass Spectrometry. Annu. Rev. Anal. Chem. 2015, 8, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Fenn, J.B. Electrospray ion source. Another variation on the free-jet theme. J. Phys. Chem. 1984, 88, 4451–4459. [Google Scholar] [CrossRef]
- Karas, M.; Bachmann, D.; Hillenkamp, F. Influence of the Wavelength in High-Irradiance Ultraviolet Laser Desorption Mass Spectrometry of Organic Molecules. Anal. Chem. 1985, 57, 2935–2939. [Google Scholar] [CrossRef]
- Gygi, S.P.; Rist, B.; Gerber, S.A.; Turecek, F.; Gelb, M.H.; Aebersold, R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 1999, 17, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.C.; Schmitt-Ulms, G.; Chalkley, R.J.; Hirsch, J.; Baldwin, M.A.; Burlingame, A.L. Mass spectrometric analysis of protein misture atlow levels using cleavable 13C-isotope-coded affinity tag and multidimensional chromatography. Mol. Cell Proteom. 2003, 2, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Boersema, P.J.; Raijmakers, R.; Lemeer, S.; Mohammed, S.; Heck, A.J. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 2009, 4, 484–494. [Google Scholar] [CrossRef]
- Schmidt, A.; Kellermann, J.; Lottspeich, F. A novel strategy for quantitative proteomics using isotope-coded protein labels. Proteomics 2005, 5, 4–15. [Google Scholar] [CrossRef]
- Rauniyar, N.; Yates, J.R., 3rd. Isobaric labeling-based relative quantification in shogun proteomics. J. Proteome Res. 2014, 13, 5293–5309. [Google Scholar] [CrossRef]
- Ong, S.-E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, silac, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Katta, W.; Chait, B.T. Hydrogen/Deuterium Exchange Electrospray Ionization Mass Spectrometry: A Method for Probing Protein Conformational Changes in Solution. J. Am. Chem. Soc. 1993, 115, 6317–6321. [Google Scholar] [CrossRef]
- Hammerschmid, D.; Calvaresi, V.; Bailey, C.; Russell, B.; Argyris Politis, L.; Morris, M.; Denbigh, L.; Anderson, M.; Reading, E. Chromatographic Phospholipid Trapping for Automated H/D Exchange Mass Spectrometry of Membrane Protein–Lipid assemblies. Anal. Chem. 2023, 95, 3002–3011. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, V.; Redsted, A.; Norais, N.; Rand, K.D. Hydrogen–Deuterium exchange mass spectrometry with integrated size-exclusion chromatography for analysis of complex protein samples. Anal. Chem. 2021, 93, 11406–11414. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, D.; Maestri, C.; Giammarinaro, P.I.; Capriotti, L.; Bartolini, E.; Veggi, D.; Petracca, R.; Scarselli, M.; Norais, N. Native state organization of outer membrane porins unraveled by HDx-MS. J. Proteome Res. 2018, 17, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Mysling, S.; Salbo, R.; Ploug, M.; Jørgensen, T.J.D. Electrochemical reduction of disulfide-containing proteins for hydrogen/deuterium exchange monitored by mass spectrometry. Anal. Chem. 2014, 86, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Bobst, C.E.; Kaltashov, I.A. Enhancing the Quality of H/D Exchange measurements with mass spectrometry detection in disulfide-rich proteins using electron capture dissociation. Anal. Chem. 2014, 86, 5225–5231. [Google Scholar] [CrossRef]
- Burns, J.A.; Butler, J.C.; Moran, J.; Whitesides, G.M. Selective reduction of disulfides by tris(2-carboxyethyl)phosphine. J. Org. Chem. 1991, 56, 2648–2650. [Google Scholar] [CrossRef]
- Comamala, G.; Wagner, C.; de la Torre, P.S.; Jakobsen, R.U.; Hilger, M.; Brouwer, H.-J.; Rand, K.D. Hydrogen/deuterium exchange mass spectrometry with improved electrochemical reduction enables comprehensive epitope mapping of a therapeutic antibody to the cysteine-knot containing vascular endothelial growth factor. Anal. Chim. Acta 2020, 1115, 41–51. [Google Scholar] [CrossRef]
- Larsen, M.R.; Højrup, P.; Roepstorff, P. Characterization of Gel-separated Glycoproteins Using Two-step Proteolytic Digestion Combined with Sequential Microcolumns and Mass Spectrometry. Mol. Cell. Proteom. 2005, 4, 107–119. [Google Scholar] [CrossRef]
- Darula, Z.; Medzihradszky, K.F. Glycan side reaction may compromise ETD-based glycopeptide identification. J. Am. Soc. Mass Spectrom. 2014, 25, 977–987. [Google Scholar] [CrossRef]
- Houel, S.; Hilliard, M.; Yu, Y.Q.; McLoughlin, N.; Martin, S.M.; Rudd, P.M.; Williams, J.P.; Chen, W. N- and O-glycosylation analysis of etanercept using liquid chromatography and quadrupole time-of-flight mass spectrometry equipped with electron-transfer dissociation functionality. Anal. Chem. 2014, 86, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Rand, K.D. Pinpointing changes in higher-order protein structure by hydrogen/deuterium exchange coupled to electron transfer dissociation mass spectrometry. Int. J. Mass Spectrom. 2013, 338, 2–10. [Google Scholar] [CrossRef]
- Javed, W.; Griffiths, D.; Politis, A. Hydrogen/deuterium exchange-mass spectrometry of integral membrane proteins in native-like environments: Current scenario and the way forward. Essays Biochem. 2023, 67, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Ahmad, A.B.; Sehar, U.; Georgieva, E.R. Lipid Membrane Mimetics in Functional and Structural Studies of Integral Membrane Proteins. Membranes 2021, 11, 685. [Google Scholar] [CrossRef]
- Popot, J.-L. Alternatives to Detergents for Handling Membrane Proteins in Aqueous Solutions Membrane Proteins in Aqueous Solutions; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Blair, J.M.; Piddock, L.J. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: An update. Curr. Opin. Microbiol. 2009, 12, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.J.F.; Maher, C.; Elbourne, L.D.H.; Eijkelkamp, B.A.; Paulsen, I.T.; Hassan, K.A. Physiological functions of bacterial ‘multidrug’ efflux pumps. Chem. Rev. 2021, 121, 5417–5478. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Pasqua, M.; Prosseda, G.; Grossi, M.; Colonna, B. AcrAB efflux pump impacts on the survival of adherent-invasive Escherichia coli strain LF82 inside macrophages. Sci. Rep. 2023, 13, 2692. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.R.; Uddin, M.R.; Moniruzzaman, M.; Kuo, K.M.; Higgins, A.J.; Shah, L.M.N.; Sobott, F.; Parks, J.M.; Hammerschmid, D.; Gumbart, J.C.; et al. Conformational restriction shapes the inhibition of a multidrug efflux adaptor protein. Nat. Commun. 2023, 14, 3900. [Google Scholar] [CrossRef]
- Lin, X.; Zmyslowski, A.M.; Gagnon, I.A.; Nakamoto, R.K.; Sosnick, T.R. Development of in vivo HDX-MS with applications to a TonB-dependent transporter and other proteins. Protein Sci. 2022, 31, e4402. [Google Scholar] [CrossRef]
- Karasek, F.W. Drift-mass spectrometer. Res. Dev. 1970, 21, 25–27. [Google Scholar]
- Alge, E.; Villinger, H.; Lindinger, W. Drift tube investigations on the reactions of O2 + with CH4 and of CO2 + with NO in various buffer gases. Plasma Chem. Plasma Process. 1981, 1, 65–71. [Google Scholar] [CrossRef]
- Smith, D.P.; Giles, K.; Bateman, R.H.; Radford, S.E.; Ashcroft, A.E. Monitoring Copopulated Conformational States during Protein Folding Events Using Electrospray Ionization-Ion Mobility Spectrometry-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- May, J.C.; Morris, C.B.; McLean, J.A. Ion mobility collision cross section compendium. Anal. Chem. 2017, 89, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Williams, J.P.; Menikarachchi, L.; Thompson, J.W.; Tyldesley-Worster, R.; Halldórsson, S.; Rolfsson, O.; Moseley, A.; Grant, D.; Langridge, J.; et al. Ion mobility derived collision cross sections to support metabolomics applications. Anal. Chem. 2014, 86, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tu, J.; Zhu, Z.-J. Advancing the large-scale CCS database for metabolomics and lipidomics at the machine-learning era. Curr. Opin. Chem. Biol. 2018, 42, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gabelica, V.; Shvartsburg, A.A.; Afonso, C.; Barran, P.; Benesch, J.L.P.; Bleiholder, C.; Bowers, M.T.; Bilbao, A.; Bush, M.F.; Campbell, J.L.; et al. Recommendations for reporting ion mobility Mass Spectrometry measurements. Mass Spectrom. Rev. 2019, 38, 291–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Luo, M.; Chen, X.; Yin, Y.; Xiong, X.; Wang, R.; Zhu, Z.-J. Ion mobility collision cross-section atlas for known and unknown metabolite annotation in untargeted metabolomics. Nat. Commun. 2020, 11, 4334. [Google Scholar] [CrossRef] [PubMed]
- Hoaglund-Hyzer, C.S.; Clemmer, D.E. Ion Trap/Ion Mobility/Quadrupole/Time-of-Flight Mass Spectrometry for Peptide Mixture Analysis. Anal. Chem. 2001, 73, 177–184. [Google Scholar] [CrossRef]
- Wyttenbach, T.; Bowers, M.T. Structural stability from solution to the gas phase: Native solution structure of ubiquitin survives analysis in a solvent-free ion mobility–mass spectrometry environment. J. Phys. Chem. B 2011, 115, 12266–12275. [Google Scholar] [CrossRef]
- Ruotolo, B.T.; Robinson, C.V. Aspects of native proteins are retained in vacuum. Curr. Opin. Chem. Biol. 2006, 10, 402–408. [Google Scholar] [CrossRef]
- Turzo, S.M.B.A.; Seffernick, J.T.; Rolland, A.D.; Donor, M.T.; Heinze, S.; Prell, J.S.; Wysocki, V.H.; Lindert, S. Protein shape sampled by ion mobility mass spectrometry consistently improves protein structure prediction. Nat. Commun. 2022, 13, 4377. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; Bonito, C.A.; Cordeiro, M.N.D.S.; Ferreira, M.-J.U.; dos Santos, D.J.V.A. Structure-function relationships in ABCG2: Insights from molecular dynamics simulations and molecular docking studies. Sci. Rep. 2017, 7, 15534. [Google Scholar] [CrossRef]
- Christofi, E.; Barran, P. Ion Mobility Mass Spectrometry (IM-MS) for Structural Biology: Insights Gained by Measuring Mass, Charge, and Collision Cross Section. Chem. Rev. 2023, 123, 2902–2949. [Google Scholar] [CrossRef] [PubMed]
- Yost, R.A.; Enke, C.G. Selected ion fragmentation with a tandem quadrupole mass spectrometer. J. Am. Chem. Soc. 1978, 100, 2274–2275. [Google Scholar] [CrossRef]
- Zubarev, R.A.; Horn, D.M.; Fridriksson, E.K.; Kelleher, N.L.; Kruger, N.A.; Lewis, M.A.; Carpenter, B.K.; McLafferty, F.W. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 2000, 72, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Syrstad, E.A.; Turecček, F. Toward a general mechanism of electron capture dissociation. J. Am. Soc. Mass Spectrom. 2005, 16, 208–224. [Google Scholar] [CrossRef]
- Zubarev, R.A.; Kelleher, N.L.; McLafferty, F.W. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc. 1998, 120, 3265–3266. [Google Scholar] [CrossRef]
- Qi, Y.; Volmer, D.A. Electron-based fragmentation methods in mass spectrometry: An overview. Mass Spectrom. Rev. 2017, 36, 4–15. [Google Scholar] [CrossRef]
- Baba, T.; Campbell, J.L.; Le Blanc, J.C.Y.; Hager, J.W.; Thomson, B.A. Electron Capture Dissociation in a Branched Radio-Frequency Ion Trap. Anal. Chem. 2015, 87, 785–792. [Google Scholar] [CrossRef]
- Voinov, V.G.; Deinzer, M.L.; Barofsky, D.F. Electron capture dissociation in a linear radiofrequency-free magnetic cell. Rapid Commun. Mass Spectrom. 2008, 22, 3087–3088. [Google Scholar] [CrossRef]
- Voinov, V.G.; Bennett, S.E.; Beckman, J.S.; Barofsky, D.F. ECD of Tyrosine Phosphorylation in a Triple Quadrupole Mass Spectrometer with a Radio-Frequency-Free Electro-magnetostatic Cell. J. Am. Soc. Mass Spectrom. 2014, 25, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Syka, J.E.P.; Coon, J.J.; Schroeder, M.J.; Shabanowitz, J.; Hunt, D.F. Peptide and Protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 2004, 101, 9528–9533. [Google Scholar] [CrossRef] [PubMed]
- Pitteri, S.J.; Chrisman, P.A.; McLuckey, S.A. Electron-Transfer Ion/Ion Reactions of Doubly Protonated Peptides: Effect of Elevated Bath Gas Temperature. Anal. Chem. 2005, 77, 5662–5669. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.J.; Brodbelt, J.S. Enhanced electron transfer dissociation of peptides modified at C-terminus with fixed charges. J. Am. Soc. Mass Spectrum. 2012, 23, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.L.; Ladror, D.T.; Sondalle, S.B.; Krusemark, C.J.; Jue, A.L.; Coon, J.J.; Smith, L.M. Chemical derivatization of peptide carboxyl groups for highly efficient electron transfer dissociation. J. Am. Soc. Mass Spectrom. 2013, 24, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Swaney, D.L.; McAlister, G.C.; Coon, J.J. Decision tree–driven tandem mass spectrometry for shotgun proteomics. Nat. Methods 2008, 5, 959–964. [Google Scholar] [CrossRef]
- Frese, C.K.; Altelaar, A.F.M.; Hennrich, M.L.; Nolting, D.; Zeller, M.; Griep-Raming, J.; Heck, A.J.R.; Mohammed, S. Improved peptide identification by targeted fragmentation using CID, HCD and ETD on an LTQ-orbitrap velos. J. Proteome Res. 2011, 10, 2377–2388. [Google Scholar] [CrossRef]
- Tureček, F.; Julian, R.R. Peptide radicals and cation radicals in the gas phase. Chem. Rev. 2013, 113, 6691–6733. [Google Scholar] [CrossRef]
- Zhurov, K.O.; Fornelli, L.; Wodrich, M.D.; Laskay, A.; Tsybin, Y.O. Principles of electron capture and transfer dissociation mass spectrometry applied to peptide and protein structure analysis. Chem. Soc. Rev. 2013, 42, 5014–5030. [Google Scholar] [CrossRef]
- Woodin, R.L.; Bomse, D.S.; Beauchamp, J.L. Multiphoton dissociation of molecules with low power continuous wave infrared laser radiation. J. Am. Chem. Soc. 1978, 100, 3248–3250. [Google Scholar] [CrossRef]
- Maitre, P.; Scuderi, D.; Corinti, D.; Chiavarino, B.; Crestoni, M.E.; Fornarini, S. Applications of Infrared Multiple Photon Dissociation (IRMPD) to the Detection of Posttranslational Modifications. Chem. Rev. 2020, 120, 3261–3295. [Google Scholar] [CrossRef] [PubMed]
- Greisch, J.-F.; van der Laarse, S.A.; Heck, A.J. Enhancing Top-Down Analysis Using Chromophore-Assisted Infrared Multiphoton Dissociation from (Phospho)peptides to Protein Assemblies. Anal. Chem. 2020, 92, 15506–15516. [Google Scholar] [CrossRef] [PubMed]
- Bowers, W.D.; Delbert, S.S.; Hunter, R.L.; Mciver, R.T. Fragmentation of oligopeptide ions using ultraviolet laser radiation and fourier transform mass spectrometry. J. Am. Chem. Soc. 1984, 106, 7288–7289. [Google Scholar] [CrossRef]
- Hunt, D.F.; Shabanowitz, J.; Yates, J.R. Peptide sequence analysis by laser photodissociation Fourier transform mass spectrometry. J. Chem. Soc. Chem. Commun. 1987, 548–550. [Google Scholar] [CrossRef]
- Thompson, M.S.; Cui, W.; Reilly, J.P. Fragmentation of Singly Charged Peptide Ions by Photodissociation at λ = 157 nm. Angew. Chem. Int. Ed. 2004, 43, 4791–4794. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Cammarata, M.B.; Robotham, S.A.; Cotham, V.C.; Shaw, J.B.; Fellers, R.T.; Early, B.P.; Thomas, P.M.; Kelleher, N.L.; Brodbelt, J.S. Ultraviolet photodissociation for characterization of whole proteins on a chromatographic time scale. Anal. Chem. 2014, 86, 2185–2192. [Google Scholar] [CrossRef]
- Smyrnakis, A.; Levin, N.; Kosmopoulou, M.; Jha, A.; Fort, K.; Makarov, A.; Papanastasiou, D.; Mohammed, S. Characterization of an Omnitrap-Orbitrap Platform Equipped with Infrared Multiphoton Dissociation, Ultraviolet Photodissociation, and Electron Capture Dissociation for the Analysis of Peptides and Proteins. Anal. Chem. 2023, 95, 12039–12046. [Google Scholar] [CrossRef]
- Fornelli, L.; Srzentić, K.; Toby, T.K.; Doubleday, P.F.; Huguet, R.; Mullen, C.; Melani, R.D.; dos Santos Seckler, H.; DeHart, C.J.; Weisbrod, C.R.; et al. Thorough Performance Evaluation of 213 nm Ultraviolet Photodissociation for Top-down Proteomics. Mol. Cell. Proteom. 2020, 19, 405–420. [Google Scholar] [CrossRef]
- Julian, R.R.; Amster, I.J.; Kong, X.; Brodbelt, J.S.; Jørgensen, T.J.D.; Wysocki, V.H.; Hendrickson, C.L.; Santos, I.; Shaw, J.B.; Boyarkin, O.V.; et al. The mechanism behind top-down uvpd experiments: Making sense of apparent contradictions. J. Am. Soc. Mass Spectrom. 2017, 28, 1823–1826. [Google Scholar] [CrossRef]
- Zabuga, A.V.; Kamrath, M.Z.; Boyarkin, O.V.; Rizzo, T.R. Fragmentation mechanism of UV-excited peptides in the gas phase. J. Chem. Phys. 2014, 141, 154309. [Google Scholar] [CrossRef]
- Papanastasiou, D.; Kounadis, D.; Lekkas, A.; Orfanopoulos, I.; Mpozatzidis, A.; Smyrnakis, A.; Panagiotopoulos, E.; Kosmopoulou, M.; Reinhardt-Szyba, M.; Fort, K.; et al. The Omnitrap Platform: A Versatile Segmented Linear Ion Trap for Multidimensional Multiple-Stage Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2022, 33, 1990–2007. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. BBA—Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Szewczyk, P.; Grimard, V.; Lee, C.-W.; Martinez, L.; Doshi, R.; Caya, A.; Villaluz, M.; Pardon, E.; Cregger, C.; et al. Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc. Natl. Acad. Sci. USA 2013, 110, 13386–13391. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Van Bambeke, F. ABC multidrug transporters: Target for modulation of drug pharmacokinetics and drug-drug interactions. Curr. Drug Targets 2011, 12, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef]
- Wen, P.-C.; Verhalen, B.; Wilkens, S.; Mchaourab, H.S.; Tajkhorshid, E. On the origin of large flexibility of P-glycoprotein in the inward-facing state. J. Biol. Chem. 2013, 288, 19211–19220. [Google Scholar] [CrossRef]
- Jin, M.S.; Oldham, M.L.; Zhang, Q.; Chen, J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 2012, 490, 566–569. [Google Scholar] [CrossRef]
- Kopcho, N.; Geoffrey Chang, G.; Komives, E.A. Dynamics of ABC transporter P-glycoprotein in three conformational states. Sci. Rep. 2019, 9, 15092. [Google Scholar] [CrossRef]
- Crowley, E.; McDevitt, C.A.; Callaghan, R. Generating Inhibitors of P-Glycoprotein: Where to, now? Multi-Drug Resist. Cancer 2010, 596, 405–432. [Google Scholar]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Leonard, G.D.; Fojo, T.; Bates, S.E. The role of ABC transporters in clinical practice. Oncologist 2003, 8, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Modok, S.; Mellor, H.R.; Callaghan, R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr. Opin. Pharmacol. 2006, 6, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J.; Erba, P.; Mariani, G.; Gomes, C.M.F. Multidrug resistance in cancer: Its mechanism and its modulation. Drug News Perspect. 2007, 20, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Van Herwaarden, A.E.; Schinkel, A.H. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol. Sci. 2006, 27, 10–16. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Khunweeraphong, N.; Stockner, T.; Kuchler, K. The structure of the human ABC transporter ABCG2 reveals a novel mechanism for drug extrusion. Sci. Rep. 2017, 7, 13767. [Google Scholar] [CrossRef]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef]
- Manolaridis, I.; Jackson, S.M.; Taylor, N.M.I.; Kowal, J.; Stahlberg, H.; Locher, K.P. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 2018, 563, 426–430. [Google Scholar] [CrossRef]
- Kim, Y.; Chen, J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science 2018, 359, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Dönmez Cakil, Y.; Khunweeraphong, N.; Parveen, Z.; Schmid, D.; Artaker, M.; Ecker, G.F.; Sitte, H.H.; Pusch, O.; Stockner, T.; Chiba, P. Pore-exposed tyrosine residues of P-glycoprotein are important hydrogen-bonding partners for drugs. Mol. Pharmacol. 2014, 85, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Zhang, Y.; Vagiannis, D.; Budagaga, Y.; Sabet, Z.; Hanke, I.; Rozkoš, T.; Hofman, J. Sonidegib potentiates the cancer cells’ sensitivity to cytostatic agents by functional inhibition of ABCB1 and ABCG2 in vitro and ex vivo. Biochem. Pharmacol. 2022, 199, 115009. [Google Scholar] [CrossRef]
- Gao, H.-L.; Cui, Q.; Wang, J.-Q.; Ashby, C.R., Jr.; Chen, Y.; Shen, Z.-X.; Chen, Z.-S. The AKT inhibitor, MK-2206, attenuates ABCG2-mediated drug resistance in lung and colon cancer cells. Front. Pharmacol. 2023, 13, 1235285. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Yang, Y.; Bates, S.; Zhang, J.-T. Characterization of Oligomeric Human Half-ABC Transporter ATP-binding Cassette G2. J. Biol. Chem. 2004, 279, 19781–19789. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xiang, D.; Wang, T.; Zhang, Y.; Pham, C.V.; Zhou, S.; Jiang, G.; Hou, Y.; Zhu, Y.; Han, Y.; et al. The inhibition of ABCB1/MDR1 or ABCG2/BCRP enables doxorubicin to eliminate liver cancer stem cells. Sci. Rep. 2021, 11, 10791. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Weinman, S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepatic Oncol. 2016, 3, 57–59. [Google Scholar] [CrossRef]
- Budagaga, Y.; Sabet, Z.; Zhang, Y.; Novotná, E.; Hanke, I.; Rozkoš, T.; Hofman, J. Tazemetostat synergistically combats multidrug resistance by the unique triple inhibition of ABCB1, ABCC1, and ABCG2 efflux transporters in vitro and ex vivo. Biochem. Pharmacol. 2023, 216, 115769. [Google Scholar] [CrossRef]
- Dey, S.; Ramachandra, M.; Pastan, I.; Gottesman, M.M.; Ambudkar, S.V. Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc. Natl. Acad. Sci. USA 1997, 94, 10594–10599. [Google Scholar] [CrossRef]
- Pleban, K.; Kopp, S.; Csaszar, E.; Peer, M.; Hrebicek, T.; Rizzi, A.; Ecker, G.F.; Chiba, P. P-glycoprotein substrate binding domains are located at the transmembrane domain/transmembrane domain interfaces: A combined photoaffinity labeling-protein homology modeling approach. Mol. Pharmacol. 2005, 67, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.E.; Ellington, A.D. Nucleic Acid Selection and the Challenge of Combinatorial Chemistry. Chem. Rev. 1997, 97, 349–370. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostini, M.; Traldi, P.; Hamdan, M. Mass Spectrometry Investigation of Some ATP-Binding Cassette (ABC) Proteins. Medicina 2024, 60, 200. https://doi.org/10.3390/medicina60020200
Agostini M, Traldi P, Hamdan M. Mass Spectrometry Investigation of Some ATP-Binding Cassette (ABC) Proteins. Medicina. 2024; 60(2):200. https://doi.org/10.3390/medicina60020200
Chicago/Turabian StyleAgostini, Marco, Pietro Traldi, and Mahmoud Hamdan. 2024. "Mass Spectrometry Investigation of Some ATP-Binding Cassette (ABC) Proteins" Medicina 60, no. 2: 200. https://doi.org/10.3390/medicina60020200
APA StyleAgostini, M., Traldi, P., & Hamdan, M. (2024). Mass Spectrometry Investigation of Some ATP-Binding Cassette (ABC) Proteins. Medicina, 60(2), 200. https://doi.org/10.3390/medicina60020200





