Coagulation Profile in Neonates with Congenital Heart Disease: A Pilot Study
Abstract
1. Introduction
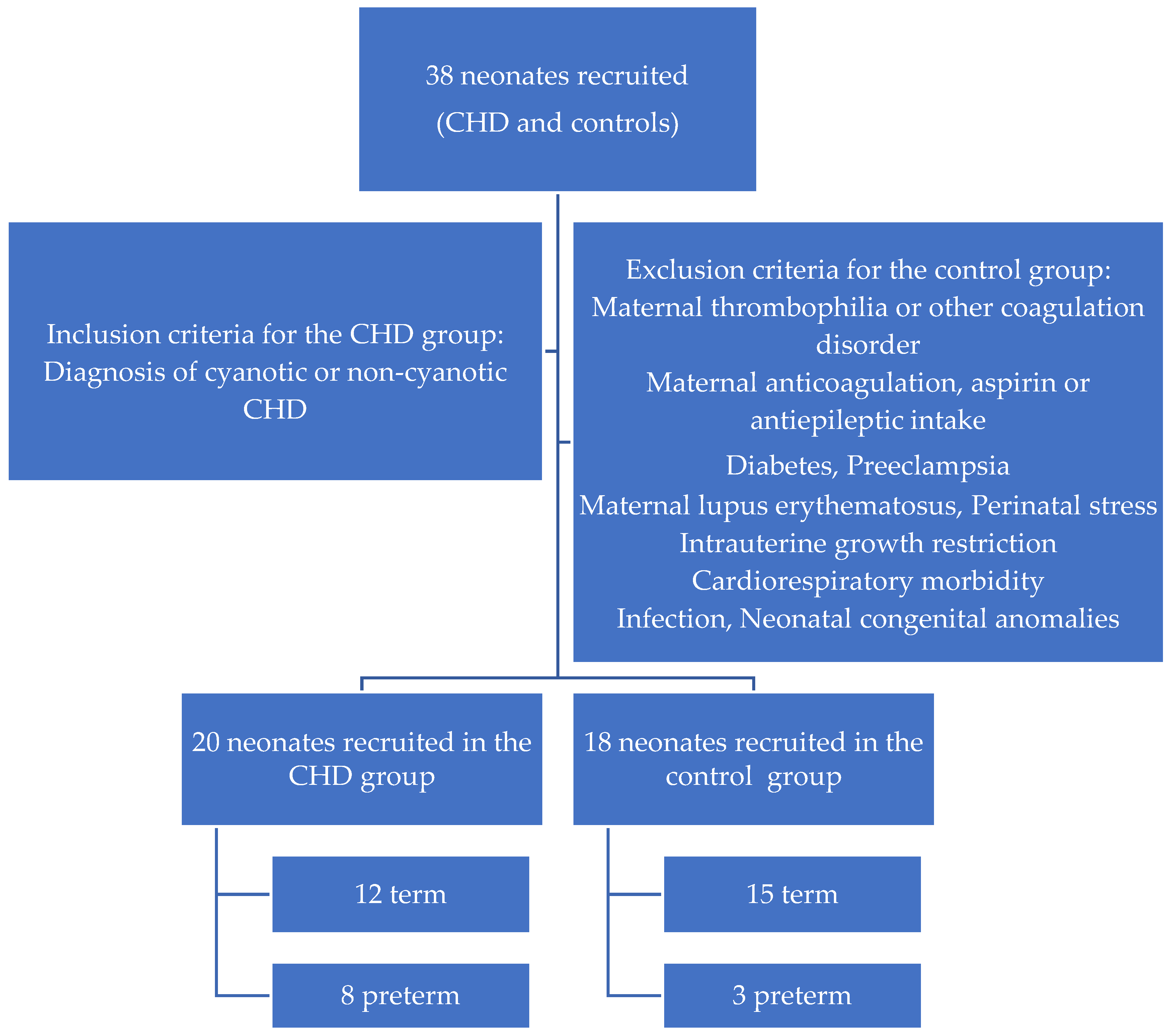
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iacobazzi, D.; Alvino, V.V.; Caputo, M.; Madeddu, P. Accelerated Cardiac Aging in Patients with Congenital Heart Disease. Front. Cardiovasc. Med. 2022, 9, 892861. [Google Scholar] [CrossRef] [PubMed]
- Nora, J.J. Multifactorial Inheritance Hypothesis for the Etiology of Congenital Heart Diseases: The Genetic-Environmental Interaction. Circulation 1968, 38, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Bahnson, H.T.; Ziegler, R.F. A Consideration of the Causes of Death Following Operation for Congenital Heart Disease of the Cyanotic Type. Surg. Gynecol. Obstet. 1950, 90, 60–76. [Google Scholar] [PubMed]
- Hartmann, R.C. A Hemorrhagic Disorder Occurring in Patients with Cyanotic Congenital Heart Disease. Bull. Johns Hopkins Hosp. 1952, 91, 49–67. [Google Scholar]
- Nelson McMillan, K.; Kramer, J.; Takemoto, C.M.; Ozment, C.P. Coagulation Disorders in Congenital Heart Disease. In Critical Heart Disease in Infants and Children; Elsevier: Amsterdam, The Netherlands, 2019; pp. 282–302.e7. ISBN 978-1-4557-0760-7. [Google Scholar]
- Karim, F.; Adil, S.N.; Afaq, B.; ul Haq, A. Deficiency of ADAMTS-13 in Pediatric Patients with Severe Sepsis and Impact on in-Hospital Mortality. BMC Pediatr. 2013, 13, 44. [Google Scholar] [CrossRef]
- Katneni, U.K.; Holcomb, D.D.; Hernandez, N.E.; Hamasaki-Katagiri, N.; Hunt, R.C.; Bar, H.; Ibla, J.C.; Kimchi-Sarfaty, C. In Silico Features of ADAMTS13 Contributing to Plasmatic ADAMTS13 Levels in Neonates with Congenital Heart Disease. Thromb. Res. 2020, 193, 66–76. [Google Scholar] [CrossRef]
- DeYoung, V.; Singh, K.; Kretz, C.A. Mechanisms of ADAMTS13 Regulation. J. Thromb. Haemost. 2022, 20, 2722–2732. [Google Scholar] [CrossRef]
- Turecek, P.L.; Peck, R.C.; Rangarajan, S.; Reilly-Stitt, C.; Laffan, M.A.; Kazmi, R.; James, I.; Dushianthan, A.; Schrenk, G.; Gritsch, H.; et al. Recombinant ADAMTS13 Reduces Abnormally Up-Regulated von Willebrand Factor in Plasma from Patients with Severe COVID-19. Thromb. Res. 2021, 201, 100–112. [Google Scholar] [CrossRef]
- Odegard, K.C.; Zurakowski, D.; Hornykewycz, S.; DiNardo, J.A.; Castro, R.A.; Neufeld, E.J.; Laussen, P.C. Evaluation of the Coagulation System in Children with Two-Ventricle Congenital Heart Disease. Ann. Thorac. Surg. 2007, 83, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.; Varendh, G.; Lundstrom, N.R. Haemostatic Defects in Cyanotic Congenital Heart Disease. Heart 1979, 41, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Cholette, J.M.; Rubenstein, J.S.; Alfieris, G.M.; McDermott, M.P.; Harmon, W.G.; Vermilion, R.; Eaton, M.P.; Gangemi, J.J.; Lerner, N.B. Elevated Risk of Thrombosis in Neonates Undergoing Initial Palliative Cardiac Surgery. Ann. Thorac. Surg. 2007, 84, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Odegard, K.C.; McGowan, F.X.; DiNardo, J.A.; Castro, R.A.; Zurakowski, D.; Connor, C.M.; Hansen, D.D.; Neufeld, E.J.; del Nido, P.J.; Laussen, P.C. Coagulation Abnormalities in Patients with Single-Ventricle Physiology Precede the Fontan Procedure. J. Thorac. Cardiovasc. Surg. 2002, 123, 459–465. [Google Scholar] [CrossRef]
- Odegard, K.C.; McGowan, F.X.; Zurakowski, D.; DiNardo, J.A.; Castro, R.A.; del Nido, P.J.; Laussen, P.C. Coagulation Factor Abnormalities in Patients with Single-Ventricle Physiology Immediately Prior to the Fontan Procedure. Ann. Thorac. Surg. 2002, 73, 1770–1777. [Google Scholar] [CrossRef]
- Hakacova, N.; Laluhova-Striezencova, Z.; Zahorec, M. Disturbances of Coagulation in Neonates with Functionally Univentricular Physiology Prior to the First Stage of Surgical Reconstruction. Cardiol. Young 2008, 18, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Majiyagbe, O.O.; Akinsete, A.M.; Adeyemo, T.A.; Salako, A.O.; Ekure, E.N.; Okoromah, C.A.N. Coagulation Abnormalities in Children with Uncorrected Congenital Heart Defects Seen at a Teaching Hospital in a Developing Country. PLoS ONE 2022, 17, e0263948. [Google Scholar] [CrossRef]
- Shebl, S.S.; El-shehaby, W.A.N.; Said, Y.S.; Darwish, A.H.; Elfadaly, N.H.; Amer, E. Thrombo-Hemorrhagic Liability in Children with Congenital Heart Diseases. Hematol./Oncol. Stem Cell Ther. 2018, 11, 123–128. [Google Scholar] [CrossRef]
- Tempe, D.K.; Virmani, S. Coagulation Abnormalities in Patients with Cyanotic Congenital Heart Disease. J. Cardiothorac. Vasc. Anesth. 2002, 16, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Horigome, H.; Hiramatsu, Y.; Shigeta, O.; Nagasawa, T.; Matsui, A. Overproduction of Platelet Microparticles in Cyanotic Congenital Heart Disease with Polycythemia. J. Am. Coll. Cardiol. 2002, 39, 1072–1077. [Google Scholar] [CrossRef]
- Averin, K.; Byrnes, J.W.; Benscoter, D.T.; Whiteside, W.; DeSena, H.; Hirsch, R.; Goldstein, B.H. Life-Threatening Airway Bleeding after Palliation of Single Ventricle Congenital Heart Disease. Heart 2018, 104, 254–260. [Google Scholar] [CrossRef]
- Eaton, M.P.; Iannoli, E.M. Coagulation Considerations for Infants and Children Undergoing Cardiopulmonary Bypass: Coagulation in Children on Bypass. Pediatr. Anesth. 2011, 21, 31–42. [Google Scholar] [CrossRef]
- Ten, V.S.; Pinsky, D.J. Endothelial Response to Hypoxia: Physiologic Adaptation and Pathologic Dysfunction. Curr. Opin. Crit. Care 2002, 8, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Piccoli, D.A.; Nadolski, G.; Rychik, J.; DeWitt, A.; Pinto, E.; Rome, J.; Dori, Y. Protein-Losing Enteropathy in Patients With Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2929–2937. [Google Scholar] [CrossRef]
- Arnold, N.; Lechner, K.; Waldeyer, C.; Shapiro, M.D.; Koenig, W. Inflammation and Cardiovascular Disease: The Future. Eur. Cardiol. 2021, 16, e20. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, H.; Nakazawa, M.; Murasaki, K.; Mori, Y.; Tanoue, K.; Kasanuki, H.; Nakanishi, T. Increased Thrombogenesity in Patients With Cyanotic Congenital Heart Disease. Circ. J. 2007, 71, 948–953. [Google Scholar] [CrossRef]
- Zabala, L.M.; Guzzetta, N.A. Cyanotic Congenital Heart Disease (CCHD): Focus on Hypoxemia, Secondary Erythrocytosis, and Coagulation Alterations. Paediatr. Anaesth. 2015, 25, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Ninivaggi, M.; de Laat, M.; Lancé, M.M.D.; Kicken, C.H.; Pelkmans, L.; Bloemen, S.; Dirks, M.L.; van Loon, L.J.C.; Govers-Riemslag, J.W.P.; Lindhout, T.; et al. Hypoxia Induces a Prothrombotic State Independently of the Physical Activity. PLoS ONE 2015, 10, e0141797. [Google Scholar] [CrossRef]
- Komp, D.M.; Sparrow, A.W. Polycythemia in Cyanotic Heart Disease—A Study of Altered Coagulation. J. Pediatr. 1970, 76, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zanolini, D.; Merlin, S.; Feola, M.; Ranaldo, G.; Amoruso, A.; Gaidano, G.; Zaffaroni, M.; Ferrero, A.; Brunelleschi, S.; Valente, G.; et al. Extrahepatic Sources of Factor VIII Potentially Contribute to the Coagulation Cascade Correcting the Bleeding Phenotype of Mice with Hemophilia A. Haematologica 2015, 100, 881–892. [Google Scholar] [CrossRef]
- Odegard, K.C.; Zurakowski, D.; DiNardo, J.A.; Castro, R.A.; McGowan, F.X.; Neufeld, E.J.; Laussen, P.C. Prospective Longitudinal Study of Coagulation Profiles in Children with Hypoplastic Left Heart Syndrome from Stage I through Fontan Completion. J. Thorac. Cardiovasc. Surg. 2009, 137, 934–941. [Google Scholar] [CrossRef]
- Hollestelle, M.J.; Thinnes, T.; Crain, K.; Stiko, A.; Kruijt, J.K.; van Berkel, T.J.; Loskutoff, D.J.; van Mourik, J.A. Tissue Distribution of Factor VIII Gene Expression In Vivo—A Closer Look. Thromb. Haemost. 2001, 86, 855–861. [Google Scholar] [CrossRef]
- McLeod, K.A.; Martin, P.; Williams, G.; Walker, D.R. Neutrophil Activation and Morbidity in Young Adults with Cyanotic Congenital Heart Disease. Blood Coagul. Fibrinolysis 1994, 5, 17–22. [Google Scholar] [CrossRef]
- Boneu, B.; Abbal, M.; Plante, J.; Bierme, R. Factor-VIII Complex And Endothelial Damage. Lancet 1975, 305, 1430. [Google Scholar] [CrossRef]
- Lip, G. Von Willebrand Factor: A Marker of Endothelial Dysfunction in Vascular Disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ismail, E.A.R.; Youssef, O.I. Platelet-Derived Microparticles and Platelet Function Profile in Children with Congenital Heart Disease. Clin. Appl. Thromb. Hemost. 2013, 19, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.V.; Rawley, O.; Smith, O.P.; O’Donnell, J.S. Elevated Factor VIII Levels and Risk of Venous Thrombosis. Br. J. Haematol. 2012, 157, 653–663. [Google Scholar] [CrossRef]
- O’Donnell, J.; Tuddenham, E.G.; Manning, R.; Kemball-Cook, G.; Johnson, D.; Laffan, M. High Prevalence of Elevated Factor VIII Levels in Patients Referred for Thrombophilia Screening: Role of Increased Synthesis and Relationship to the Acute Phase Reaction. Thromb. Haemost. 1997, 77, 825–828. [Google Scholar] [CrossRef]
- Davidson, S.J. Inflammation and Acute Phase Proteins in Haemostasis. In Acute Phase Proteins; Janciauskiene, S., Ed.; InTech: Vienna, Austria, 2013; ISBN 978-953-51-1185-6. [Google Scholar]
- Ihenacho, H.N.C.; Breeze, G.R.; Fletcher, D.J.; Stuart, J. Consumption Coagulopathy in Congenital Heart-Disease. Lancet 1973, 301, 231–234. [Google Scholar] [CrossRef]
- Odegard, K.C.; McGowan, F.X.; Zurakowski, D.; DiNardo, J.A.; Castro, R.A.; del Nido, P.J.; Laussen, P.C. Procoagulant and Anticoagulant Factor Abnormalities Following the Fontan Procedure: Increased Factor VIII May Predispose to Thrombosis. J. Thorac. Cardiovasc. Surg. 2003, 125, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, M.; Kreutzer, J.; Zurakowski, D.; Bacha, E.; Jonas, R.A. Evaluation of Hemostatic and Coagulation Factor Abnormalities in Patients Undergoing the Fontan Operation. J. Thorac. Cardiovasc. Surg. 2000, 120, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Rauch, R.; Ries, M.; Hofbeck, M.; Buheitel, G.; Singer, H.; Klinge, J. Hemostatic Changes Following the Modified Fontan Operation (Total Cavopulmonary Connection). Thromb. Haemost. 2000, 83, 678–682. [Google Scholar] [PubMed]
- Binotto, M.A.; Maeda, N.Y.; Lopes, A.A. Evidence of Endothelial Dysfunction in Patients with Functionally Univentricular Physiology before Completion of the Fontan Operation. Cardiol. Young 2005, 15, 26–30. [Google Scholar] [CrossRef]
- Caramuru, L.H.; Maeda, N.Y.; Bydlowski, S.P.; Lopes, A.A. Age-Dependent Likelihood of In Situ Thrombosis in Secondary Pulmonary Hypertension. Clin. Appl. Thromb. Hemost. 2004, 10, 217–223. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, Q.-W.; Shen, Y.-M.; Liu, J.-F.; Li, W.-Z.; Jia, K.-G. [Application Value of Plasma vWF and ADAMTS-13 in Evaluating Postoprative Vascular Endothelial Injury and Prognosis in Children with VSD]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2023, 31, 189–196. [Google Scholar]
- Soares, R.P.S.; Bydlowski, S.P.; Nascimento, N.M.; Thomaz, A.M.; Bastos, E.N.M.; Lopes, A.A. Plasmatic ADAMTS-13 Metalloprotease and von Willebrand Factor in Children with Cyanotic Congenital Heart Disease. Braz. J. Med. Biol. Res. 2013, 46, 375–381. [Google Scholar] [CrossRef]
- Papadogeorgou, P.; Boutsikou, T.; Boutsikou, M.; Pergantou, E.; Mantzou, A.; Papassotiriou, I.; Iliodromiti, Z.; Sokou, R.; Bouza, E.; Politou, M.; et al. A Global Assessment of Coagulation Profile and a Novel Insight into Adamts-13 Implication in Neonatal Sepsis. Biology 2023, 12, 1281. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A.; et al. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediat. Inflamm. 2017, 2017, 5620314. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.T.B.; Lam, J.K.; Rance, J.B.; Mollica, L.R.; O’Donnell, J.S.; Lane, D.A. Proteolytic Inactivation of ADAMTS13 by Thrombin and Plasmin. Blood 2005, 105, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Monagle, P. Thrombosis in Children with BT Shunts, Glenns and Fontans. Progress Pediatr. Cardiol. 2005, 21, 17–21. [Google Scholar] [CrossRef]
- Revel-Vilk, S.; Chan, A.; Bauman, M.; Massicotte, P. Prothrombotic Conditions in an Unselected Cohort of Children with Venous Thromboembolic Disease. J. Thromb. Haemost. 2003, 1, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Silvey, M.; Hall, M.; Bilynsky, E.; Carpenter, S.L. Increasing Rates of Thrombosis in Children with Congenital Heart Disease Undergoing Cardiac Surgery. Thromb. Res. 2018, 162, 15–21. [Google Scholar] [CrossRef]
- Attard, C.; Huang, J.; Monagle, P.; Ignjatovic, V. Pathophysiology of Thrombosis and Anticoagulation Post Fontan Surgery. Thromb. Res. 2018, 172, 204–213. [Google Scholar] [CrossRef]
- Flinterman, L.E.; Van Hylckama Vlieg, A.; Rosendaal, F.R.; Doggen, C.J.M. Venous Thrombosis of the Upper Extremity: Effect of Blood Group and Coagulation Factor Levels on Risk. Br. J. Haematol. 2010, 149, 118–123. [Google Scholar] [CrossRef]
- Koster, T.; Vandenbroucke, J.P.; Rosendaal, F.R.; Briët, E.; Rosendaal, F.R.; Blann, A.D. Role of Clotting Factor VIII in Effect of von Willebrand Factor on Occurrence of Deep-Vein Thrombosis. Lancet 1995, 345, 152–155. [Google Scholar] [CrossRef]
- Kraaijenhagen, R.A.; in’t Anker, P.S.; Koopman, M.M.; Reitsma, P.H.; Prins, M.H.; van den Ende, A.; Büller, H.R. High Plasma Concentration of Factor VIIIc is a Major Risk Factor for Venous Thromboembolism. Thromb. Haemost. 2000, 83, 5–9. [Google Scholar] [PubMed]
- Ota, S.; Yamada, N.; Ogihara, Y.; Tsuji, A.; Ishikura, K.; Nakamura, M.; Wada, H.; Ito, M. High Plasma Level of Factor VIII—An Important Risk Factor for Venous Thromboembolism. Circ. J. 2011, 75, 1472–1475. [Google Scholar] [CrossRef]
- Goldenberg, N.A.; Knapp-Clevenger, R.; Manco-Johnson, M.J. Elevated Plasma Factor VIII and D-Dimer Levels as Predictors of Poor Outcomes of Thrombosis in Children. N. Engl. J. Med. 2004, 351, 1081–1088. [Google Scholar] [CrossRef]
- Kreuz, W.; Stoll, M.; Junker, R.; Heinecke, A.; Schobess, R.; Kurnik, K.; Kelsch, R.; Nowak-Göttl, U. Familial Elevated Factor VIII in Children with Symptomatic Venous Thrombosis and Post-Thrombotic Syndrome: Results of a Multicenter Study. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Laffan, M.A.; Manning, R. The Influence of Factor VIII on Measurement of Activated Protein C Resistance. Blood Coagul. Fibrinolysis 1996, 7, 761–765. [Google Scholar] [CrossRef]
- Hunt, R.; Hoffman, C.M.; Emani, S.; Trenor, C.C.; Emani, S.M.; Faraoni, D.; Kimchi-Sarfaty, C.; Ibla, J.C. Elevated Preoperative von Willebrand Factor Is Associated with Perioperative Thrombosis in Infants and Neonates with Congenital Heart Disease. J. Thromb. Haemost. 2017, 15, 2306–2316. [Google Scholar] [CrossRef]
- Rajpal, S.; Ahluwalia, J.; Kumar, N.; Malhotra, P.; Uppal, V. Elevated Von Willebrand Factor Antigen Levels Are an Independent Risk Factor for Venous Thromboembolism: First Report from North India. Indian. J. Hematol. Blood Transfus. 2019, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, G.; Biasco, L.; Sulzer, I.; Anesini, A.; Moccetti, T.; Kremer Hovinga, J.A.; Alberio, L. Acquired Intracoronary ADAMTS13 Deficiency and VWF Retention at Sites of Critical Coronary Stenosis in Patients with STEMI. Blood 2016, 127, 2934–2936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonneveld, M.A.H.; Kavousi, M.; Ikram, M.A.; Hofman, A.; Rueda Ochoa, O.L.; Turecek, P.L.; Franco, O.H.; Leebeek, F.W.G.; de Maat, M.P.M. Low ADAMTS-13 Activity and the Risk of Coronary Heart Disease—A Prospective Cohort Study: The Rotterdam Study. J. Thromb. Haemost. 2016, 14, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, M.A.H.; de Maat, M.P.M.; Portegies, M.L.P.; Kavousi, M.; Hofman, A.; Turecek, P.L.; Rottensteiner, H.; Scheiflinger, F.; Koudstaal, P.J.; Ikram, M.A.; et al. Low ADAMTS13 Activity Is Associated with an Increased Risk of Ischemic Stroke. Blood 2015, 126, 2739–2746. [Google Scholar] [CrossRef]
- Pagliari, M.T.; Boscarino, M.; Cairo, A.; Mancini, I.; Martinelli, I.; Bucciarelli, P.; Rossi, F.; Rosendaal, F.R.; Peyvandi, F. ADAMTS13 Activity, High VWF and FVIII Levels in the Pathogenesis of Deep Vein Thrombosis. Thromb. Res. 2021, 197, 132–137. [Google Scholar] [CrossRef]
- Sonneveld, M.A.H.; de Maat, M.P.M.; Leebeek, F.W.G. Von Willebrand Factor and ADAMTS13 in Arterial Thrombosis: A Systematic Review and Meta-Analysis. Blood Rev. 2014, 28, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Feys, H.B.; Deckmyn, H.; Vanhoorelbeke, K. ADAMTS13 in Health and Disease. Acta Haematol. 2009, 121, 183–185. [Google Scholar] [CrossRef]
- Nowak-Göttl, U.; Kotthoff, S.; Hagemeyer, E.; Junker, R.; Kehl, H.; Vielhaber, H.; Kececioglu, D. Interaction of Fibrinolysis and Prothrombotic Risk Factors in Neonates, Infants and Children with and without Thromboembolism and Underlying Cardiac Disease. Thromb. Res. 2001, 103, 93–101. [Google Scholar] [CrossRef]
- Alioglu, B.; Avci, Z.; Tokel, K.; Atac, F.B.; Ozbek, N. Thrombosis in Children with Cardiac Pathology: Analysis of Acquired and Inherited Risk Factors. Blood Coagul. Fibrinolysis 2008, 19, 294–304. [Google Scholar] [CrossRef]
- Sokou, R.; Parastatidou, S.; Konstantinidi, A.; Tsantes, A.G.; Iacovidou, N.; Piovani, D.; Bonovas, S.; Tsantes, A.E. Contemporary Tools for Evaluation of Hemostasis in Neonates. Where Are We and Where Are We Headed? Blood Rev. 2023, 101157. [Google Scholar] [CrossRef]
- Appel, I.M.; Grimminck, B.; Geerts, J.; Stigter, R.; Cnossen, M.H.; Beishuizen, A. Age Dependency of Coagulation Parameters during Childhood and Puberty: Pediatric Reference Levels of Coagulation Parameters. J. Thromb. Haemost. 2012, 10, 2254–2263. [Google Scholar] [CrossRef]
- Camet, C.N.; Yee, D.L. Focus on Diagnosis. Pediatr. Rev. 2011, 32, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Dennis, L.; Stewart, J.; Conrad, M. Heparin Treatment of Hæmorrhagic Diathesis in Cyanotic Congenital Heart-Disease. Lancet 1967, 289, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Abildgaard, C.F.; Schulman, I. Absence of Coagulation Abnormalities in Children with Cyanotic Congenital Heart-Disease. Lancet 1968, 292, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, A.L. Coagulation in Cyanotic Congenital Heart Disease. Arch. Pediatr. Adolesc. Med. 1972, 124, 656. [Google Scholar] [CrossRef]
- Ekert, H.; Gilchrist, G.S.; Stanton, R.; Hammond, D. Hemostasis in Cyanotic Congenital Heart Disease. J. Pediatr. 1970, 76, 221–230. [Google Scholar] [CrossRef]
| Variable | CHD Group | Control Group |
|---|---|---|
| N | 20 | 18 |
| Gestational age (weeks) | 37.1 ± 2.5 | 38.2 ± 1.5 |
| Birthweight (g) | 2604 ± 753 | 3090 ± 461 |
| Premature | ||
| No | 12(60.0) | 15(83.3) |
| Yes | 8(40.0) | 3(16.7) |
| Underlying genetic disorder | ||
| No | 10(71.4) | |
| Yes | 4(28.6) | |
| Prenatal diagnosis | ||
| No | 11(55.0) | |
| Yes | 9(45.0) | |
| Obstetric FU | ||
| No | 1(5.0) | |
| Yes | 19(95.0) | |
| Gender N (%) | ||
| Male | 13(65) | 8(44.4) |
| Female | 7(35) | 10(55.6) |
| Type of conception N (%) | ||
| Normal | 18(90) | 16(88.9) |
| IVF | 2(10) | 2(11.1) |
| Bethesda classification | ||
| Simple | 0 | |
| Moderate complexity | 13(65.0) | |
| Complex | 7(35.0) | |
| Type of delivery | ||
| Vaginal | 11(55) | 11(61.1) |
| Cesarean | 9(45) | 7(38.9) |
| Liver pathology | ||
| No | 16(80) | |
| Yes | 4(20) | |
| SGA | ||
| No | 13(65) | 17(94.4) |
| Yes | 7(35) | 1(5.6) |
| Prostaglandin-dependent CHD | ||
| No | 6(30) | |
| Yes | 14(70) | |
| Inotrope administration | ||
| No | 4(20) | |
| Yes | 16(80) | |
| Hemorrhage | ||
| No | 17(85) | |
| Yes | 3(15) | |
| Thrombosis | ||
| No | 18(90) | |
| Yes | 2(10) | |
| Outcome | ||
| Survival | 11(73.3) | |
| Non-survival | 4(26.7) |
| Diagnosis | No |
|---|---|
| PA/CAVC | 1 |
| CoA | 4 |
| CoA/VSD | 2 |
| CoA/APW | 1 |
| TAPVR | 1 |
| Ebstein anomaly | 1 |
| VSD | 1 |
| CCTGA/VSD/PS | 1 |
| TGA | 1 |
| PA | 1 |
| PA/VSD | 1 |
| Fallot tetralogy | 2 |
| CAVC | 1 |
| Single ventricle physiology | 1 |
| DORV/VSD | 1 |
| Variable | CHD | Control | p Value |
|---|---|---|---|
| Chronological neonatal age (days) | 13.8 ± 14.0 | 4.6 ± 2.2 | - |
| PT (s) | 14.4 ± 3.8 | 11.9 ± 1.8 | 0.012 |
| aPPT (s) | 29.7 ± 3.2 | 29.5 ± 2.7 | 0.809 |
| Fibrinogen (mg%) | 258.9 ± 85.2 | 268.8 ± 55.8 | 0.677 |
| FII (%) | 60.0 ± 16.5 | 59.8 ± 17.1 | 0.976 |
| FVII (%) | 41.6 ± 22.4 | 60.1 ± 16.0 | 0.006 |
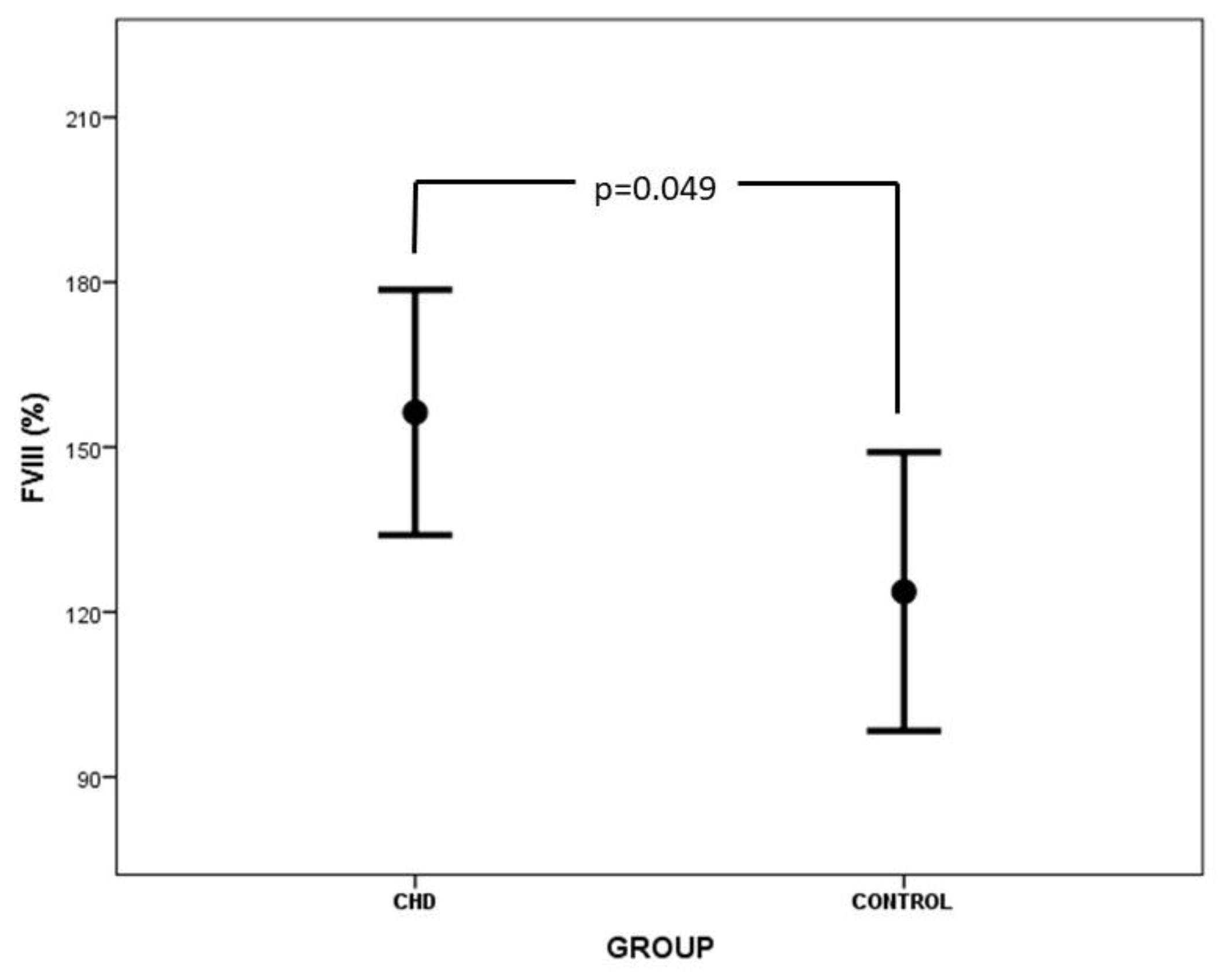
| FVIII (%) | 156.3 ± 47.7 | 123.7 ± 51.0 | 0.049 |
| FIX (%) | 58.7 ± 14.2 | 61.3 ± 25.0 | 0.688 |
| FX (%) | 56.8 ± 12.9 | 53.3 ± 12.8 | 0.419 |
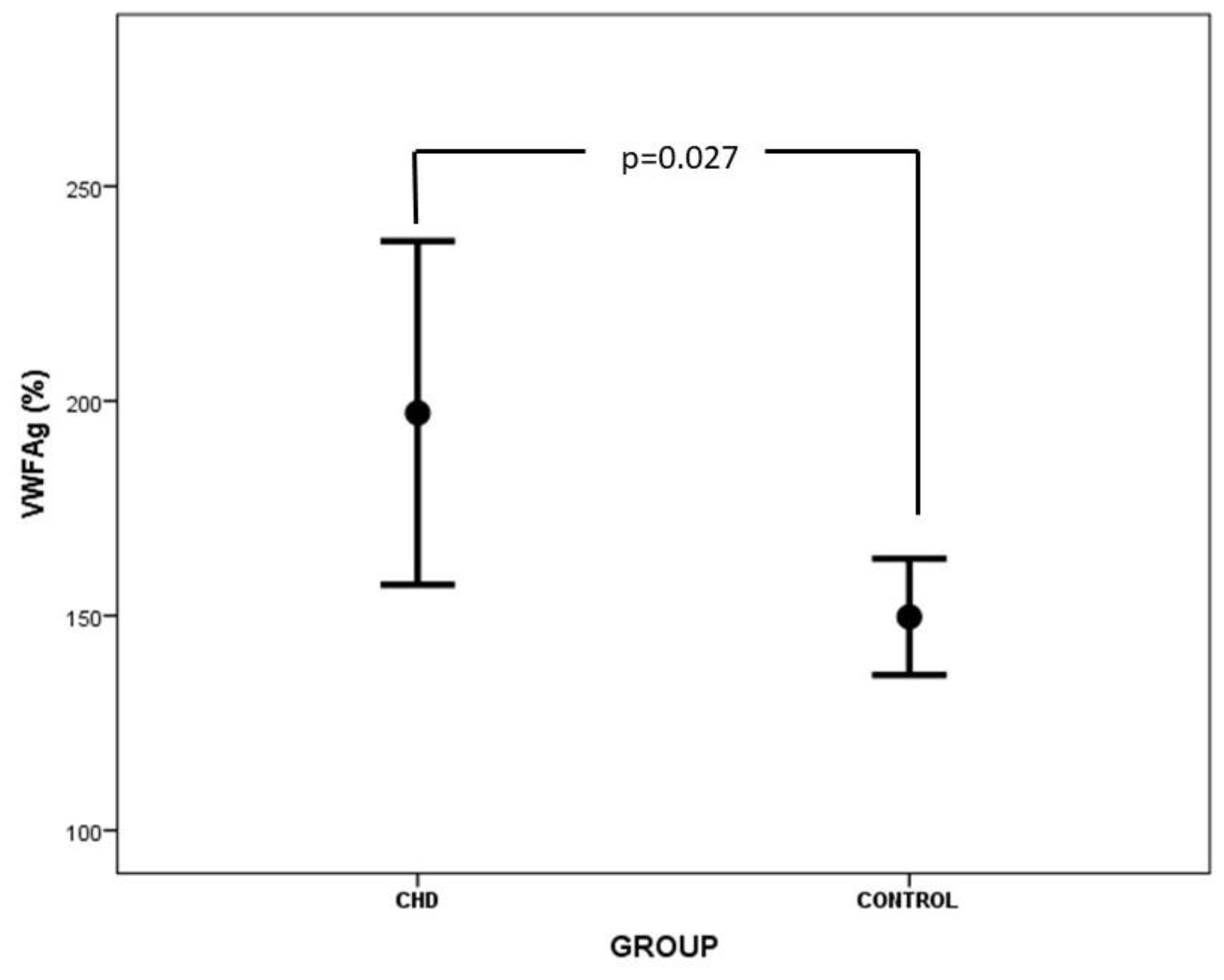
| VWFAg(%) | 207.6 ± 73.8 | 147.8 ± 26.0 | 0.027 |
| Rcof(%) | 175.6 ± 64.9 | 137.1 ± 23.6 | 0.022 |
| Antithrombin (%) | 65.0 ± 18.9 | 63.1 ± 13.3 | 0.718 |
| Protein C (%) | 39.6 ± 14.1 | 40.1 ± 8.3 | 0.884 |
| Protein S (%) | 53.1 ± 18.2 | 44.5 ± 11.2 | 0.091 |
| D-dimers (μg/mL) | 1.8 ± 1.6 | 6.4 ± 7.5 | 0.036 |
| ADAMTS-13 (ng/mL) | 490.4 ± 167.4 | 577.2 ± 113.6 | 0.073 |
| Variable | Non-Prostaglandin Dependent | Prostaglandin Dependent | p-Value |
|---|---|---|---|
| PT (s) | 11.4 ± 0.5 | 16.0 ± 4.0 | 0.022 |
| FVII (%) | 68.6 ± 11.6 | 29.2 ± 15.8 | <0.001 |
| FX (%) | 72.0 ± 12.2 | 51.0 ± 8.5 | 0.002 |
| Protein C (%) | 50.6 ± 7.9 | 34.1 ± 12.8 | 0.017 |
| Protein S (%) | 67.8 ± 11.3 | 47.1 ± 18.0 | 0.031 |
| ADAMTS-13 (ng/mL) | 503 ± 127 | 452 ± 126 | 0.455 |
| D-dimers (μg/mL) | 1.0 ± 0.7 | 2.2 ± 1.8 | 0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadogeorgou, P.; Valsami, S.; Boutsikou, M.; Pergantou, E.; Mantzou, A.; Papassotiriou, I.; Iliodromiti, Z.; Sokou, R.; Bouza, E.; Politou, M.; et al. Coagulation Profile in Neonates with Congenital Heart Disease: A Pilot Study. Medicina 2024, 60, 268. https://doi.org/10.3390/medicina60020268
Papadogeorgou P, Valsami S, Boutsikou M, Pergantou E, Mantzou A, Papassotiriou I, Iliodromiti Z, Sokou R, Bouza E, Politou M, et al. Coagulation Profile in Neonates with Congenital Heart Disease: A Pilot Study. Medicina. 2024; 60(2):268. https://doi.org/10.3390/medicina60020268
Chicago/Turabian StylePapadogeorgou, Paraskevi, Serena Valsami, Maria Boutsikou, Eleni Pergantou, Aimilia Mantzou, Ioannis Papassotiriou, Zoi Iliodromiti, Rozeta Sokou, Elena Bouza, Marianna Politou, and et al. 2024. "Coagulation Profile in Neonates with Congenital Heart Disease: A Pilot Study" Medicina 60, no. 2: 268. https://doi.org/10.3390/medicina60020268
APA StylePapadogeorgou, P., Valsami, S., Boutsikou, M., Pergantou, E., Mantzou, A., Papassotiriou, I., Iliodromiti, Z., Sokou, R., Bouza, E., Politou, M., Iacovidou, N., & Boutsikou, T. (2024). Coagulation Profile in Neonates with Congenital Heart Disease: A Pilot Study. Medicina, 60(2), 268. https://doi.org/10.3390/medicina60020268








