Menstrual Changes Following COVID-19 Vaccination: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
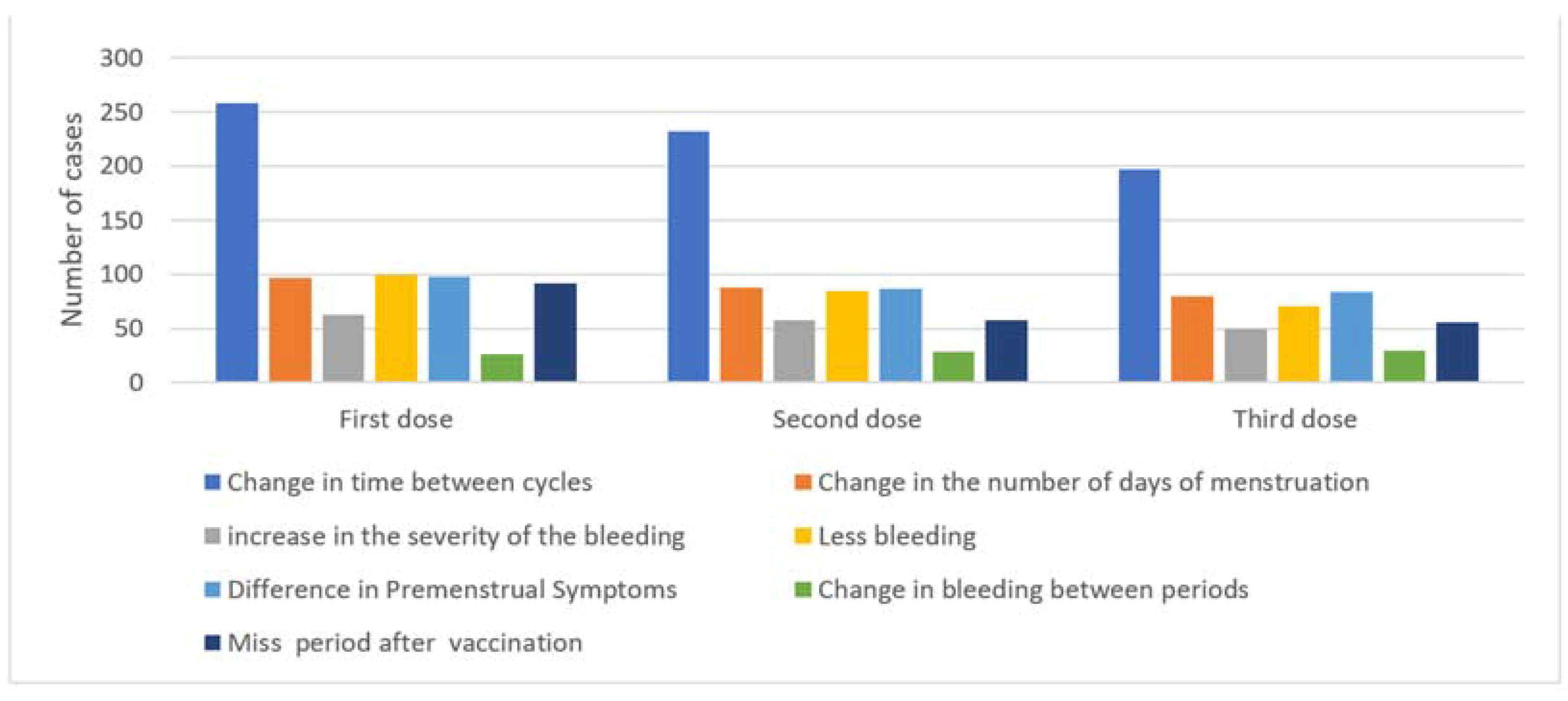
3.1. Menstrual Changes after the First, Second, and Third Vaccine Doses
3.2. Menstrual Cycle Characteristics of the Participants
3.3. Association between Menstrual Cycle Changes Following COVID-19 Vaccination and Participant Characteristics
3.4. Menstrual Cycle Changes According to Vaccine Brand
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McIntosh, K. COVID-19: Epidemiology, Virology, and Prevention; UpToDate Inc.: Waltham, MA, USA, 2023. [Google Scholar]
- Edwards, K.M.; Orenstein, W.A. COVID-19: Vaccines; UpToDate Inc.: Waltham, MA, USA, 2023. [Google Scholar]
- World Health Organization. Coronavirus Disease (COVID-19): Vaccines and Vaccine Safety; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- VIPER Group COVID19 Vaccine Tracker Team. Saudi Arabia—COVID19 Vaccine Tracker. 2020. Available online: https://covid19.trackvaccines.org/ (accessed on 21 January 2024).
- National Institute of Child Health and Human Development. Menstruation and Menstrual Problems; National Institute of Child Health and Human Development: Bethesda, MD, USA, 2017. [Google Scholar]
- National Institute of Child Health and Human Development. Notice of Special Interest (NOSI) to Encourage Administrative Supplement Applications to Investigate COVID-19 Vaccination and Menstruation National Institutes of Health; National Institute of Child Health and Human Development: Bethesda, MD, USA, 2021. [Google Scholar]
- National Institutes of Health. COVID-19 Vaccines and the Menstrual Cycle; National Institutes of Health: Bethesda, MD, USA, 2023. [Google Scholar]
- Edelman, A.; Boniface, E.R.; Benhar, E.; Han, L.; Matteson, K.A.; Favaro, C.; Pearson, J.T.; Darney, B.G. Association between Menstrual Cycle Length and Coronavirus Disease 2019 (COVID-19) Vaccination: A U.S. Cohort. Obstet. Gynecol. 2022, 139, 481–489. [Google Scholar] [CrossRef] [PubMed]
- General Authority for Statistics. Sixteenth Directory of Services-Al Madinah; General Authority for Statistics: Riyadh, Saudi Arabia, 2017. [Google Scholar]
- Taibah University. About Taibah University; Taibah University: Tayba, Saudi Arabia, 2023. [Google Scholar]
- Dean, A.G.; Arner, T.G.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; et al. Epi Info™, a Database and Statistics Program for Public Health Professionals; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2011. [Google Scholar]
- Edelman, A.; Boniface, E.R.; Male, V.; Cameron, S.T.; Benhar, E.; Han, L.; Matteson, K.A.; Van Lamsweerde, A.; Pearson, J.T.; Darney, B.G. Association between menstrual cycle length and COVID-19 vaccination: Global, retrospective cohort study of prospectively collected data. BMJ Med. 2022, 1, e000297. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, S.; Akiyama, N.; Baba, H.; Ohta, M. Association between COVID-19 vaccines and the menstrual cycle in young Japanese women. J. Infect. Chemother. 2023, 29, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Muhaidat, N.; Alshrouf, M.A.; Azzam, M.I.; Karam, A.M.; Al-Nazer, M.W.; Al-Ani, A. Menstrual Symptoms After COVID-19 Vaccine: A Cross-Sectional Investigation in the MENA Region. Int. J. Womens Health 2022, 14, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Qashqari, F.S.I.; Dahlawi, M.; Assaggaf, H.M.; Alsafi, R.; Gari, A.; Abudawood, A.; Al-Doboke, A.; Alsulami, S.; Bukhari, R.; Majeed, S.A.; et al. Effect of the COVID-19 Vaccine on the Menstrual Cycle among Females in Saudi Arabia. Ethiop. J. Health Sci. 2022, 32, 1083–1092. [Google Scholar] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Alghamdi, A.N.; Alotaibi, M.I.; Alqahtani, A.S.; Al Aboud, D.; Abdel-Moneim, A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front. Med. 2021, 8, 760047. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Asghar, S.; Rathore, M.A.; Shahzad, A.; Shahid, A.; Ashraf Khan, A.; Malik, A.; Fakhar, T.; Kausar, H.; Malik, J. Menstrual abnormalities after COVID-19 vaccines: A systematic review. Vacunas 2022, 23, S77–S87. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Pang, R.D.; Nelson, A.L.; Pearson, J.T.; Benhar Noccioli, E.; Reissner, H.R.; von Schwarzenfeld, A.K.; Acuna, J. Detecting variations in ovulation and menstruation during the COVID-19 pandemic, using real-world mobile app data. PLoS ONE 2021, 16, e0258314. [Google Scholar] [CrossRef] [PubMed]
- Medicines and Healthcare Products Regulatory Agency. Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting; Medicines and Healthcare Products Regulatory Agency: London, UK, 2022. [Google Scholar]
- Al-Hatamleh, M.A.; Abusalah, M.A.; Ma’mon, M.H.; Alshaer, W.; Ahmad, S.; Mohd-Zahid, M.H.; Rahman, E.N.; Yean, C.Y.; Alias, I.Z.; Uskoković, V.; et al. Understanding the challenges to COVID-19 vaccines and treatment options, herd immunity and probability of reinfection. J. Taibah Univ. Med. Sci. 2023, 18, 600–638. [Google Scholar] [CrossRef] [PubMed]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; O’Keeffe, A.; Phelan, N.; Behan, L.A.; Collier, S.; Hevey, D.; Owens, L. Female Reproductive Health Disturbance Experienced during the COVID-19 Pandemic Correlates with Mental Health Disturbance and Sleep Quality. Front. Endocrinol. 2022, 13, 838886. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Ji, H.H.; Tang, X.W.; Pan, L.Y.; Chen, X.; Jia, Y.T. Human papillomavirus vaccine-associated premature ovarian insufficiency and related adverse events: Data mining of Vaccine Adverse Event Reporting System. Sci. Rep. 2020, 10, 10762. [Google Scholar] [CrossRef] [PubMed]
- Lebar, V.; Lagana, A.S.; Chiantera, V.; Kunic, T.; Lukanovic, D. The Effect of COVID-19 on the Menstrual Cycle: A Systematic Review. J. Clin. Med. 2022, 11, 3800. [Google Scholar] [CrossRef] [PubMed]
- Berbic, M.; Fraser, I.S. Immunology of normal and abnormal menstruation. Womens Health 2013, 9, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.Y.; Lavender, M.; Marsh, E.E.; et al. Menstruation: Science and society. Am. J. Obstet. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef]
- Abusam, A. Dynamics of COVID-19 in the Gulf Cooperation Council (GCC) countries. J. Taibah Univ. Med. Sci. 2022, 17, 461–466. [Google Scholar] [CrossRef]
| Variable | Parameters | Frequency (%) |
|---|---|---|
| Age | Mean (SD) | 20.9 (4.4) * |
| Marital status | Unmarried (single, widowed, divorced) | 450 (95.3%) |
| Married | 22 (4.7%) | |
| Education | High-school level or below | 335 (71.0%) |
| University | 129 (27.3%) | |
| Postgraduate | 8 (1.7%) | |
| Occupation | Working | 18 (3.8%) |
| Student | 454 (96.2%) | |
| BMI classes | Underweight | 112 (23.7%) |
| Normal weight | 252 (53.4%) | |
| Overweight | 67 (14.2%) | |
| Obesity class 1 | 28 (5.9%) | |
| Obesity class 2 | 7 (1.5%) | |
| Obesity class 3 | 6 (1.3%) | |
| Medical history | I don’t have any diseases | 374 (79.2%) |
| Yes | 99 (20.8%) | |
| Type of disease | Diabetes mellitus | 7 (1.5%) |
| Hypertension | 7 (1.5%) | |
| Thyroid diseases | 9 (1.9%) | |
| Anemia | 73 (15.5%) | |
| Smoking history | I have never smoked in my life | 442 (93.6%) |
| I used to smoke before, but I stopped smoking | 11 (2.3%) | |
| Yes, I currently smoke less than 10 cigarettes a day | 4 (0.8%) | |
| Yes, I currently smoke non-daily | 15 (3.2%) | |
| Have you had a COVID-19 infection? | Yes | 185 (39.2%) |
| No | 287 (60.8%) | |
| How many times have you had a COVID-19 infection? | Once | 154 (32.6%) |
| Twice | 27 (5.7%) | |
| Three times | 2 (0.4%) | |
| Never | 289 (61.2%) |
| Variable | Parameters | Frequency (%) n = 472 |
|---|---|---|
| Cycle length variability | ≤8 days | 306 (64.8%) |
| >8 days | 166 (35.2%) | |
| Cycle length | Less than 24 days | 96 (20.3%) |
| Between 24 and 38 days | 345 (73.1%) | |
| More than 38 days | 31 (6.6%) | |
| Menstrual flow | Low (less than 5 mL) | 49 (10.4%) |
| Moderate (5–80 mL) | 382 (91.3%) | |
| Severe (more than 80 mL/clots) | 41 (8.7%) | |
| Bleeding days | Less than 8 days | 403 (85.4%) |
| More than 8 days | 23 (4.9%) | |
| Too irregular | 46 (9.7%) | |
| Mid-cycle spotting | Yes | 53 (11.2%) |
| No | 419 (88.8%) | |
| PMS symptoms | Yes | 447(94.7%) |
| No | 25 (5.3%) | |
| Do you use a smart device application to monitor your menstrual cycle? | Yes | 215 (45.6%) |
| No | 257 (54.4%) |
| Variables | Reporting Cycle Change after First Dose 258 (54.7%) | Reporting Cycle Change after Second Dose 232 (49.6%) | Reporting Cycle Change after Third Dose 197 (52.3%) | |||
|---|---|---|---|---|---|---|
| Yes | p-Value | Yes | p-Value | Yes | p-Value * | |
| Marital Status | ||||||
| Single | 243 (54.6%) | 0.99 | 218 (49.4%) | 0.531 | 182 (51.3%) | 0.286 |
| Married | 12 (54.5%) | 10 (45.5%) | 12 (63.2%) | |||
| Occupation | ||||||
| Employee | 13 (72.2%) | 0.15 | 8 (44.4%) | 0.657 | 8 (61.5%) | 0.58 |
| Student | 245 (54%) | 224 (49.8%) | 189 (51.9%) | |||
| BMI Classes | ||||||
| Non-overweight/non-obese | 197 (54.3%) | 0.814 | 179 (49.9%) | 0.755 | 141 (49.8%) | 0.122 |
| Overweight/obese | 60 (55.6%) | 52 (48.1%) | 55 (59.1%) | |||
| Medical History | ||||||
| Healthy | 208 (55.6%) | 0.427 | 184 (49.7%) | 0.91 | 159 (52.8%) | 0.377 |
| Diagnosed with a medical problem | 50 (51%) | 48 (49.0%) | 38 (50.0%) | |||
| Prolonged Menstruation | Menorrhagia | Change in PMS | Intermenstrual Bleeding | Missed Period | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | p-Value | n (%) | p-Value | n (%) | p-Value | n (%) | p-Value | n (%) | p-Value * | |
| First dose | ||||||||||
| Pfizer | 83 (19.9%) | 0.341 | 52 (12.4%) | 0.206 | 90 (21.5%) | 0.563 | 24 (5.7%) | 0.687 | 86 (20.6%) | 0.344 |
| AstraZeneca | 14 (28.6%) | 11 (22.4%) | 8 (16.3%) | 1 (2%) | 5 (10.2%) | |||||
| Second dose | ||||||||||
| Pfizer | 69 (17.7%) | 0.417 | 49 (12.6%) | 0.458 | 75 (19.2%) | 0.58 | 25 (6.4%) | 0.598 | 44 (11.3%) | 0.338 |
| AstraZeneca | 14 (27.5%) | 8 (15.7%) | 6 (11.8%) | 2 (3.9%) | 10 (19.6%) | |||||
| Third dose | ||||||||||
| Pfizer | 63 (20.2%) | 0.327 | 42 (13.5%) | 0.98 | 69 (22.1%) | 0.671 | 26 (8.3%) | 0.014 | 48 (15.4%) | 0.103 |
| Moderna | 14 (29.8%) | 6 (12.8%) | 10 (21.3%) | 0 (0%) | 4 (8.5%) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallatah, N.I.; Alrehaili, B.O.; Alsulami, S.S.; Al-Zalabani, A.H. Menstrual Changes Following COVID-19 Vaccination: A Cross-Sectional Study. Medicina 2024, 60, 206. https://doi.org/10.3390/medicina60020206
Fallatah NI, Alrehaili BO, Alsulami SS, Al-Zalabani AH. Menstrual Changes Following COVID-19 Vaccination: A Cross-Sectional Study. Medicina. 2024; 60(2):206. https://doi.org/10.3390/medicina60020206
Chicago/Turabian StyleFallatah, Nahid Ibrahim, Bushra Omar Alrehaili, Salhah Saleh Alsulami, and Abdulmohsen Hamdan Al-Zalabani. 2024. "Menstrual Changes Following COVID-19 Vaccination: A Cross-Sectional Study" Medicina 60, no. 2: 206. https://doi.org/10.3390/medicina60020206
APA StyleFallatah, N. I., Alrehaili, B. O., Alsulami, S. S., & Al-Zalabani, A. H. (2024). Menstrual Changes Following COVID-19 Vaccination: A Cross-Sectional Study. Medicina, 60(2), 206. https://doi.org/10.3390/medicina60020206







