A Narrative Review of Self-Reported Scales to Evaluate Depression and Anxiety Symptoms in Adult Obstructive Sleep Apnea Patients
Abstract
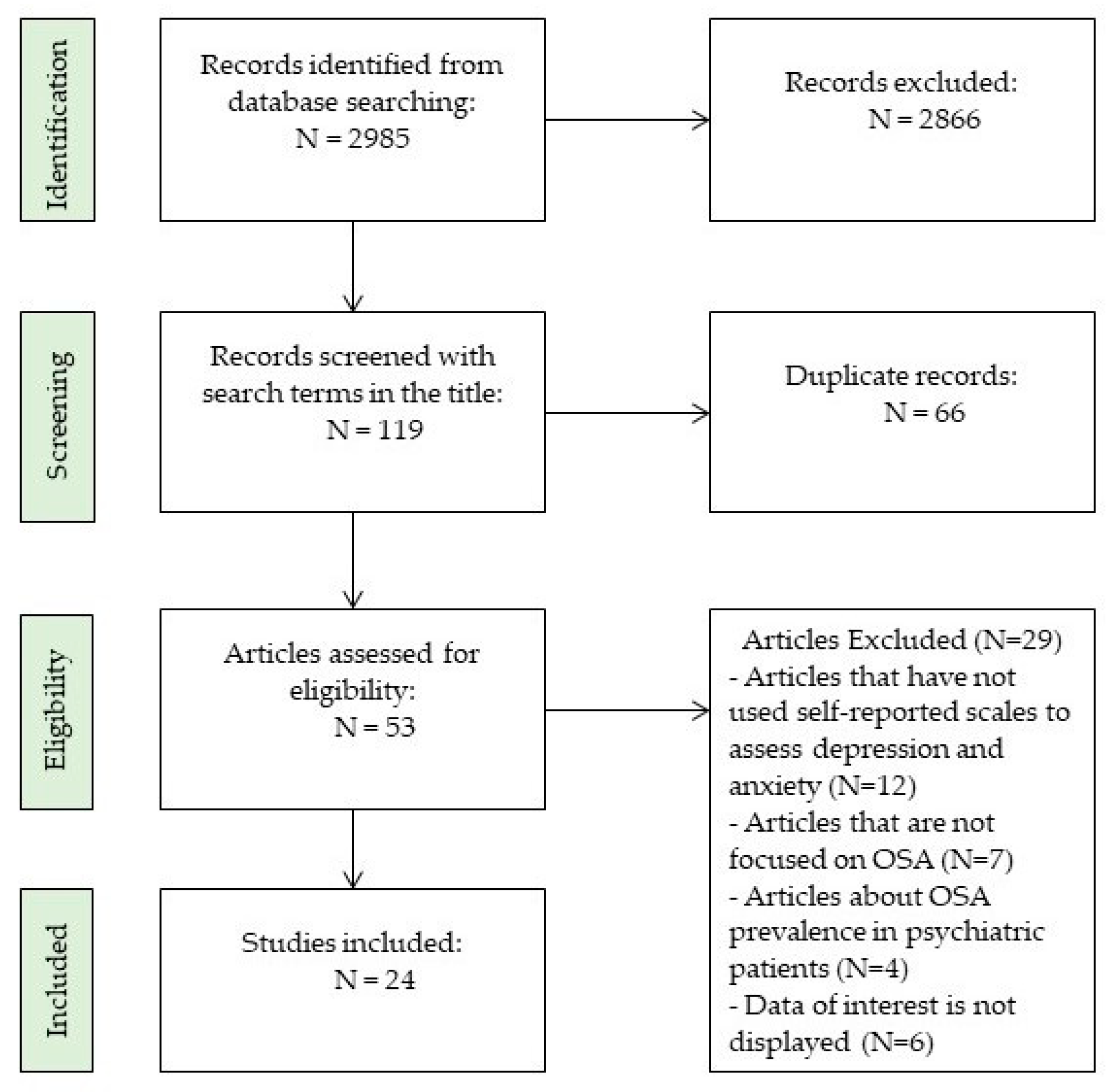
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
3. Results
3.1. Depression, Anxiety, and Obstructive Sleep Apnea
3.1.1. Prevalence and Risk Factors
3.1.2. Assessment of Depression and Anxiety
3.1.3. Scales for Depression and Anxiety in OSA Patients
- Center for Epidemiologic Studies Depression Scale (CES-D)
- Hospital Anxiety and Depression Scale (HADS)
- Patient Health Questionnaire-9 (PHQ-9)
- Beck Depression Inventory (BDI-II)
- Zung Self-Rating Depression Scale (SDS)
- Generalized Anxiety Disorder-7 (GAD-7)
- State-Trait Anxiety Inventory (STAI)
- Beck Anxiety Inventory (BAI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2016, 34, 70–81. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Bhuiyan, A.R.; Jones, E.A. Association and Risk Factors for Obstructive Sleep Apnea and Cardiovascular Diseases: A Systematic Review. Diseases 2021, 9, 88. [Google Scholar] [CrossRef]
- Moula, A.I.; Parrini, I.; Tetta, C.; Lucà, F.; Parise, G.; Rao, C.M.; Mauro, E.; Parise, O.; Matteucci, F.; Gulizia, M.M.; et al. Obstructive Sleep Apnea and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1242. [Google Scholar] [CrossRef]
- Javaheri, S.; Javaheri, S. Obstructive Sleep Apnea in Heart Failure: Current Knowledge and Future Directions. J. Clin. Med. 2022, 11, 3458. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Sapiña-Beltrán, E.; Gavaldà, R.; Barbé, F.; Torres, G.; Sauret, A.; Dalmases, M.; López-Cano, C.; Gutiérrez-Carrasquilla, L.; Bermúdez-López, M.; et al. Prediabetes Is Associated with Increased Prevalence of Sleep-Disordered Breathing. J. Clin. Med. 2022, 11, 1413. [Google Scholar] [CrossRef]
- Wang, C.; Tan, J.; Miao, Y.; Zhang, Q. Obstructive sleep apnea, prediabetes and progression of type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Investig. 2022, 13, 1396–1411. [Google Scholar] [CrossRef]
- Legault, J.; Thompson, C.; Martineau-Dussault, M.È.; André, C.; Baril, A.A.; Martinez Villar, G.; Carrier, J.; Gosselin, N. Obstructive Sleep Apnea and Cognitive Decline: A Review of Potential Vulnerability and Protective Factors. Brain Sci. 2021, 11, 706. [Google Scholar] [CrossRef]
- Garbarino, S.; Bardwell, A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav. Sleep Med. 2018, 18, 35–37. [Google Scholar] [CrossRef]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J. Clin. Sleep Med. 2009, 5, 573–581. [Google Scholar] [CrossRef]
- Cheng, A.C.; Wu, G.J.; Chung, C.H.; Wu, K.H.; Sun, C.A.; Wang, I.D.; Chien, W.C. Effect of Obstructive Sleep Apnea on the Risk of Injuries—A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 13416. [Google Scholar] [CrossRef]
- Kaufmann, C.N.; Susukida, R.; Depp, C.A. Sleep apnea, psychopathology, and mental health care. Sleep Health 2017, 3, 244–249. [Google Scholar] [CrossRef]
- Schröder, C.M.; O’Hara, R. Depression and Obstructive Sleep Apnea (OSA). Ann. Gen. Psychiatry 2005, 4, 13. [Google Scholar] [CrossRef]
- Gupta, M.A.; Simpson, F.C. Obstructive sleep apnea and psychiatric disorders: A systematic review. J. Clin. Sleep Med. 2015, 11, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.L.; Tsao, H.M.; Hsu, C.C.; Wu, K.M.; Hsu, T.S.; Wu, Y.T.; Hu, G.C. Bidirectional association between obstructive sleep apnea and depression: A population-based longitudinal study. Medicine 2016, 95, e4833. [Google Scholar] [CrossRef]
- Edwards, C.; Almeida, O.P.; Ford, A.H. Obstructive sleep apnea and depression: A systematic review and meta-analysis. Maturitas 2020, 142, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Karakuła, K.; Ryczkowski, A.; Sitarz, E.; Januszewski, J.; Juchnowicz, D. The relationships between obstructive sleep apnea and psychiatric disorders: A narrative review. Curr. Probl. Psychiatry 2021, 22, 46–53. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 4 March 2023).
- Ejaz, S.M.; Khawaja, I.S.; Bhatia, S.; Hurwitz, T.D. Obstructive sleep apnea and depression: A review. Innov. Clin. Neurosci. 2011, 8, 17–25. [Google Scholar]
- Bardwell, W.A.; Norman, D.; Ancoli-Israel, S.; Loredo, J.S.; Lowery, A.; Lim, W.; Dimsdale, J.E. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: A randomized placebo-controlled study. Behav. Sleep Med. 2007, 5, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. The neurotransmitters of sleep. J. Clin. Psychiatry 2004, 65 (Suppl. 16), 4–7. [Google Scholar]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Cross, R.L.; Kumar, R.; Macey, P.M.; Doering, L.V.; Alger, J.R.; Yan-Go, F.L.; Harper, R.M. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep 2008, 31, 1103–1109. [Google Scholar]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Bozic, J.; Borovac, J.A.; Galic, T.; Kurir, T.T.; Supe-Domic, D.; Dogas, Z. Adropin and Inflammation Biomarker Levels in Male Patients with Obstructive Sleep Apnea: A Link with Glucose Metabolism and Sleep Parameters. J. Clin. Sleep Med. 2018, 14, 1109–1118. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Pevernagie, D. Obstructive sleep apnea: Transition from pathophysiology to an integrative disease model. J. Sleep Res. 2022, 31, e13616. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; McNicholas, W.T. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur. Respir. Rev. 2022, 31, 210256. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. JAMA 1994, 272, 828–829. [Google Scholar] [CrossRef]
- American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013. [Google Scholar]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbauch, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Braz. J. Psychiatry 2013, 35, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W.K. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Plummer, F.; Manea, L.; Trepel, D.; McMillan, D. Screening for anxiety disorders with the GAD-7 and GAD-2: A systematic review and diagnostic metaanalysis. Gen. Hosp. Psychiatry 2016, 39, 24–31. [Google Scholar] [CrossRef]
- Carneiro-Barrera, A.; Amaro-Gahete, F.J.; Sáez-Roca, G.; Martín-Carrasco, C.; Ruiz, R.J.; Buela-Casal, G. Anxiety and Depression in Patients with Obstructive Sleep Apnoea before and after Continuous Positive Airway Pressure: The ADIPOSA Study. J. Clin. Med. 2019, 8, 2099. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R. Beck Anxiety Inventory [Database Record]; APA PsycTests; American Psychological Association: Washington, DC, USA, 1988. [Google Scholar] [CrossRef]
- Smarr, K.L.; Keefer, A.L. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center Epidemiological Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res. 2011, 63, S454–S466. [Google Scholar] [CrossRef]
- Andresen, E.M.; Malmgren, J.A.; Carter, W.B.; Patrick, D.L. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am. J. Prev. Med. 1944, 10, 77–84. [Google Scholar] [CrossRef]
- Vilagut, G.; Forero, C.G.; Barbaglia, G.; Alonso, J. Screening for Depression in the General Population with the Center for Epidemiologic Studies Depression (CES-D): A Systematic Review with Meta-Analysis. PLoS ONE 2016, 11, e0155431. [Google Scholar] [CrossRef]
- Cosco, T.D.; Prina, M.; Stubbs, B.; Wu, Y.T. Reliability and Validity of the Center for Epidemiologic Studies Depression Scale in a Population-Based Cohort of Middle-Aged, U.S. Adults. J. Nurs. Meas. 2017, 25, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, A.S.; Gilbody, S.; McMillan, D.; Manea, L. Screening and case finding for major depressive disorder using the Patient Health Questionnaire (PHQ-9): A meta-analysis. Gen. Hosp. Psychiatry 2015, 37, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med. Care 2003, 41, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef]
- Löwe, B.; Schenkel, I.; Carney-Doebbeling, C.; Göbel, C. Responsiveness of the PHQ-9 to Psychopharmacological Depression Treatment. Psychosomatics 2006, 47, 62–67. [Google Scholar] [CrossRef]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ 2012, 184, E191–E196. [Google Scholar] [CrossRef]
- Manea, L.; Boehnke, J.R.; Gilbody, S.; Moriarty, A.S.; McMillan, D. Are there researcher allegiance effects in diagnostic validation studies of the PHQ-9? A systematic review and meta-analysis. BMJ Open 2017, 7, e015247. [Google Scholar] [CrossRef]
- Salkind, M.R. Beck depression inventory in general practice. J. R. Coll. Gen. Pract. 1969, 18, 267–271. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Garbin, M.G. Psychometric proprieties of the Beck Depressive Inventory: Twenty-five years of evaluation. Clin. Psichol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gorenstein, C. The Beck depression inventory: Uses and applications. In The Neuroscience of Depression: Features, Diagnosis and Treatment; Martin, C.R., Hunter, L.A., Patel, V.B., Preedy, V.R., Rajendram, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 154–196. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI–Fast Screen for Medical Patients [Database Record]; APA PsycTests; American Psychological Association: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Deforge, B.R.; Sobal, J. Self-report depression scales in the elderly: The relationship between the CES-D and Zung. Int. J. Psychiatry Med. 1989, 18, 325–338. [Google Scholar] [CrossRef]
- Dunstan, D.A.; Scott, N. Clarification of the cut-off score for Zung’s self-rating depression scale. BMC Psychiatry 2019, 19, 177. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Spielberger, C.D. Manual for the State-Trait Anxiety Inventory; Mind Garden, Inc.: Menlo Park, CA, USA, 1983. [Google Scholar]
- Oh, H.; Park, K.; Yoon, S.; Kim, Y.; Lee, S.H.; Choi, Y.Y.; Choi, K.H. Clinical Utility of Beck Anxiety Inventory in Clinical and Nonclinical Korean Samples. Front. Psychiatry 2018, 9, 666. [Google Scholar] [CrossRef]
- Chin, W.Y.; Choi, E.P.; Chan, K.T.; Wong, C.K. The Psychometric Properties of the Center for Epidemiologic Studies Depression Scale in Chinese Primary Care Patients: Factor Structure, Construct Validity, Reliability, Sensitivity and Responsiveness. PLoS ONE 2015, 10, e0135131. [Google Scholar] [CrossRef]
- Sharif Nia, H.; Rezapour, M.; Allen, K.A.; Pahlevan Sharif, S.; Jafari, A.; Torkmandi, H.; Goudarzian, A.H. The Psychometric Properties of the Center for Epidemiological Studies Depression Scale (CES-D) for Iranian Cancer Patients. Asian Pac. J. Cancer Prev. 2019, 20, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ting, R.Z.; Lam, M.H.; Lam, S.P.; Yeung, R.O.; Nan, H.; Ozaki, R.; Luk, A.O.; Kong, A.P.; Wing, Y.K.; et al. Measuring depression with CES-D in Chinese patients with type 2 diabetes: The validity and its comparison to PHQ-9. BMC Psychiatry 2015, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Milette, K.; Hudson, M.; Baron, M.; Thombs, B.D.; Canadian Scleroderma Research Group. Comparison of the PHQ-9 and CES-D depression scales in systemic sclerosis: Internal consistency reliability, convergent validity and clinical correlates. Rheumatology 2001, 49, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Meader, N.; Moe-Byrne, T.; Llewellyn, A.; Mitchell, A.J. Screening for poststroke major depression: A meta-analysis of diagnostic validity studies. J. Neurol. Neurosurg. Psychiatry 2014, 85, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Nikolovski, A.; Gamgoum, L.; Deol, A.; Quilichini, S.; Kazemir, E.; Rhodenizer, J.; Oliveira, A.; Brooks, D.; Alsubheen, S. Psychometric properties of the Hospital Anxiety and Depression Scale (HADS) in individuals with stable chronic obstructive pulmonary disease (COPD): A systematic review. Disabil. Rehabil. 2023, 1–9, advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Krebber, A.M.; Buffart, L.M.; Kleijn, G.; Riepma, I.C.; de Bree, R.; Leemans, C.R.; Becker, A.; Brug, J.; van Straten, A.; Cuijpers, P.; et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psycho-Oncology 2014, 23, 121–130. [Google Scholar] [CrossRef]
- Litster, B.; Fiest, K.M.; Patten, S.B.; Fisk, J.D.; Walker, J.R.; Graff, L.A.; Bolton, J.M.; Sareen, J.; Marriott, J.J.; Berrigan, L.I.; et al. Screening Tools for Anxiety in People with Multiple Sclerosis: A Systematic Review. Int. J. MS Care 2016, 18, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Marinus, J.; Leentjens, A.F.; Visser, M.; Stiggelbout, A.M.; van Hilten, J.J. Evaluation of the hospital anxiety and depression scale in patients with Parkinson’s disease. Clin. Neuropharmacol. 2002, 25, 318–324. [Google Scholar] [CrossRef]
- Rosemann, T.; Backenstrass, M.; Joest, K.; Rosemann, A.; Szecsenyi, J.; Laux, G. Predictors of depression in a sample of 1021 primary care patients with osteoarthritis. Arthritis Rheum. 2007, 57, 415–422. [Google Scholar] [CrossRef]
- Pitanupong, J.; Phirom, W.; Kittichet, R. Prevalence and associated factors of depressive symptoms among patients with cancer receiving radiotherapy in southern Thailand: A university hospital-based cross-sectional study. BMC Palliat. Care 2023, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Cholera, R.; Gaynes, B.N.; Pence, B.W.; Bassett, J.; Qangule, N.; Macphail, C.; Bernhardt, S.; Pettifor, A.; Miller, W.C. Validity of the Patient Health Questionnaire-9 to screen for depression in a high-HIV burden primary healthcare clinic in Johannesburg, South Africa. J. Affect. Disord. 2014, 167, 160–166. [Google Scholar] [CrossRef]
- Biracyaza, E.; Habimana, S.; Rusengamihigo, D. Psychometric Properties of the Beck Depression Inventory (BDI-II) in Cancer Patients: Cancer Patients from Butaro Ambulatory Cancer Center, Rwanda. Psychol. Res. Behav. Manag. 2021, 14, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Elben, S.; Dimenshteyn, K.; Trenado, C.; Folkerts, A.K.; Ophey, A.; Sulzer, P.; Becker, S.; Schmidt, N.; Tödt, I.; Witt, K.; et al. Screen Fast, Screen Faster: A Pilot Study to Screen for Depressive Symptoms Using the Beck Depression Inventory Fast Screen in Parkinson’s Disease with Mild Cognitive Impairment. Front. Neurol. 2021, 12, 640137. [Google Scholar] [CrossRef]
- Jokelainen, J.; Timonen, M.; Keinänen-Kiukaanniemi, S.; Härkönen, P.; Jurvelin, H.; Suija, K. Validation of the Zung self-rating depression scale (SDS) in older adults. Scand. J. Prim. Health Care 2019, 37, 353–357. [Google Scholar] [CrossRef]
- Löwe, B.; Decker, O.; Müller, S.; Brähler, E.; Schellberg, D.; Herzog, W.; Herzberg, P.Y. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med. Care 2008, 46, 266–274. [Google Scholar] [CrossRef]
- Esser, P.; Hartung, T.J.; Friedrich, M.; Johansen, C.; Wittchen, H.U.; Faller, H.; Koch, U.; Härter, M.; Keller, M.; Schulz, H.; et al. The Generalized Anxiety Disorder Screener (GAD-7) and the anxiety module of the Hospital and Depression Scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psycho-Oncology 2018, 27, 1509–1516. [Google Scholar] [CrossRef]
- Seo, J.G.; Cho, Y.W.; Lee, S.J.; Lee, J.J.; Kim, J.E.; Moon, H.J.; Park, S.P. Validation of the generalized anxiety disorder-7 in people with epilepsy: A MEPSY study. Epilepsy Behav. 2014, 35, 59–63. [Google Scholar] [CrossRef]
- Liu, M.; Wang, D.; Fang, J.; Chang, Y.; Hu, Y.; Huang, K. Validation of the Generalized Anxiety Disorder-7 in patients with COPD: A cross-sectional study. BMC Psychiatry 2023, 23, 593. [Google Scholar] [CrossRef] [PubMed]
- Quek, K.F.; Low, W.Y.; Razack, A.H.; Loh, C.S.; Chua, C.B. Reliability and validity of the Spielberger State-Trait Anxiety Inventory (STAI) among urological patients: A Malaysian study. Med. J. Malays. 2004, 59, 258–267. [Google Scholar]
- Gustafson, L.W.; Gabel, P.; Hammer, A.; Lauridsen, H.H.; Petersen, L.K.; Andersen, B.; Bor, P.; Larsen, M.B. Validity and reliability of State-Trait Anxiety Inventory in Danish women aged 45 years and older with abnormal cervical screening results. BMC Med. Res. Methodol. 2020, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Bardhoshi, G.; Duncan, K.; Erford, B.T. Psychometric meta-analysis of the English version of the beck anxiety inventory. J. Couns. Dev. 2016, 94, 356–373. [Google Scholar] [CrossRef]
- Bardwell, W.A.; Moore, P.; Ancoli-Israel, S.; Dimsdale, J.E. Fatigue in obstructive sleep apnea: Driven by depressive symptoms instead of apnea severity? Am. J. Psychiatry 2003, 160, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, C.; Manali, E.; Ginieri-Coccossis, M.; Vougas, K.; Cholidou, K.; Markozannes, E.; Bakakos, P.; Liappas, I.; Alchanatis, M. Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep Breath. 2013, 17, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Dabis, R.; Gharraf, H. Predictors of anxiety and depression in patients with obstructive sleep apnea. Egypt. J. Chest Dis. Tuberc. 2012, 61, 171–177. [Google Scholar] [CrossRef]
- Surani, S.; Rao, S.; Surani, S.; Guntupalli, B.; Subramanina, S. Anxiety and depression in obstructive sleep apnea: Prevalence and gender/ethnic variance. Curr. Respir. Med. Rev. 2013, 9, 274–279. [Google Scholar] [CrossRef]
- Akberzie, W.; Hesselbacher, S.; Aiyer, I.; Surani, S.; Surani, Z.S. The Prevalence of Anxiety and Depression Symptoms in Obstructive Sleep Apnea. Cureus 2020, 12, e11203. [Google Scholar] [CrossRef] [PubMed]
- Lundetræ, R.S.; Saxvig, I.W.; Lehmann, S.; Bjorvatn, B. Effect of continuous positive airway pressure on symptoms of anxiety and depression in patients with obstructive sleep apnea. Sleep Breath. 2021, 25, 1277–1283. [Google Scholar] [CrossRef]
- Walker, A.; Naughton, M.T.; Shaw, L.; Jeklin, A.T.; Martin, C.; Dabscheck, E. Depression scores improve with continuous positive airway pressure in specialized sleep clinics: Real-world data. J. Clin. Sleep Med. 2021, 17, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Mukherjee, S.; Simpson, L.J.; Palmer, L.J.; Almeida, O.P.; Hillman, D.R. Depressive Symptoms before and after Treatment of Obstructive Sleep Apnea in Men and Woman. J. Clin. Sleep Med. 2015, 11, 1029–1038. [Google Scholar] [CrossRef]
- Velescu, D.R.; Marc, M.; Manolescu, D.; Trăilă, D.; Oancea, C. CPAP Therapy on Depressive and Anxiety Symptoms in Patients with Moderate to Severe Obstructive Sleep Apnea Syndrome. Medicina 2022, 58, 1402. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Im, K.; Seo, J.Y.; Jung, M. Association between sleep apnea severity and symptoms of depression and anxiety among individuals with obstructive sleep apnea. Sleep Med. 2023, 101, 11–18. [Google Scholar] [CrossRef] [PubMed]
- McCall, W.V.; Harding, D.; O’Donovan, C. Correlates of depressive symptoms in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2006, 2, 424–426. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.A.; Chung, Y.S.; Kim, W.S. The relation between apnea and depressive symptoms in men with severe obstructive sleep apnea: Mediational effects of sleep quality. Lung 2015, 193, 261–267. [Google Scholar] [CrossRef]
- Yosunkaya, S.; Kutlu, R.; Cihan, F.G. Evaluation of depression and quality of life in patients with obstructive sleep apnea syndrome. Niger. J. Clin. Pract. 2016, 19, 573–579. [Google Scholar] [CrossRef]
- Karamanlı, H.; Kayhan, F.; Akgedik, R. Depressive Symptoms in Patients with Obstructive Sleep Apnea. Turk. Thorac. J. 2016, 17, 109–113. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Karatinos, G. For individuals with obstructive sleep apnea, institution of CPAP therapy is associated with an amelioration of symptoms of depression which is sustained long term. J. Clin. Sleep Med. 2007, 3, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Aloia, M.S.; Arnedt, J.T.; Smith, L.; Skrekas, J.; Stanchina, M.; Millman, R.P. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005, 6, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ishman, S.L.; Benke, J.R.; Cohen, A.P.; Stephen, M.J.; Ishii, L.E.; Gourin, C.G. Does surgery for obstructive sleep apnea improve depression and sleepiness? Laryngoscope 2014, 124, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, D.A.; Gurubhagavatula, I.; Broderick, P.; Chirinos, J.A.; Teff, K.; Wadden, T.; Maislin, G.; Saif, H.; Chittams, J.; Cassidy, C.; et al. Depressive symptoms in patients with obstructive sleep apnea: Biological mechanistic pathways. J. Behav. Med. 2017, 40, 955–963. [Google Scholar] [CrossRef]
- Yamamoto, H.; Akashiba, T.; Kosaka, N.; Ito, D.; Horie, T. Long-term effects nasal continuous positive airway pressure on daytime sleepiness, mood and traffic accidents in patients with obstructive sleep apnoea. Respir. Med. 2000, 94, 87–90. [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.; Zhang, X.; Wang, S.; Sang, J.; Tian, X.; Cao, H. Prevalence and Predisposing Factors for Depressive Status in Chinese Patients with Obstructive Sleep Apnoea: A Large-Sample Survey. PLoS ONE 2016, 11, e0149939. [Google Scholar] [CrossRef]
- Balcan, B.; Thunström, E.; Strollo, P.J., Jr.; Peker, Y. Determinants of depressive mood in coronary artery disease patients with obstructive sleep apnea and response to continuous positive airway pressure treatment in non-sleepy and sleepy phenotypes in the RICCADSA cohort. J. Sleep Res. 2019, 28, e12818. [Google Scholar] [CrossRef]
- Lee, S.A.; Han, S.H.; Ryu, H.U. Anxiety and its relationship to quality of life independent of depression in patients with obstructive sleep apnea. J. Psychosom. Res. 2015, 79, 32–36. [Google Scholar] [CrossRef]
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J. Res. Med. Sci. 2014, 19, 205–210. [Google Scholar]
- Saunamäki, T.; Jehkonen, M. Depression and anxiety in obstructive sleep apnea syndrome: A review. Acta Neurol. Scand. 2007, 116, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Boström, K.B.; Gustavsson, P.; Ekselius, L. Which instruments to support diagnosis of depression have sufficient accuracy? A systematic review. Nord. J. Psychiatry 2015, 69, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ding, R.; Wang, L.; Sheng, L.; Li, J.; Hu, D. Screening for depression in acute coronary syndrome patients: A comparison of Patient Health Questionnaire-9 versus Hospital Anxiety and Depression Scale-Depression. J. Psychosom. Res. 2019, 121, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Gorenstein, C. Assessment of depression in medical patients: A systematic review of the utility of the Beck Depression Inventory-II. Clinics 2013, 68, 1274–1287. [Google Scholar] [CrossRef]
- Mughal, A.Y.; Devadas, J.; Ardman, E.; Levis, B.; Go, V.F.; Gaynes, B.N. A systematic review of validated screening tools for anxiety disorders and PTSD in low to middle income countries. BMC Psychiatry 2020, 20, 338. [Google Scholar] [CrossRef] [PubMed]
| Scales | Overview | Number of Items | Scoring | Cut-Off | Time Frame | Administration |
|---|---|---|---|---|---|---|
| CES-D [33] | Screening for depression | 20 | Responses use a 4-point scale (0—rarely or none of the time (less than 1 day), 1—some or a little of the time (1–2 days), 2—occasionally or a moderate amount of time (3–4 days), and 3—most or all of the time (5–7 days)), with a total score ranging from 0 to 60. | ≥16 | Past week | 10 min |
| HADS-A HADS-D [34] | Screening for depression and anxiety | 14 | It comprises 14 items, 7 for anxiety and 7 for depression, and is rated from 0 to 3, according to how the respondent has felt during the past week. Total scores range from 0 to 42 and subscales range from 0 to 21. | ≥8 | Past week | 2–5 min |
| PHQ-9 [35] | Screening for depression | 9 | A 4-point scale indicates the degree of severity; items are rated from 0 (not at all) to 3 (nearly daily). Total score ranges between 0 and 27. | ≥10 | Past 2 weeks | 3–5 min |
| BDI-II [36,37] | Rating depression | 21 | It questions how respondents have felt recently. It is rated using a scale of increasing ordinal severity, ranging from 0 to 3. The total score varies from 0 to 63. | ≥11 | Not established | 5–10 min |
| SDS [38] | Rating depression | 20 | The standardized score is calculated by multiplying the total of the raw item scores of the 20 items by a factor of 1.25. They are scored from 0 (some of the time) to 4 (most of the time). The range is 0–100, where higher scores indicate more severe depression. | ≥53 | Past several days | 5–10 min |
| GAD-7 [39] | Screening for anxiety | 7 | The patient’s response options include “not at all,” “several days,” “more than half the days,” and “nearly daily,” which are assigned scores of 0, 1, 2, and 3, respectively. Mild anxiety is defined as a score of 5, while a score of 10 indicates moderate anxiety. | ≥10 | Past 2 weeks | 3–5 min |
| STAI [40] | Rating anxiety | 40 | STAI-State responses use a scale of 0 to 3, ranging from “not at all” to “very much so”. These responses reflect the individual’s current emotional state. STAI-Trait responses range from 0 (almost never) to 3 (almost always). Scores range from 20 to 80, and higher scores indicate more severe anxiety. | Not established | State anxiety Trait anxiety | 10–20 min |
| BAI [41] | Rating anxiety | 21 | The total score varies from 0 to 63. A score of 0 to 7 indicates minimal anxiety, 8 to 15 indicates mild anxiety, 16 to 25 indicates moderate anxiety, and 30 to 63 indicates severe anxiety. | ≥16 | Past week | 10 min |
| Score Range | BDI-II | BDI-FS | BDI-IA |
|---|---|---|---|
| No/minimal depression | 0–13 | 0–3 | 0–9 |
| Mild depression | 14–19 | 4–8 | 10–16 |
| Moderate depression | 20–28 | 9–12 | 17–29 |
| Severe depression | 29–63 | 13–21 | 30–63 |
| Tool | Strengths | Weaknesses | Validity in Study Populations |
|---|---|---|---|
| CES-D [33] | Alpha: 0.90 Sensitivity: 74.6% Specificity: 73.4% Free to use | A cut-off of 20 may be better than the value of 16 which is typically recommended [44]. | General population [33], primary care [62], oncology [63], diabetes [64], systemic sclerosis [65], stroke [66] |
| HADS [34] | Alpha: 0.83 Sensitivity: 80% Specificity: 80% | A license must be purchased for use. Absence of suicidal ideation item. Variation of cut-off points in studies. | General population, primary care [47], COPD [67], oncology [68], multiple sclerosis [69], Parkinson’s disease [70] |
| PHQ-9 [35] | Alpha: 0.86 Sensitivity: 88% Specificity: 88% Free to use Suicidal ideation item | A cut-off ≥ 10 could overestimate the symptoms [18]. | Diabetes [64], systemic sclerosis [65], rheumatological disorders [71], oncology [72], immunodeficiency disorder [73] |
| BDI [36,37] | Alpha: 0.92–0.93 Test–retest reliability: 0.73–0.96 Sensitivity: >70% | A license must be purchased for use. Aligns with DSM-IV but not DSM-5. Usually used as the first application without having a previous depression diagnosis. | General population [37], diabetes [64], oncology [74], Parkinson’s disease [75] |
| SDS [38] | Alpha: 0.92–0.93 Test–retest reliability: 0.73–0.96 Sensitivity: >70% | A license must be purchased for use. The large number of somatic items is likely to inflate depression rates. Requires more evidence of validity in various populations. | Older adults [76] |
| GAD-7 [39] | Alpha: 0.83 Sensitivity: 89% Specificity: 82% Free to use | Scores can be easily exaggerated. | General population [77], multiple sclerosis [69], oncology [78], epilepsy [79], COPD [80] |
| STAI [60] | Alpha: 0.86–0.95 Test–retest reliability: 0.65–0.75 Sensitivity: 78.3% Specificity: 71.2% | A license must be purchased for use. Based on DSM-IV criteria. More items. | Urologic diseases [81], oncology [82], multiple sclerosis [69] |
| BAI [41,83] | Alpha: 0.91 Test–retest reliability: 0.58–0.66 Sensitivity: >70% | A license must be purchased for use. Aligns with DSM-IV but not DSM-5. Requires more evidence of validity in various populations. | Multiple sclerosis [69] |
| Study ID | Tool for Evaluation | Study Design | Sample Size (Participants) | Mean Age (SD) % Females | Prevalence of Mental Disorders | Cut-Off and Severity |
|---|---|---|---|---|---|---|
| Bardwell et al., 2003 [84] | CES-D | Cross-sectional | 60 | 49.1 (7.5) 15.6% | In total, 33.3% of OSA patients presented depressive symptoms. | ≥16 |
| Diamanti et al., 2013 [85] | CES-D | Prospective observational | 41 | 51.9 (10.5) 14.6% | In total, 53.6% of OSA patients presented depressive symptoms. | ≥16 |
| Daabis et al., 2012 [86] | HADS-A HADS-D | Case–control | 102 | 48.8 (11.73) 17% | In total, 33% of OSA patients presented anxiety symptoms, and 51% presented depressive symptoms. | ≥11 |
| Surani et al., 2013 [87] | HADS-A HADS-D | Cross-sectional | 51 | No data | In total, 52.9% of OSA patients presented anxiety symptoms, and 39.2% presented depressive symptoms. | ≥10 |
| Akberzie et al., 2018 [88] | HADS-A HADS-D | Cross-sectional | 45 | 47 (No data) 64% | In total, 62.2% of OSA patients presented anxiety symptoms, and 64.4% presented depressive symptoms. | ≥8 |
| Lundetræ et al., 2021 [89] | HADS-A HADS-D | Prospective observational | 468 | 55.5 (12) 28.8% | In total, 26.3% of OSA patients presented anxiety symptoms, and 17.5% presented depressive symptoms. | ≥8 |
| Walker et al., 2021 [90] | HADS-A HADS-D | Prospective observational | 108 | 56 (12.8) 27.8% | In total, 17.6% of OSA patients presented anxiety symptoms, and 37% presented depressive symptoms. | HADS-A ≥ 8 HADS-D ≥ 11 |
| Edwards et al., 2015 [91] | PHQ-9 | Prospective observational | 293 | 52 (No data) 38.3% | In total, 72.6% of OSA patients presented depressive symptoms. | ≥10 |
| Velescu et al., 2022 [92] | PHQ-9 GAD-7 | Prospective observational | 99 | 56 (10.92) 32.67% | In total, 48.5% of OSA patients presented depressive symptoms, and 27.3% presented anxiety symptoms. | ≥10 |
| Lee et al., 2023 [93] | PHQ-9 GAD-7 | Cross-sectional | 1390 | 50 (12.4) 19.6% | In total, 15.9% of OSA patients presented anxiety symptoms, and 14.4% presented depressive symptoms. | PHQ-9 ≥ 10 GAD-7 ≥ 8 |
| McCall et al., 2006 [94] | BDI | Cross-sectional | 121 | 51.7 (14.1) 24% | In total, 44.6% of OSA patients presented depressive symptoms. | ≥10 |
| Lee, W et al., 2015 [95] | BDI | Cross-sectional | 302 | 48.4 (11.3) Only men | In total, 39% of OSA patients presented depressive symptoms. | ≥10 |
| Yosunkaya et al., 2016 [96] | BDI | Cross-sectional | 200 | 45.5 (9.9) 12.5% | In total, 16.4% of OSA patients presented moderate depressive symptoms. | ≥17 |
| Karamanli et al., 2016 [97] | BDI | Case–control | 96 | 51.4 (1.3) 41.6% | In total, 59,7% of OSA patients presented depressive symptoms, and 25% had moderate to severe depression. | mild (10–15), moderate (16–23), and severe (24–63) |
| Schwartz et al., 2007 [98] | BDI-FS | Prospective observational | 50 | 53 (11.3) 22% | In total, 33% of OSA patients presented depressive symptoms. | ≥4 |
| Aloia et al., 2005 [99] | BDI-II | Cross-sectional | 93 | 52.2 (11.1) 34.4% | In total, 33.3% of OSA patients presented depressive symptoms. | ≥14 |
| Cross et al., 2008 [25] | BDI-II | Case–control | 101 | 47.6 (11) No data | In total, 33% of OSA patients presented elevated depressive symptoms (BDI-II ≥ 12). | ≥12 symptomatic ≤10 asymptomatic |
| Ishman et al., 2014 [100] | BDI-II | Prospective observational | 104 | 46.8 (9.1) 24.5% | In total, 27.3% of OSA patients presented depressive symptoms. | ≥12 |
| Chirinos et al., 2017 [101] | BDI-II | Cross-sectional | 181 | 48.9 (11.2) No data | 88.3% minimal 8.9% mild 2.2% moderate 0.6% severe depression | Minimal: 0–13 Mild: 14–19 Moderate: 20–28 Severe: 29–63 |
| Yamatoto et al., 2000 [102] | SDS | Prospective observational | 47 | 49.5 (10.8) No data | In total, 63.4% of OSA patients presented depressive symptoms. | ≥41 |
| Dai et al., 2016 [103] | SDS | Cross-sectional | 1327 | 47 (No data) 19.3% | In total, 47.4% of OSA patients presented depressive symptoms. | ≥53 |
| Balcan et al., 2019 [104] | SDS | Cross-sectional | 493 | 63.9 (8.6) 16.8% | In total, 29.3% of OSA patients presented depressive symptoms. | ≥50 |
| Lee et al., 2015 [105] | STAI BDI | Cross-sectional | 655 | 49.8 (11.70) 13.1% | In total, 48.4% of OSA patients presented anxiety symptoms, and 46.4% presented depressive symptoms. | STAI ≥ 40 BDI ≥ 10 |
| Rezaeitalab et al., 2014 [106] | BAI BDI | Cross-sectional | 178 | 50.3 (No data) 14.4% | In total, 53.9% of OSA patients presented anxiety symptoms, and 46.1% presented depressive symptoms. | BAI ≥ 8 BDI ≥ 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velescu, D.R.; Marc, M.S.; Traila, D.; Pescaru, C.C.; Hogea, P.; Suppini, N.; Crisan, A.F.; Wellmann, N.; Oancea, C. A Narrative Review of Self-Reported Scales to Evaluate Depression and Anxiety Symptoms in Adult Obstructive Sleep Apnea Patients. Medicina 2024, 60, 261. https://doi.org/10.3390/medicina60020261
Velescu DR, Marc MS, Traila D, Pescaru CC, Hogea P, Suppini N, Crisan AF, Wellmann N, Oancea C. A Narrative Review of Self-Reported Scales to Evaluate Depression and Anxiety Symptoms in Adult Obstructive Sleep Apnea Patients. Medicina. 2024; 60(2):261. https://doi.org/10.3390/medicina60020261
Chicago/Turabian StyleVelescu, Diana Raluca, Monica Steluta Marc, Daniel Traila, Camelia Corina Pescaru, Patricia Hogea, Noemi Suppini, Alexandru Florian Crisan, Norbert Wellmann, and Cristian Oancea. 2024. "A Narrative Review of Self-Reported Scales to Evaluate Depression and Anxiety Symptoms in Adult Obstructive Sleep Apnea Patients" Medicina 60, no. 2: 261. https://doi.org/10.3390/medicina60020261
APA StyleVelescu, D. R., Marc, M. S., Traila, D., Pescaru, C. C., Hogea, P., Suppini, N., Crisan, A. F., Wellmann, N., & Oancea, C. (2024). A Narrative Review of Self-Reported Scales to Evaluate Depression and Anxiety Symptoms in Adult Obstructive Sleep Apnea Patients. Medicina, 60(2), 261. https://doi.org/10.3390/medicina60020261









