One Anastomosis Gastric Bypass versus Roux-en-Y Gastric Bypass: A Randomized Prospective Trial
Abstract
:1. Introduction
2. Materials and Methods
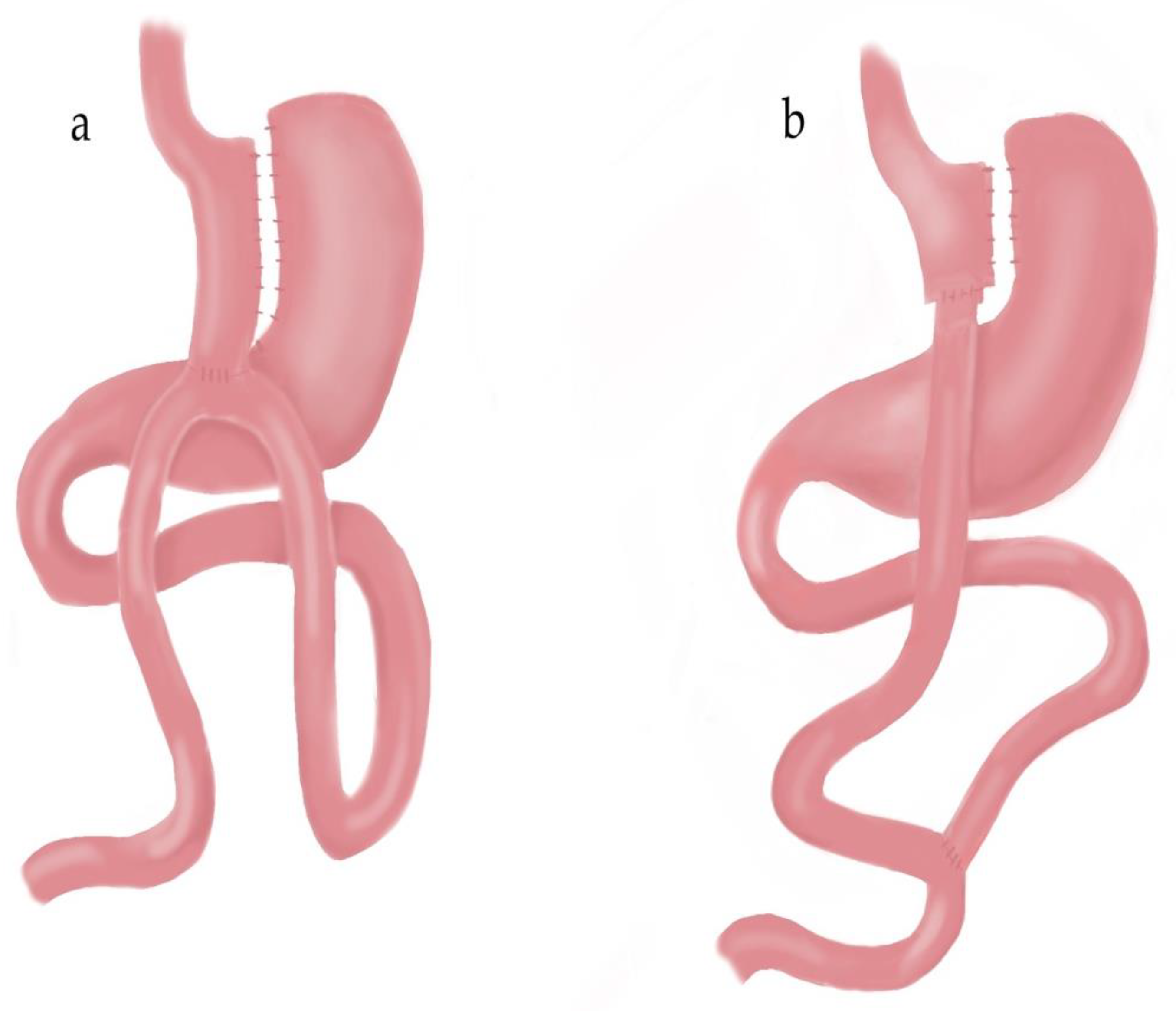
2.1. Surgical Techniques
2.2. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14 (Suppl. S2), 21–28. [Google Scholar] [CrossRef]
- Askari, M.; Heshmati, J.; Shahinfar, H.; Tripathi, N.; Daneshzad, E. Ultra-processed food and the risk of overweight and obesity: A systematic review and meta-analysis of observational studies. Int. J. Obes. 2020, 44, 2080–2091. [Google Scholar] [CrossRef]
- Juul, F.; Martinez-Steele, E.; Parekh, N.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 2018, 120, 90–100. [Google Scholar] [CrossRef]
- Silveira, E.A.; Mendonça, C.R.; Delpino, F.M.; Elias Souza, G.V.; Pereira de Souza Rosa, L.; de Oliveira, C.; Noll, M. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 50, 63–73. [Google Scholar] [CrossRef]
- Day, K.; Alfonzo, M.; Chen, Y.; Guo, Z.; Lee, K.K. Overweight, obesity, and inactivity and urban design in rapidly growing Chinese cities. Heal. Place 2013, 21, 29–38. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 May 2023).
- Li, H.; Boakye, D.; Chen, X.; Hoffmeister, M.; Brenner, H. Association of Body Mass Index with Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2021, 116, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Shambhu, S.; Arterburn, D.E.; McTigue, K.M.; Haynes, K. Interventions and Operations after Bariatric Surgery in a Health Plan Research Network Cohort from the PCORnet, the National Patient-Centered Clinical Research Network. Obes. Surg. 2021, 31, 3531–3540. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Kamocka, A.; Patel, D.; Dexter, S.; Finlay, I.; Hopkins, J.C.; Khan, O.; Reddy, M.; Sedman, P.; Small, P.; et al. Obesity surgery makes patients healthier and more functional: Real world results from the United Kingdom National Bariatric Surgery Registry. Surg. Obes. Relat. Dis. 2018, 14, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.P.; Feigelson, H.S.; Koebnick, C.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann. Surg. 2019, 269, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Vitiello, A.; Angrisani, L.; Santonicola, A.; Iovino, P.; Pilone, V.; Forestieri, P. Bariatric Surgery versus Lifestyle Intervention in Class I Obesity: 7–10-Year Results of a Retrospective Study. World J. Surg. 2019, 43, 758–762. [Google Scholar] [CrossRef]
- Hofsø, D.; Nordstrand, N.; Johnson, L.K.; Karlsen, T.I.; Hager, H.; Jenssen, T.; Bollerslev, J.; Godang, K.; Sandbu, R.; Røislien, J.; et al. Obesity-related cardiovascular risk factors after weight loss: A clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur. J. Endocrinol. 2010, 163, 735–745. [Google Scholar] [CrossRef]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; Hardy, E.; Mitchell, S.; Batson, S. Semaglutide 2.4 Mg for the Management of Overweight and Obesity: Systematic Literature Review and Meta-Analysis. Diabetes Metab. Syndr. Obes. 2022, 15, 3961–3987. [Google Scholar] [CrossRef] [PubMed]
- Anam, M.; Maharjan, S.; Amjad, Z.; Abaza, A.; Vasavada, A.M.; Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I. Efficacy of Semaglutide in Treating Obesity: A Systematic Review of Randomized Controlled Trials (RCTs). Cureus 2022, 14, e32610. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.; Klarenbach, S.; Wiebe, N.; Birch, D.; Karmali, S.; Manns, B.; Hazel, M.; Sharma, A.M.; Tonelli, M. Bariatric surgery: A systematic review and network meta-analysis of randomized trials. Obes. Rev. 2011, 12, 602–621. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Edholm, D.; Svensson, F.; Näslund, I.; Karlsson, F.A.; Rask, E.; Sundbom, M. Long-term results 11 years after primary gastric bypass in 384 patients. Surg. Obes. Relat. Dis. 2013, 9, 708–713. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Root, J.; Zainabadi, K.; Sabio, A.; Chalifoux, S.; Stevens, C.M.; Mavandadi, S.; Longoria, M.; Wilson, S.E. Accelerated growth of bariatric surgery with the introduction of minimally invasive surgery. Arch. Surg. 2005, 140, 1198–1202. [Google Scholar] [CrossRef]
- Sharples, A.J.; Mahawar, K. Systematic Review and Meta-Analysis of Randomised Controlled Trials Comparing Long-Term Outcomes of Roux-En-Y Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2020, 30, 664–672. [Google Scholar] [CrossRef]
- Himpens, J.; Verbrugghe, A.; Cadière, G.B.; Everaerts, W.; Greve, J.W. Long-term results of laparoscopic Roux-en-Y Gastric bypass: Evaluation after 9 years. Obes. Surg. 2012, 22, 1586–1593. [Google Scholar] [CrossRef]
- Shoar, S.; Saber, A.A. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: A systematic review and meta-analysis of comparative studies. Surg. Obes. Relat. Dis. 2017, 13, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Lai, D.D.; Lin, Z.H.; Jiang, T.Y.; Zhang, A.M.; Dai, J.F. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: A systematic review and meta-analysis of randomized and nonrandomized trials. Surg. Laparosc. Endosc. Percutan Tech. 2014, 24, 1–11. [Google Scholar] [CrossRef]
- Rutledge, R. The mini-gastric bypass: Experience with the first 1274 cases. Obes. Surg. 2001, 11, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Musella, M.; Susa, A.; Manno, E.; De Luca, M.; Greco, F.; Raffaelli, M.; Cristiano, S.; Milone, M.; Bianco, P.; Vilardi, A.; et al. Complications Following the Mini/One Anastomosis Gastric Bypass (MGB/OAGB): A Multi-institutional Survey on 2678 Patients with a Mid-term (5 Years) Follow-up. Obes. Surg. 2017, 27, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Ser, K.H.; Lee, Y.C.; Tsou, J.J.; Chen, S.C.; Chen, J.C. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: A 10-year experience. Obes. Surg. 2012, 22, 1827–1834. [Google Scholar] [CrossRef]
- Singh, B.; Saikaustubh, Y.; Singla, V.; Kumar, A.; Ahuja, V.; Gupta, Y.; Kashyap, L.; Aggarwal, S. One Anastomosis Gastric Bypass (OAGB) vs Roux en Y Gastric Bypass (RYGB) for Remission of T2DM in Patients with Morbid Obesity: A Randomized Controlled Trial. Obes. Surg. 2023, 33, 1218–1227. [Google Scholar] [CrossRef]
- Level, L.; Rojas, A.; Piñango, S.; Avariano, Y. One anastomosis gastric bypass vs. Roux-en-Y gastric bypass: A 5-year follow-up prospective randomized trial. Langenbecks Arch. Surg. 2021, 406, 171–179. [Google Scholar] [CrossRef]
- Balamurugan, G.; Leo, S.J.; Sivagnanam, S.T.; Balaji Prasad, S.; Ravindra, C.; Rengan, V.; Arora, E.; Bindal, V. Comparison of Efficacy and Safety between Roux-en-Y Gastric Bypass (RYGB) vs One Anastomosis Gastric Bypass (OAGB) vs Single Anastomosis Duodeno-ileal Bypass with Sleeve Gastrectomy (SADI-S): A Systematic Review of Bariatric and Metabolic Surgery. Obes. Surg. 2023, 33, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, K.K.; Borg, C.M.; Kular, K.S.; Courtney, M.J.; Sillah, K.; Carr, W.R.J.; Jennings, N.; Madhok, B.; Singhal, R.; Small, P.K. Understanding Objections to One Anastomosis (Mini) Gastric Bypass: A Survey of 417 Surgeons Not Performing this Procedure. Obes. Surg. 2017, 27, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Rheinwalt, K.P.; Plamper, A.; Rückbeil, M.V.; Kroh, A.; Neumann, U.P.; Ulmer, T.F. One Anastomosis Gastric Bypass-Mini-Gastric Bypass (OAGB-MGB) Versus Roux-en-Y Gastric Bypass (RYGB)-a Mid-Term Cohort Study with 612 Patients. Obes. Surg. 2020, 30, 1230–1240. [Google Scholar] [CrossRef]
- Fahmy, M.H.; Sarhan, M.D.; Salman, M.A.; Fathy, E. Gastro-Esophageal Reflux Disease After Laparoscopic Mini-Gastric Bypass and Roux-en-Y Gastric Bypass: Is There a Difference? Bariatr. Surg. Pr. Patient Care 2018, 13, 109–114. [Google Scholar] [CrossRef]
- Robert, M.; Espalieu, P.; Pelascini, E.; Caiazzo, R.; Sterkers, A.; Khamphommala, L.; Poghosyan, T.; Chevallier, J.M.; Malherbe, V.; Chouillard, E.; et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): A multicentre, randomised, open-label, non-inferiority trial. Lancet 2019, 393, 1299–1309. [Google Scholar] [CrossRef]
- Lee, W.J.; Yu, P.J.; Wang, W.; Chen, T.C.; Wei, P.L.; Huang, M.T. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: A prospective randomized controlled clinical trial. Ann. Surg. 2005, 242, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Eskandaros, M.S.; Abbass, A.; Zaid, M.H.; Darwish, A.A. Laparoscopic One Anastomosis Gastric Bypass versus Laparoscopic Roux-en-Y Gastric Bypass Effects on Pre-existing Mild-to-Moderate Gastroesophageal Reflux Disease in Patients with Obesity: A Randomized Controlled Study. Obes. Surg. 2021, 31, 4673–4681. [Google Scholar] [CrossRef]
- Katayama, R.C.; Arasaki, C.H.; Herbella, F.A.M.; Neto, R.A.; Lopes Filho, G.J. One-Anastomosis and Roux-en-Y Gastric Bypass Promote Similar Weight Loss, Patient Satisfaction, Quality of Life, Inflammation Grade, and Cellular Damage in the Esophagus and Gastric Pouch in a Short-term Follow-up. J. Obes. Metab. Syndr. 2021, 30, 396–402. [Google Scholar] [CrossRef]
- Kim, M.S.; Kwon, Y.; Park, E.P.; An, L.; Park, H.; Park, S. Revisiting Laparoscopic Reconstruction for Billroth 1 Versus Billroth 2 Versus Roux-en-Y after Distal Gastrectomy: A Systematic Review and Meta-Analysis in the Modern Era. World J. Surg. 2019, 43, 1581–1593. [Google Scholar] [CrossRef]
- Karagul, S.; Kayaalp, C.; Kirmizi, S.; Tardu, A.; Ertugrul, I.; Tolan, K.; Sumer, F. Influence of repeated measurements on small bowel length. Springerplus 2016, 5, 1828. [Google Scholar] [CrossRef]
- Karagul, S.; Kayaalp, C. Repeated stretched or non-stretched small bowel length measurements in healthy individuals. Turk. J. Surg. 2018, 35, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bellicha, A.; Ciangura, C.; Roda, C.; Torcivia, A.; Aron-Wisnewsky, J.; Poitou, C.; Oppert, J.M. Effect of exercise training after bariatric surgery: A 5-year follow-up study of a randomized controlled trial. PLoS ONE 2022, 17, e0271561. [Google Scholar] [CrossRef] [PubMed]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training before and after bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. S4), e13296. [Google Scholar] [CrossRef] [PubMed]




| Operation Type | OAGB (n = 20) | RYGB (n = 18) | p Value |
|---|---|---|---|
| Age, mean ± SD | 43.60 ± 11.10 | 42.72 ± 13.77 | 0.829 a |
| Sex, n (%) | |||
| Female | 17 (85.0) | 14 (77.8) | 0.437 b |
| Male | 3 (15.0) | 4 (22.2) | |
| BMI_initial, mean ± SD | 44.75 ± 6.10 | 46.69 ± 6.62 | 0.353 a |
| DM, n (%) | 13 (65.0) | 10 (55.6) | 0.396 b |
| HT, n (%) | 9 (45.0) | 6 (33.3) | 0.345 b |
| Asthma, n (%) | 3 (15.0) | 3 (16.7) | 0.616 b |
| Sleep apnea, n (%) | 3 (15.0) | 2 (11.1) | 0.552 b |
| Dispnea, n (%) | 7 (35.0) | 5 (27.8) | 0.450 b |
| Operation Type | OAGB (n = 20) | RYGB (n = 18) | p Value |
|---|---|---|---|
| DM, n (%) | 1 (5.0) | 3 (16.7) | 0.263 a |
| HT, n (%) | 3 (15.0) | 2 (11.1) | 0.552 a |
| Asthma, n (%) | - | - | NA |
| Sleep apnea, n (%) | - | - | NA |
| Dispnea, n (%) | - | - | NA |
| Denovo reflux, n (%) | 4 (20.0) | - | 0.066 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karagul, S.; Senol, S.; Karakose, O.; Uzunoglu, K.; Kayaalp, C. One Anastomosis Gastric Bypass versus Roux-en-Y Gastric Bypass: A Randomized Prospective Trial. Medicina 2024, 60, 256. https://doi.org/10.3390/medicina60020256
Karagul S, Senol S, Karakose O, Uzunoglu K, Kayaalp C. One Anastomosis Gastric Bypass versus Roux-en-Y Gastric Bypass: A Randomized Prospective Trial. Medicina. 2024; 60(2):256. https://doi.org/10.3390/medicina60020256
Chicago/Turabian StyleKaragul, Servet, Serdar Senol, Oktay Karakose, Kevser Uzunoglu, and Cuneyt Kayaalp. 2024. "One Anastomosis Gastric Bypass versus Roux-en-Y Gastric Bypass: A Randomized Prospective Trial" Medicina 60, no. 2: 256. https://doi.org/10.3390/medicina60020256
APA StyleKaragul, S., Senol, S., Karakose, O., Uzunoglu, K., & Kayaalp, C. (2024). One Anastomosis Gastric Bypass versus Roux-en-Y Gastric Bypass: A Randomized Prospective Trial. Medicina, 60(2), 256. https://doi.org/10.3390/medicina60020256






