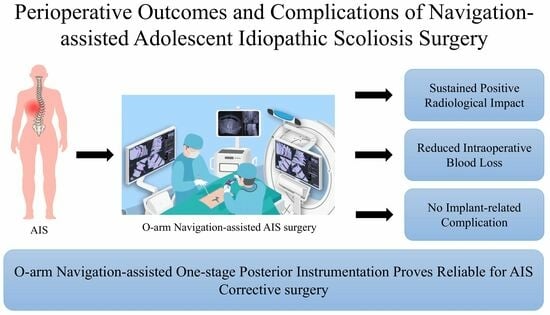
Navigation-Assisted One-Staged Posterior Spinal Fusion Using Pedicle Screw Instrumentation in Adolescent Idiopathic Scoliosis—A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Variables
2.4. Surgical Procedure and Postoperative Care
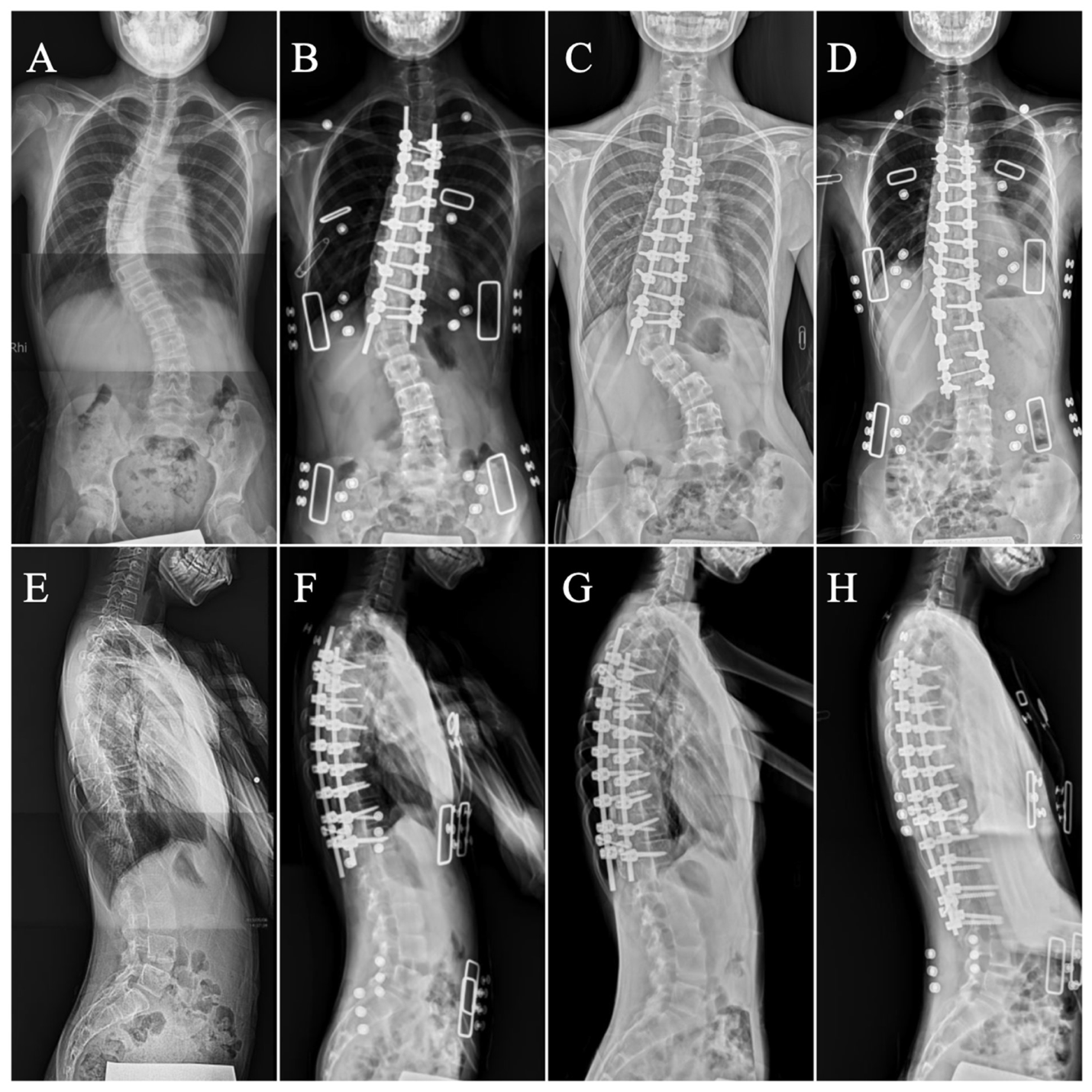
2.5. Measurements
2.6. Statistical Analysis
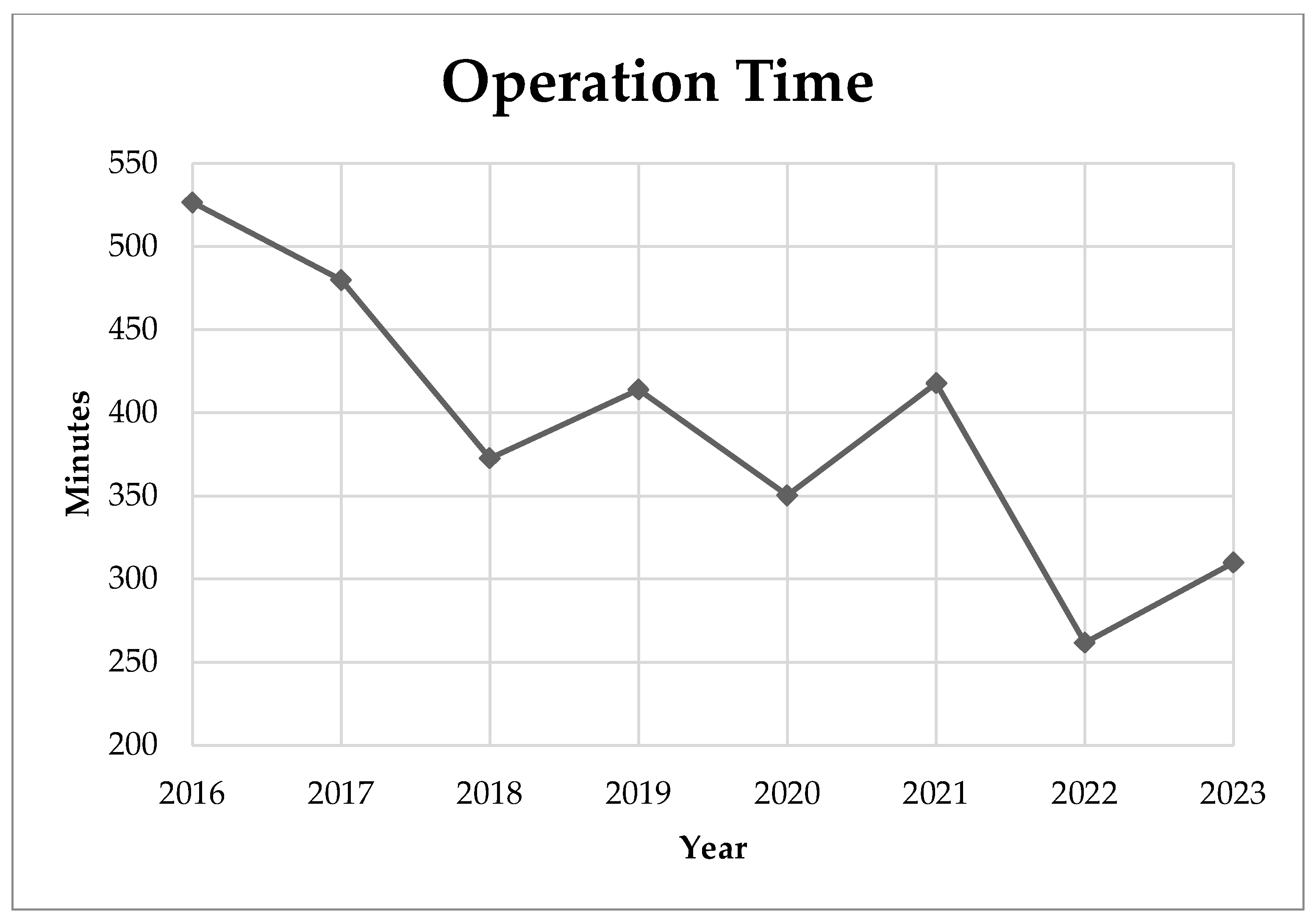
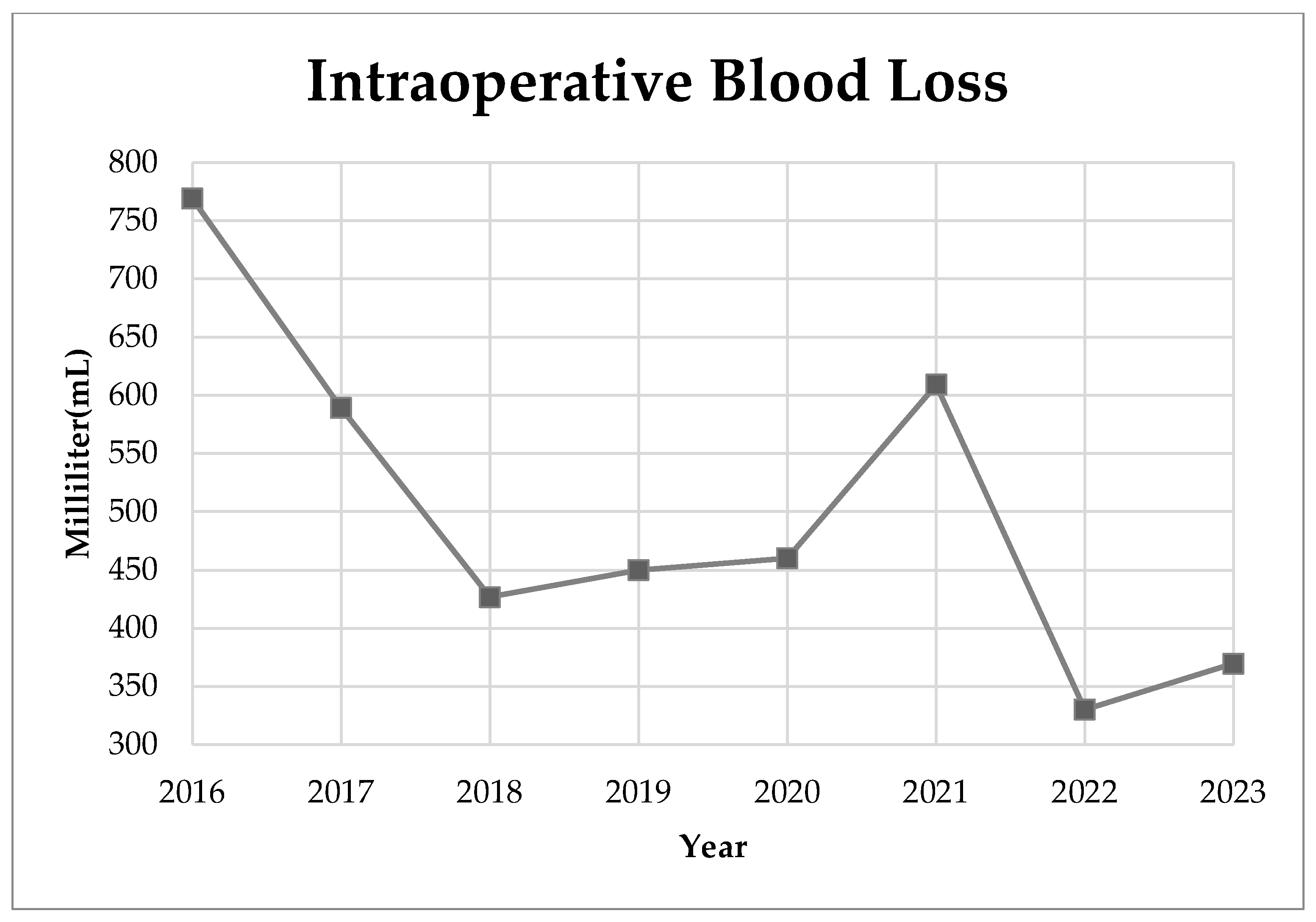
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konieczny, M.R.; Senyurt, H.; Krauspe, R. Epidemiology of adolescent idiopathic scoliosis. J. Child. Orthop. 2013, 7, 3–9. [Google Scholar] [CrossRef]
- Kamtsiuris, P.; Atzpodien, K.; Ellert, U.; Schlack, R.; Schlaud, M. Prevalence of somatic diseases in German children and adolescents. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 686–700. [Google Scholar] [CrossRef]
- Daruwalla, J.S.; Balasubramaniam, P.; Chay, S.O.; Rajan, U.; Lee, H.P. Idiopathic scoliosis. Prevalence and ethnic distribution in Singapore schoolchildren. J. Bone Jt. Surg. Br. Vol. 1985, 67, 182–184. [Google Scholar] [CrossRef]
- Sung, S.; Chae, H.W.; Lee, H.S.; Kim, S.; Kwon, J.W.; Lee, S.B.; Moon, S.H.; Lee, H.M.; Lee, B.H. Incidence and Surgery Rate of Idiopathic Scoliosis: A Nationwide Database Study. Int. J. Environ. Res. Public Health 2021, 18, 8152. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, J.F.; Rimetz, A.; Genevieve, F.; Renaud, G.; Mounet, N. Idiopathic adolescent scoliosis and obesity: Prevalence study. Eur. Spine J. 2023, 32, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Zaydman, A.M.; Strokova, E.L.; Pahomova, N.Y.; Gusev, A.F.; Mikhaylovskiy, M.V.; Shevchenko, A.I.; Zaidman, M.N.; Shilo, A.R.; Subbotin, V.M. Etiopathogenesis of adolescent idiopathic scoliosis: Review of the literature and new epigenetic hypothesis on altered neural crest cells migration in early embryogenesis as the key event. Med. Hypotheses 2021, 151, 110585. [Google Scholar] [CrossRef]
- Kouwenhoven, J.W.; Castelein, R.M. The pathogenesis of adolescent idiopathic scoliosis: Review of the literature. Spine 2008, 33, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Machado, G.; Berenguer-Pascual, E.; Bovea-Marco, M.; Rubio-Belmar, P.A.; García-López, E.; Garzón, M.J.; Mena-Mollá, S.; Pallardó, F.V.; Bas, T.; Viña, J.R.; et al. From genetics to epigenetics to unravel the etiology of adolescent idiopathic scoliosis. Bone 2020, 140, 115563. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, S.R.; Qiu, G.X.; Zhang, J.G.; Zhuang, Q.Y. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin. Med. J. 2020, 133, 483–493. [Google Scholar] [CrossRef]
- Seifert, J.; Thielemann, F.; Bernstein, P. Adolescent idiopathic scoliosis: Guideline for practical application. Orthopade 2016, 45, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.W. Adolescent idiopathic scoliosis. Orthop Clin. N. Am. 1999, 30, 353–365, vii–viii. [Google Scholar] [CrossRef]
- Alzakri, A.; AlMuhid, F.; Almousa, N.; Aljehani, M.; Alhalabi, H. Saudi patients outcomes after surgical treatment of adolescent idiopathic scoliosis. J. Orthop. Surg. Res. 2023, 18, 450. [Google Scholar] [CrossRef] [PubMed]
- Di Silvestre, M.; Zanirato, A.; Greggi, T.; Scarale, A.; Formica, M.; Vallerga, D.; Legrenzi, S.; Felli, L. Severe adolescent idiopathic scoliosis: Posterior staged correction using a temporary magnetically-controlled growing rod. Eur. Spine J. 2020, 29, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.K.; Loh, K.W.; Chung, W.H.; Chiu, C.K.; Hasan, M.S.; Chan, C.Y.W. Perioperative outcome and complications following single-staged Posterior Spinal Fusion (PSF) using pedicle screw instrumentation in Adolescent Idiopathic Scoliosis (AIS): A review of 1057 cases from a single centre. BMC Musculoskelet. Disord. 2021, 22, 413. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.K.; Kaucha, D.; Banskota, B.; Barakoti, R.K.; Banskota, A.K. Correlation between Radiological Outcome and Health Related Quality of Life after Posterior Spinal Fusion for Adolescent Idiopathic Scoliosis. J. Nepal Health Res. Counc. 2021, 19, 44–47. [Google Scholar] [CrossRef] [PubMed]
- St-Georges, M.; Teles, A.R.; Rabau, O.; Saran, N.; Ouellet, J.A.; Ferland, C.E. Adolescent idiopathic scoliosis: Evaluating perioperative back pain through a simultaneous morphological and biomechanical approach. BMC Musculoskelet. Disord. 2020, 21, 466. [Google Scholar] [CrossRef] [PubMed]
- Sarwahi, V.; Galina, J.; Atlas, A.; Gecelter, R.; Hasan, S.; Amaral, T.D.; Maguire, K.; Lo, Y.; Kalantre, S. Scoliosis Surgery Normalizes Cardiac Function in Adolescent Idiopathic Scoliosis Patients. Spine 2021, 46, E1161–E1167. [Google Scholar] [CrossRef] [PubMed]
- Pietton, R.; Bouloussa, H.; Langlais, T.; Taytard, J.; Beydon, N.; Skalli, W.; Vergari, C.; Vialle, R. Estimating pulmonary function after surgery for adolescent idiopathic scoliosis using biplanar radiographs of the chest with 3D reconstruction. Bone Jt. J. 2022, 104-b, 112–119. [Google Scholar] [CrossRef] [PubMed]
- De Bodman, C.; Ansorge, A.; Tabard, A.; Amirghasemi, N.; Dayer, R. Clinical and radiological outcomes of minimally-invasive surgery for adolescent idiopathic scoliosis at a minimum two years’ follow-up. Bone Jt. J. 2020, 102-b, 506–512. [Google Scholar] [CrossRef]
- Lonner, B.S.; Ren, Y.; Yaszay, B.; Cahill, P.J.; Shah, S.A.; Betz, R.R.; Samdani, A.F.; Shufflebarger, H.L.; Newton, P.O. Evolution of Surgery for Adolescent Idiopathic Scoliosis Over 20 Years: Have Outcomes Improved? Spine 2018, 43, 402–410. [Google Scholar] [CrossRef]
- Carreon, L.Y.; Puno, R.M.; Lenke, L.G.; Richards, B.S.; Sucato, D.J.; Emans, J.B.; Erickson, M.A. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J. Bone Jt. Surg. Am. 2007, 89, 2427–2432. [Google Scholar] [CrossRef]
- Tang, C.Y.K.; Kamath, V.H.D.; Cheung, P.W.H.; Cheung, J.P.Y. Predictive factors for intraoperative blood loss in surgery for adolescent idiopathic scoliosis. BMC Musculoskelet. Disord. 2021, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xiao, H.; Wang, R.; Huang, Y. Prediction of massive blood loss in scoliosis surgery from preoperative variables. Spine 2013, 38, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, R.; Takeshita, K.; Takahashi, J.; Arai, Y.; Watanabe, K.; Yamato, Y.; Oba, H.; Matsumoto, M. The complication trends of pediatric spinal deformity surgery in Japan—The Japanese Scoliosis Society Morbidity and Mortality survey from 2012 to 2017. J. Orthop. Sci. 2021, 26, 744–749. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Li, D.; Tian, Y.; Yuan, S.; Wang, L.; Liu, X. Safety and accuracy of cannulated pedicle screw placement in scoliosis surgery: A comparison of robotic-navigation, O-arm-based navigation, and freehand techniques. Eur. Spine J. 2023, 32, 3094–3104. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ando, K.; Ito, K.; Tsushima, M.; Morozumi, M.; Tanaka, S.; Machino, M.; Ota, K.; Ishiguro, N.; Imagama, S. Intraoperative radiation exposure in spinal scoliosis surgery for pediatric patients using the O-arm® imaging system. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 579–583. [Google Scholar] [CrossRef]
- Abdaliyev, S.S.; Yestay, D.Z.; Vissarionov, S.V.; Baitov, D.T.; Serikov, S.Z.; Chsherbina, A.Y. Computed tomography-guided intraoperative navigation in children with congenital scoliosis versus freehand/fluoroscopy methods. Pediatr. Traumatol. Orthop. Reconstr. Surg. 2023, 11, 307–314. [Google Scholar] [CrossRef]
- Feng, W.; Wang, W.; Chen, S.; Wu, K.; Wang, H. O-arm navigation versus C-arm guidance for pedicle screw placement in spine surgery: A systematic review and meta-analysis. Int. Orthop. 2020, 44, 919–926. [Google Scholar] [CrossRef]
- Linden, G.S.; Ghessese, S.; Cook, D.; Hedequist, D.J. Pedicle Screw Placement in Adolescent Idiopathic Scoliosis: A Comparison between Robotics Coupled with Navigation versus the Freehand Technique. Sensors 2022, 22, 5204. [Google Scholar] [CrossRef]
- Kaur, J.; Koltsov, J.C.B.; Kwong, J.W.; Cheng, I.; Vorhies, J.S. Does Navigation Make Spinal Fusion for Adolescent Idiopathic Scoliosis Safer? Insights From a National Database. Spine 2021, 46, E1049–E1057. [Google Scholar] [CrossRef]
- Bartley, C.E.; Yaszay, B.; Bastrom, T.P.; Shah, S.A.; Lonner, B.S.; Asghar, J.; Miyanji, F.; Samdani, A.; Newton, P.O. Perioperative and Delayed Major Complications Following Surgical Treatment of Adolescent Idiopathic Scoliosis. J. Bone Jt. Surg. Am. 2017, 99, 1206–1212. [Google Scholar] [CrossRef]
- Darnis, A.; Grobost, P.; Roussouly, P. Very long-term clinical and radiographic outcomes after posterior spinal fusion with pedicular screws for thoracic adolescent idiopathic scoliosis. Spine Deform. 2021, 9, 441–449. [Google Scholar] [CrossRef]
- Uehara, M.; Kuraishi, S.; Ikegami, S.; Oba, H.; Takizawa, T.; Munakata, R.; Hatakenaka, T.; Koseki, M.; Takahashi, J. Long-Term Surgical Results of Skip Pedicle Screw Fixation for Patients with Adolescent Idiopathic Scoliosis: A Minimum-Ten-Year Follow-Up Study. J. Clin. Med. 2020, 9, 4002. [Google Scholar] [CrossRef] [PubMed]
- Benli, I.T.; Ates, B.; Akalin, S.; Citak, M.; Kaya, A.; Alanay, A. Minimum 10 years follow-up surgical results of adolescent idiopathic scoliosis patients treated with TSRH instrumentation. Eur. Spine J. 2007, 16, 381–391. [Google Scholar] [CrossRef]
- Kwan, M.K.; Chiu, C.K.; Tan, P.H.; Chian, X.H.; Ler, X.Y.; Ng, Y.H.; Ng, S.J.; Goh, S.H.; Chan, C.Y.W. Radiological and clinical outcome of selective thoracic fusion for patients with Lenke 1C and 2C adolescent idiopathic scoliosis with a minimum follow-up of 2 years. Spine J. 2018, 18, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.H.; Betz, R.R.; Newton, P.O.; Rohmiller, M.; Marks, M.C.; Bastrom, T. Correlation of scoliosis curve correction with the number and type of fixation anchors. Spine 2009, 34, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.N.; Polly, D.W., Jr.; Diamond, B.; Ledonio, C.; Richards, B.S., III; Emans, J.B.; Sucato, D.J.; Johnston, C.E.; Minimize Implants Maximize Outcomes Study Group. Does higher anchor density result in increased curve correction and improved clinical outcomes in adolescent idiopathic scoliosis? Spine 2014, 39, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jones-Quaidoo, S.M.; Eager, M.; Griffin, J.W.; Reddi, V.; Novicoff, W.; Shilt, J.; Bersusky, E.; Defino, H.; Ouellet, J.; et al. Right adolescent idiopathic thoracic curve (Lenke 1 A and B): Does cost of instrumentation and implant density improve radiographic and cosmetic parameters? Eur. Spine J. 2011, 20, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.M.; Gibson, M.J. Correction of main thoracic adolescent idiopathic scoliosis using pedicle screw instrumentation: Does higher implant density improve correction? Spine 2010, 35, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, N.J.; Lonner, B.S.; Auerbach, J.D.; Kean, K.E.; Trobisch, P.D. Low-density versus high-density thoracic pedicle screw constructs in adolescent idiopathic scoliosis: Do more screws lead to a better outcome? Spine J. 2013, 13, 375–381. [Google Scholar] [CrossRef]
- Larson, A.N.; Polly, D.W.; Sponseller, P.D.; Kelly, M.P.; Richards, B.S.; Garg, S.; Parent, S.; Shah, S.A.; Weinstein, S.L.; Crawford, C.H.; et al. The Effect of Implant Density on Adolescent Idiopathic Scoliosis Fusion: Results of the Minimize Implants Maximize Outcomes Randomized Clinical Trial. J. Bone Jt. Surg. Am. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Delman, C.; Cage, J.M.; Lausé, G.; Roberto, R.; Gupta, M.C.; Klineberg, E. Anterior and Posterior Fusion for Large, Rigid Idiopathic Scoliosis: Does Implant Density Matter? World Neurosurg. 2020, 134, e37–e45. [Google Scholar] [CrossRef]
- Vavruch, L.; Brink, R.C.; Malmqvist, M.; Schlösser, T.P.; van Stralen, M.; Abul-Kasim, K.; Ohlin, A.; Castelein, R.M.; Tropp, H. Surgical outcomes of anterior versus posterior fusion in lenke type 1 adolescent idiopathic scoliosis. Spine 2019, 44, E823–E832. [Google Scholar] [CrossRef]
- Gu, H.; Li, Y.; Dai, Y.; Wang, B. Anterior versus posterior approach in Lenke type 1 adolescent idiopathic scoliosis: A comparison of long-term follow-up outcomes. Ann. Transl. Med. 2022, 10, 405. [Google Scholar] [CrossRef]
- Boylan, M.R.; Riesgo, A.M.; Chu, A.; Paulino, C.B.; Feldman, D.S. Costs and complications of increased length of stay following adolescent idiopathic scoliosis surgery. J. Pediatr. Orthop. B 2019, 28, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lonner, B.S.; Ren, Y.; Bess, S.; Kelly, M.; Kim, H.J.; Yaszay, B.; Lafage, V.; Marks, M.; Miyanji, F.; Shaffrey, C.I. Surgery for the adolescent idiopathic scoliosis patients after skeletal maturity: Early versus late surgery. Spine Deform. 2019, 7, 84–92. [Google Scholar] [CrossRef] [PubMed]
- LaBarge, M.E.; Chanbour, H.; Waddell, W.H.; Vickery, J.; Jonzzon, S.; Roth, S.G.; Croft, A.J.; Abtahi, A.M.; Louer, C.R.; Martus, J.E. Clinical and radiographic outcomes following correction of idiopathic scoliosis in adolescence vs. young adulthood. Spine Deform. 2023, 11, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.; Gibson, A.; Dannawi, Z.; Noordeen, H. Adolescent idiopathic scoliosis. BMJ 2013, 346, f2508. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Borkhuu, B.; Dhawale, A.A.; Oto, M.; Littleton, A.G.; Mason, D.E.; Gabos, P.G.; Shah, S.A. Comparative analysis of hook, hybrid, and pedicle screw instrumentation in the posterior treatment of adolescent idiopathic scoliosis. J. Pediatr. Orthop. 2012, 32, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Wagala, N.N.; Marasigan, J.A.M.; Mian, H.M.; Schwend, R.M. Operative time in adolescent idiopathic scoliosis surgery: A need for a standard definition. J. Pediatr. Orthop. B 2021, 30, 205–210. [Google Scholar] [CrossRef]
- Uribe, J.S.; Kolla, J.; Omar, H.; Dakwar, E.; Abel, N.; Mangar, D.; Camporesi, E. Brachial plexus injury following spinal surgery: A review. J. Neurosurg. Spine 2010, 13, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.I.; Vaghela, K.R.; Brown, H.; Adams, K.; Sinisi, M.; Fox, M.; Quick, T. Reducing the risk and impact of brachial plexus injury sustained from prone positioning—A clinical commentary. J. Intensive Care Med. 2020, 35, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Dolan, L.; Weinstein, S. Unanticipated revision surgery in adolescent idiopathic scoliosis. Spine 2012, 37, 1048–1053. [Google Scholar] [CrossRef] [PubMed]

| Radiological Measurement | Total (n = 55) | Age 12–16 (n = 32) | Age > 16 (n = 23) | p-Value (Age) 12–16 vs. >16 |
|---|---|---|---|---|
| Preoperative major Cobb angle (°) | 58.24 ± 15.99 | 61.87 ± 17.51 | 53.19 ± 12.25 | p = 0.046 * |
| Postoperative major Cobb angle (°) | 23.47 ± 8.02 | 24.27 ± 8.80 | 22.36 ± 6.82 | p = 0.390 |
| Last follow-up major Cobb angle (°) | 24.62 ± 7.72 | 25.20 ± 8.22 | 23.82 ± 7.07 | p = 0.517 |
| Initial correction rate (%) | 58.88 ± 12.88 | 60.00 ± 13.35 | 57.16 ± 12.30 | p = 0.426 |
| Loss of correction rate (%) Last follow-up correction rate (%) | 3.36 ± 11.09 | 2.82 ± 11.40 | 4.10 ± 10.87 | p = 0.675 |
| 56.56 ± 13.27 | 57.99 ± 13.93 | 54.56 ± 12.31 | p = 0.348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-L.; Chen, M.J.-W.; Hsiao, P.-H.; Lin, C.-Y.; Lo, Y.-S.; Tseng, C.; Li, L.-Y.; Lai, C.-Y.; Chen, H.-T. Navigation-Assisted One-Staged Posterior Spinal Fusion Using Pedicle Screw Instrumentation in Adolescent Idiopathic Scoliosis—A Case Series. Medicina 2024, 60, 300. https://doi.org/10.3390/medicina60020300
Chang P-L, Chen MJ-W, Hsiao P-H, Lin C-Y, Lo Y-S, Tseng C, Li L-Y, Lai C-Y, Chen H-T. Navigation-Assisted One-Staged Posterior Spinal Fusion Using Pedicle Screw Instrumentation in Adolescent Idiopathic Scoliosis—A Case Series. Medicina. 2024; 60(2):300. https://doi.org/10.3390/medicina60020300
Chicago/Turabian StyleChang, Pao-Lung, Michael Jian-Wen Chen, Pang-Hsuan Hsiao, Chia-Yu Lin, Yuan-Shun Lo, Chun Tseng, Ling-Yi Li, Chien-Ying Lai, and Hsien-Te Chen. 2024. "Navigation-Assisted One-Staged Posterior Spinal Fusion Using Pedicle Screw Instrumentation in Adolescent Idiopathic Scoliosis—A Case Series" Medicina 60, no. 2: 300. https://doi.org/10.3390/medicina60020300