Assessment of Radiation Dosage to the Hippocampi during Treatment of Multiple Brain Metastases Using Gamma Knife Therapy
Abstract
:1. Introduction
2. Materials and Methods
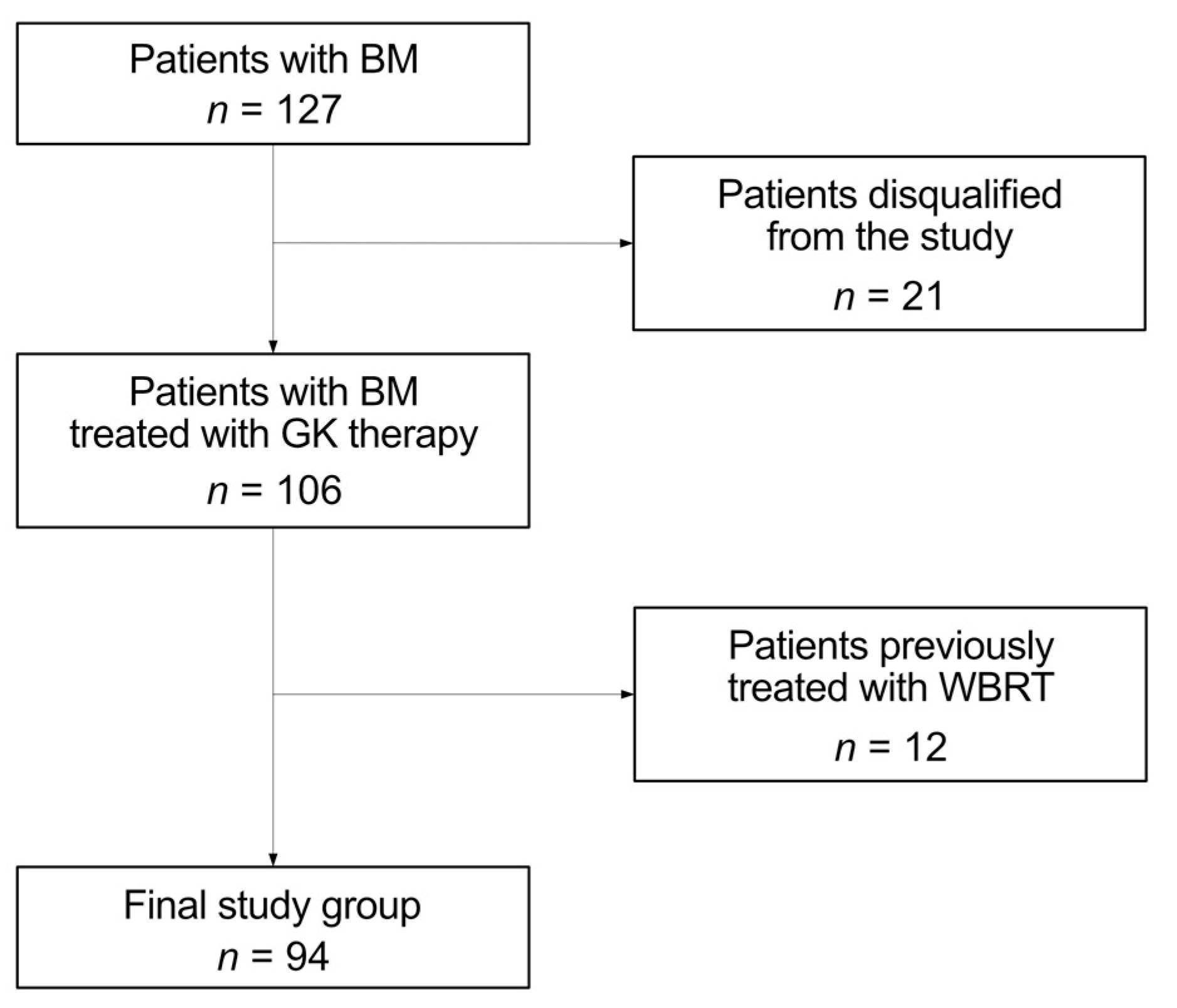
2.1. Patient Selection, Inclusion and Exclusion Criteria
2.2. Indications for the Gamma Knife Therapy
2.3. Gamma Knife Therapy
2.4. Post-Treatment Management
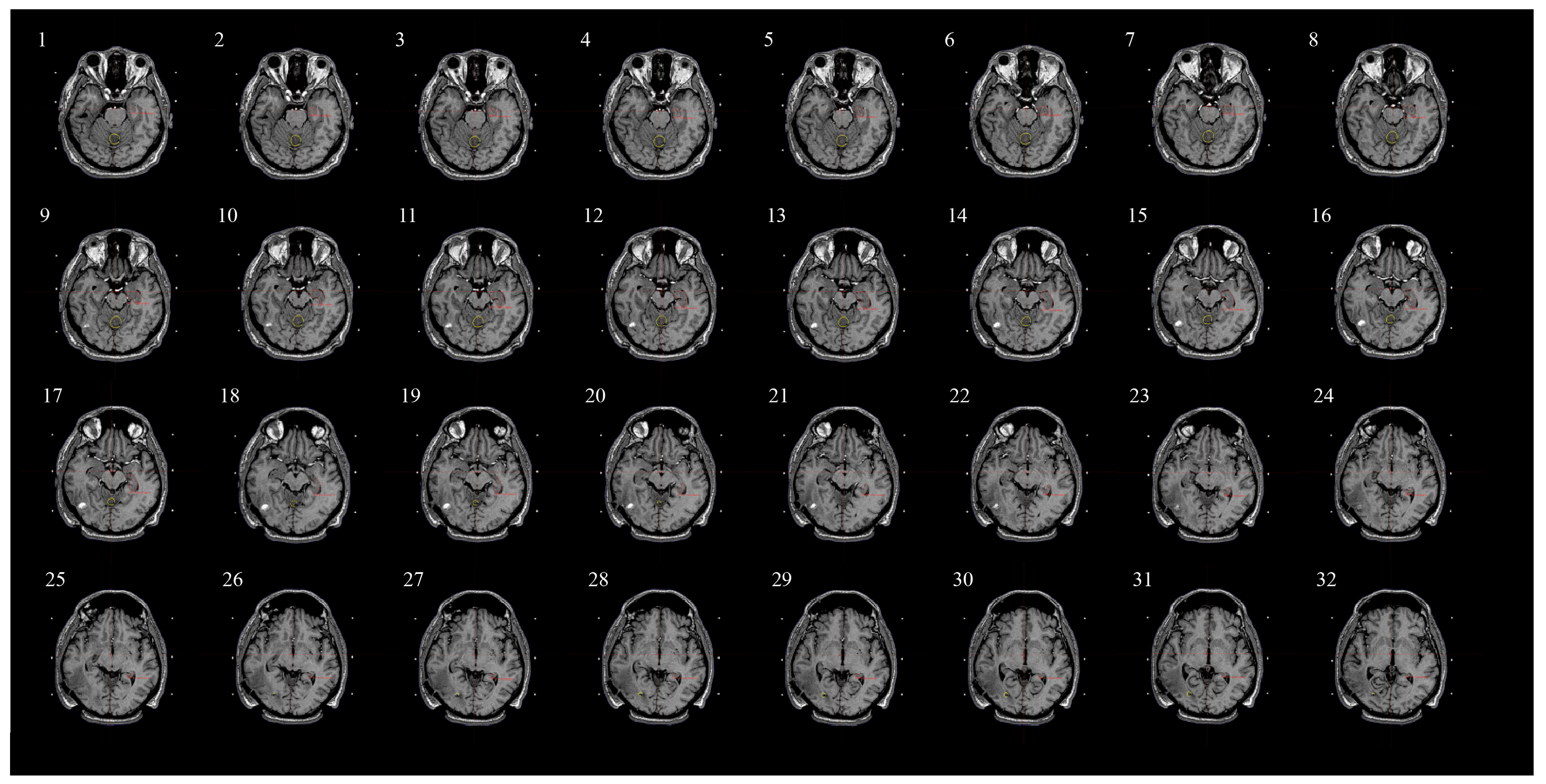
2.5. Assessing Radiation Dose to Hippocampi and Contouring Process
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boire, A. Metastasis to the Central Nervous System. Continuum 2020, 26, 1584–1601. [Google Scholar] [CrossRef]
- Lauko, A.; Rauf, Y.; Ahluwalia, M.S. Medical Management of Brain Metastases. Neuro-Oncol. Adv. 2020, 2, vdaa015. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Vogelbaum, M.V.; Chao, S.T.; Mehta, M.M. Brain Metastasis and Treatment. F1000Prime Rep. 2014, 6, 114. [Google Scholar] [CrossRef]
- Rogers, L.R. Neurologic Complications of Cancer, 2nd Ed. Contemporary Neurology Series. Neuro Oncol. 2009, 11, 96–97. [Google Scholar] [CrossRef]
- Langer, C.J.; Mehta, M.P. Current Management of Brain Metastases, with a Focus on Systemic Options. J. Clin. Oncol. 2005, 23, 6207–6219. [Google Scholar] [CrossRef]
- Noh, T.; Walbert, T. Brain Metastasis: Clinical Manifestations, Symptom Management, and Palliative Care. Handb. Clin. Neurol. 2018, 149, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovic, I.T.; Posner, J.B. Brain Metastases: Epidemiology and Pathophysiology. J. Neuro-Oncol. 2005, 75, 5–14. [Google Scholar] [CrossRef]
- Sawada, K. [Lung cancer: Classification by cell types]. Nihon Rinsho 1980, 38, 2574–2580. [Google Scholar] [PubMed]
- Li, N.; Chu, Y.; Song, Q. Brain Metastasis in Patients with Small Cell Lung Cancer. Int. J. Gen. Med. 2021, 14, 10131–10139. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Abrouk, N.; Caroen, S.; Lybeck, M.; Guo, X.; Wang, X.; Yu, Z.; Reid, T. A 2022 Update on Extensive Stage Small-Cell Lung Cancer (SCLC). J. Cancer 2022, 13, 2945–2953. [Google Scholar] [CrossRef]
- Konopka-Filippow, M.; Hempel, D.; Sierko, E. Actual, Personalized Approaches to Preserve Cognitive Functions in Brain Metastases Breast Cancer Patients. Cancers 2022, 14, 3119. [Google Scholar] [CrossRef] [PubMed]
- Kazda, T.; Jancalek, R.; Pospisil, P.; Sevela, O.; Prochazka, T.; Vrzal, M.; Burkon, P.; Slavik, M.; Hynkova, L.; Slampa, P.; et al. Why and How to Spare the Hippocampus during Brain Radiotherapy: The Developing Role of Hippocampal Avoidance in Cranial Radiotherapy. Radiat. Oncol. 2014, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Satyamitra, M.M.; DiCarlo, A.L.; Taliaferro, L. Understanding the Pathophysiology and Challenges of Development of Medical Countermeasures for Radiation-Induced Vascular/Endothelial Cell Injuries: Report of a NIAID Workshop, August 20, 2015. Radiat. Res. 2016, 186, 99–111. [Google Scholar] [CrossRef]
- Monje, M.L.; Mizumatsu, S.; Fike, J.R.; Palmer, T.D. Irradiation Induces Neural Precursor-Cell Dysfunction. Nat. Med. 2002, 8, 955–962. [Google Scholar] [CrossRef]
- DeAngelis, L.M.; Delattre, J.Y.; Posner, J.B. Radiation-Induced Dementia in Patients Cured of Brain Metastases. Neurology 1989, 39, 789–796. [Google Scholar] [CrossRef]
- Kotecha, R.; Gondi, V.; Ahluwalia, M.S.; Brastianos, P.K.; Mehta, M.P. Recent Advances in Managing Brain Metastasis. F1000Res 2018, 7, F1000 Faculty Rev-1772. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, F.; Ma, J.; Zhao, D. Palliative Whole-Brain Radiotherapy and Health- Related Quality of Life for Patients with Brain Metastasis in Cancer. Neuropsychiatr. Dis. Treat. 2015, 11, 2185–2190. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Fu, J. Treatment of Radiation-Induced Brain Necrosis. Oxid. Med. Cell Longev. 2021, 2021, 4793517. [Google Scholar] [CrossRef] [PubMed]
- Haldbo-Classen, L.; Amidi, A.; Lukacova, S.; Wu, L.M.; von Oettingen, G.; Lassen-Ramshad, Y.; Zachariae, R.; Kallehauge, J.F.; Høyer, M. Cognitive Impairment Following Radiation to Hippocampus and Other Brain Structures in Adults with Primary Brain Tumours. Radiother. Oncol. 2020, 148, 1–7. [Google Scholar] [CrossRef]
- Goda, J.S.; Dutta, D.; Krishna, U.; Goswami, S.; Kothavade, V.; Kannan, S.; Maitre, M.; Bano, N.; Gupta, T.; Jalali, R. Hippocampal Radiotherapy Dose Constraints for Predicting Long-Term Neurocognitive Outcomes: Mature Data from a Prospective Trial in Young Patients with Brain Tumors. Neuro-Oncology 2020, 22, 1677–1685. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Acharya, M.M.; Lan, M.L.; Kan, V.H.; Patel, N.H.; Giedzinski, E.; Tseng, B.P.; Limoli, C.L. Consequences of Ionizing Radiation-Induced Damage in Human Neural Stem Cells. Free Radic. Biol. Med. 2010, 49, 1846–1855. [Google Scholar] [CrossRef]
- Mineyeva, O.A.; Bezriadnov, D.V.; Kedrov, A.V.; Lazutkin, A.A.; Anokhin, K.V.; Enikolopov, G.N. Radiation Induces Distinct Changes in Defined Subpopulations of Neural Stem and Progenitor Cells in the Adult Hippocampus. Front. Neurosci. 2018, 12, 1013. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of Memory with Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment during Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Popp, I.; Rau, A.; Kellner, E.; Reisert, M.; Fennell, J.T.; Rothe, T.; Nieder, C.; Urbach, H.; Egger, K.; Grosu, A.L.; et al. Hippocampus-Avoidance Whole-Brain Radiation Therapy Is Efficient in the Long-Term Preservation of Hippocampal Volume. Front. Oncol. 2021, 11, 714709. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J. Prophylactic Cranial Irradiation for Small-Cell Lung Cancer: Time for a Reassessment. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Le Fèvre, C.; Cheng, X.; Loit, M.-P.; Keller, A.; Cebula, H.; Antoni, D.; Thiery, A.; Constans, J.-M.; Proust, F.; Noel, G. Role of Hippocampal Location and Radiation Dose in Glioblastoma Patients with Hippocampal Atrophy. Radiat. Oncol. 2021, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Tolakanahalli, R.; Mehta, M.P.; Tewatia, D.; Rowley, H.; Kuo, J.S.; Khuntia, D.; Tomé, W.A. Hippocampal-Sparing Whole-Brain Radiotherapy: A “How-to” Technique Using Helical Tomotherapy and Linear Accelerator-Based Intensity-Modulated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Slade, A.N.; Stanic, S. The Impact of RTOG 0614 and RTOG 0933 Trials in Routine Clinical Practice: The US Survey of Utilization of Memantine and IMRT Planning for Hippocampus Sparing in Patients Receiving Whole Brain Radiotherapy for Brain Metastases. Contemp. Clin. Trials 2016, 47, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Liu, T.; Li, A.; Zhao, M.; Yu, X.; Qh, O. Long-Term Follow up of Very Low-Dose LINAC Based Stereotactic Radiotherapy in Temporal Lobe Epilepsy. Epilepsy Res. 2010, 90, 60–67. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic Radiosurgery for Patients with Multiple Brain Metastases (JLGK0901): A Multi-Institutional Prospective Observational Study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Leksell, L. Stereotactic Radiosurgery. J. Neurol. Neurosurg. Psychiatry 1983, 46, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Rich, K.M. Therapeutic Role of Gamma Knife Stereotactic Radiosurgery in Neuro-Oncology. Mo. Med. 2020, 117, 33–38. [Google Scholar]
- Sanders, J.; Nordström, H.; Sheehan, J.; Schlesinger, D. Gamma Knife Radiosurgery: Scenarios and Support for Re-Irradiation. Phys. Med. 2019, 68, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, A.; Monaco, E.; Flickinger, J.; Lunsford, L.D. Guidelines for Multiple Brain Metastases Radiosurgery. Prog. Neurol. Surg. 2019, 34, 100–109. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative Radiotherapy in the Treatment of Single Metastases to the Brain: A Randomized Trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Basina, B.R.; Olson, C.; Roy, D.K.; Yen, C.-P.; Schlesinger, D.; Nagayama, K.; Sheehan, J.P. Radiation Dose and Incidence of New Metastasis in the Anterior Temporal Lobe Structures of Radiosurgically Treated Patients. J. Neurosurg. 2010, 112, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.A.; Squires, B.S.; Johnson, M.D.; Baschnagel, A.M.; Chen, P.Y.; Krauss, D.J.; Olson, R.E.; Meyer, K.D.; Grills, I.S. Predictors of Radiation Necrosis in Long-Term Survivors after Gamma Knife Stereotactic Radiosurgery for Brain Metastases. Neuro-Oncol. Pract. 2020, 7, 400–408. [Google Scholar] [CrossRef]
- Gaebe, K.; Li, A.Y.; Park, A.; Parmar, A.; Lok, B.H.; Sahgal, A.; Chan, K.K.W.; Erickson, A.W.; Das, S. Stereotactic Radiosurgery versus Whole Brain Radiotherapy in Patients with Intracranial Metastatic Disease and Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lancet Oncol. 2022, 23, 931–939. [Google Scholar] [CrossRef]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal Dosimetry Predicts Neurocognitive Function Impairment after Fractionated Stereotactic Radiotherapy for Benign or Low-Grade Adult Brain Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e487–e493. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.; Shen, Z.L.; Xu, Z.; Zhang, H.; Natsuaki, Y.; Cheng, K.; Li, V.; Kim, N.; Ma, L.; Chang, E. Hippocampal Sparing Radiation Therapy for Brain Metastases: Treatment Techniques and Clinical Implementation. Chin. Clin. Oncol. 2023, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Sahgal, A.; Detsky, J.; Soliman, H.; Myrehaug, S.; Tseng, C.-L.; Husain, Z.A.; Carty, A.; Das, S.; Yang, V.; et al. Single-Fraction Stereotactic Radiosurgery Versus Hippocampal-Avoidance Whole Brain Radiation Therapy for Patients with 10 to 30 Brain Metastases: A Dosimetric Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Susko, M.S.; Garcia, M.A.; Ma, L.; Nakamura, J.L.; Raleigh, D.R.; Fogh, S.; Theodosopoulos, P.; McDermott, M.; Sneed, P.K.; Braunstein, S.E. Stereotactic Radiosurgery to More Than 10 Brain Metastases: Evidence to Support the Role of Radiosurgery for Ideal Hippocampal Sparing in the Treatment of Multiple Brain Metastases. World Neurosurg. 2020, 135, e174–e180. [Google Scholar] [CrossRef]
| Histopathological Diagnosis | Frequency, n (%) | Mean Age (Years) | Male/Female |
|---|---|---|---|
| Non-Small Cell Lung Cancer | 53 (56.4) | 66.3 | 25|28 |
| Small Cell Lung Cancer | 5 (5.3) | 61.8 | 1|4 |
| Breast Cancer | 21 (22.3) | 62.7 | 0|21 |
| Clear-cell Renal Cell Carcinoma | 4 (4.3) | 70 | 4|0 |
| Adenocarcinoma Colon Cancer | 4 (4.3) | 58.5 | 2|2 |
| Melanoma | 2 (2.1) | 54 | 1|1 |
| Ovarian Cancer | 2 (2.1) | 73.5 | 0|2 |
| Urothelial Carcinoma | 1 (1.1) | 70 | 1|0 |
| Gastric Cancer | 1 (1.1) | 50 | 1|0 |
| Cervical Cancer | 1 (1.1) | 67 | 0|1 |
| All | 94 (100) | 63.6 | 35|59 |
| Number of Therapies | Number of Metastases | |
|---|---|---|
| N-valid | 94 | 94 |
| N-missing | 0 | 0 |
| Median | 2 | 3 |
| Minimum | 1 | 2 |
| Maximum | 9 | 20 |
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Radiation dose to the left hippocampus | 94 | 0.0 | 6.9 | 0.906 | 1.2517 |
| Radiation dose to the right hippocampus | 94 | 0.0 | 7.0 | 0.806 | 1.0779 |
| Radiation dose to the bilateral hippocampi | 94 | 0.0 | 8.3 | 1.713 | 1.7964 |
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Radiation dose to the left hippocampus | 66 | 0.0 | 6.9 | 1.068 | 1.4096 |
| Radiation dose to the right hippocampus | 66 | 0.0 | 7.0 | 0.968 | 1.2188 |
| Radiation dose to the bilateral hippocampi | 66 | 0.1 | 8.3 | 2.036 | 0.2394 |
| Name of the Study | Hippocampal Avoidance Recommendation | Number of Cases within the Limit | Number of Cases Exceeding the Limit |
|---|---|---|---|
| Goda (2020) [21] | Mean dose of ≤30 Gy to the left hippocampus | 94 | 0 |
| NRG Oncology CC001 (2020) [22] | Dose to 100% of the hippocampus ≤9 Gy, maximal hippocampal dose ≤16 Gy | 94 | 0 |
| RTOG 0933 (2014) [25] | Dose to 100% of the hippocampus ≤9 Gy, maximal hippocampal dose ≤16 Gy | 94 | 0 |
| Gondi (2012) [41] | EQD(2) to 40% of the bilateral hippocampi lesser than 7.3 Gy | 92 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowski, M.; Błaszczyk, B.; Setlak, M.; Kuca, M.; Lech, A.; Kłos, K.; Rudnik, A. Assessment of Radiation Dosage to the Hippocampi during Treatment of Multiple Brain Metastases Using Gamma Knife Therapy. Medicina 2024, 60, 246. https://doi.org/10.3390/medicina60020246
Laskowski M, Błaszczyk B, Setlak M, Kuca M, Lech A, Kłos K, Rudnik A. Assessment of Radiation Dosage to the Hippocampi during Treatment of Multiple Brain Metastases Using Gamma Knife Therapy. Medicina. 2024; 60(2):246. https://doi.org/10.3390/medicina60020246
Chicago/Turabian StyleLaskowski, Maciej, Bartłomiej Błaszczyk, Marcin Setlak, Maciej Kuca, Arkadiusz Lech, Kamil Kłos, and Adam Rudnik. 2024. "Assessment of Radiation Dosage to the Hippocampi during Treatment of Multiple Brain Metastases Using Gamma Knife Therapy" Medicina 60, no. 2: 246. https://doi.org/10.3390/medicina60020246





